Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.30 no.1 Bogotá ene./mar. 2015
Seroprotection for Hepatitis B after Three Doses of Vaccine in Colombian HIV Patients under the Age of 18
Maira Patricia Sánchez Pérez MD. (1), Carlos Alberto Velasco Benítez MD. (2), Pío López López MD. (3)
(1) Pediatrician and Gastroenterologist at the Fundación Valle del Lili and the Imbanaco Medical Center in Cali, Colombia. Email: mairasanchez@yahoo.com.
(2) Pediatrician, Gastroenterologist and Nutritionist at Universidad del Valle in Cali, Colombia. Email: carlos.velasco@correounivalle.edu.co.
(3) Pediatrician and Infectious Disease Specialist at the Universidad del Valle in Cali, Colombia. Email: piolo@emcali.net.co.
Received: 04-03-14 Accepted: 02-02-15
Abstract
Introduction: Seroprotection against hepatitis B is defined as anti-hepatitis B surface antigen titer ≥ 10 IU/L), but in children with human immunodeficiency virus (HIV) this is low.
Objective: The objective of this study was to establish SP against HBV and potential associated factors in children with HIV in Cali, Colombia.
Methodology: This is a study of seroprotection against HBV in 85 children under 18 years of age after receiving three doses of vaccine. Clinical, paraclinical, environmental and sociodemographic variables were considered. Statistical analysis included estimation of proportion per occurrence, 95% confidence intervals, percentages, means, standard deviations, and univariate analysis between variable exposure and effect. Confounding factors were evaluated with 2x2 contingency tables and estimation of odds ratios with 95% confidence intervals. The Chi2 test was used with significance of p <0.05. The multivariate model used backward elimination. Variables with p> 0.10 were eliminated by likelihood-ratio test.
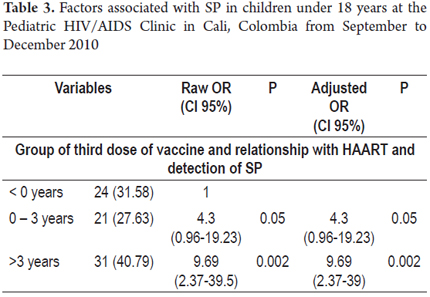
Results: SP was 35.3% (95% CI: 25.2 to 46.4, n = 30) in children with a mean age of 101 ± 44 months. Patients were predominantly female gender and mixed race and had Stage C HIV (according to US Centers for Disease Control and Prevention (CDC) standards). Average treatment time was 5 years. No relationship was found between the time between the first, second and third doses of vaccine and SP, nor among diagnosis, time of vaccination, and SP. The multivariate model showed an association of SP with the temporal relationship between start of treatment (0-3 years), last dose of vaccination (OR = 4.3, 95% CI: 0.96 to 19.23; p = 0.05) and more than three years after starting treatment (OR = 9.69, 95% CI: 2.37 to 39.5; p = 0.00).
Conclusion: The prevalence of SP was 35.3%, with a possible risk factor associated with the temporal relationship between onset of treatment and time of the last dose of vaccine.
Keywords
Hepatitis B, HIV infection, seroprotection, children.
INTRODUCTION
HIV (Human Immunodeficiency Virus) is transmitted through contact with blood and through sexual activity which are also two of the most important risk factors for acquiring hepatitis B virus (HBV) (1-4).
According to the World Health Organization (WHO) coinfections of HIV with HBV and hepatitis C virus (HCV) are associated with accelerated progression and worse prognoses for liver diseases. The results can be chronic infections, cirrhosis, cancer and death (2). Around 600,000 people die each year due to the effects of acute or chronic hepatitis. About 25% of the adults who became chronically infected during childhood die from liver cancer or cirrhosis (2). The incidence of hepatitis B can be reduced by using the vaccine according to the schemes proposed by WHO (2).
A study by Simani et al. in Sub-Saharan Africa found high levels of seroprotection (SP) for HBV in children 5 to 24 months of age. In fact, 89.3% had quantitative titers of antibodies to surface antigen anti-HBsAg higher than 10 IU/L (3). A study by Pessoa et al. in 2010 in Brazil found that 40.5% of adolescents with HIV had SP whereas 78.3% of healthy adolescents had SP (4). In 2009 Abzug et al. found that 24% of school children had SP and that after revaccination this increased to 46% at 8 weeks of the new scheme (5). A study of 121 children with HIV by Fernández-Ibieta et al. in 2009 in Spain observed a prevalence of Anti-HBsAg> 10 IU/L for HBV of 16.5%. Patients in stage A had the highest proportion of SP (20%), followed by those in stage B (16%) and then stage C (9.4%) (Stages according to the Centers for Disease Control and Prevention (CDC) in the United States). This percentage decreased in relation to the time following vaccination in these children. While it was 36.8% among children vaccinated within three years versus, it was only 7.4% for children vaccinated more than 12 years earlier (6). An unpublished study by P. Lopez P in Cali, Colombia reports a 20% SP rate, but this study did not take into account any risk factors or confounders associated with this inadequate response.
The objectives of this study are first to determine the prevalence of SP for HBV after 3 doses of vaccine in children under eighteen years of age at the Pediatrics HIV/AIDS clinic in Cali, Colombia, and then to look at possible associations with sociodemographic, clinical, laboratory, and environmental factors.
METHODOLOGY
A study of prevalence of SP for HBV in children under 18 years of age who had been diagnosed with HIV infection was conducted among the patients of the Pediatric HIV/AIDS Clinic in Cali, Colombia between September and December 2010. The study determined possible risk factors and measured SP for HBV after each of three doses of vaccine. The target population consisted of children in southwestern Colombia who were undergoing Highly Active Antiretroviral Therapy (HAART) or who were being observed. Both male and female children were included. Their ages ranged from 8 months to 18 years old. HIV had been diagnosed with ELISA and positive viral loads were confirmed by Western Blot tests taken at least 15 days before or after testing for anti-HBsAg. Children who had not been transfused within the previous six months were also included. The minimum interval between doses was four weeks with the time lag between vaccine one and three not exceeding 18 months. All patients had had their final dose of vaccine within the last 10 years. Children were excluded with other confirmed types of immunodeficiency and children hospitalized for severe infections concomitant to HIV that compromised their lives. These included basic septic shock, multisystem organ failure, sepsis, electrolyte imbalances, acid-base imbalances, severe respiratory distress syndrome and children with prior histories of HBV infection.
Assuming an expected prevalence of 24%, with an estimation error of ± 5%, a 95% confidence level, and the total population in the Pediatric HIV/AIDS Clinic in Cali, Colombia on September 1, 2010 of 122 children, the sample size was 84 children.
The study's main interests were viral load and the percentage of CD4 protein. Sociodemographic, clinical, paraclinical and environmental variables were also evaluated. Sociodemographic variables included age, sex, origin, race, and history while clinical variables included HIV mode of transmission, nutritional status (weight, height, BMI, height for age), signs and symptoms of chronic liver disease, stage of HIV infection (In accordance with the CDC staging system), hospitalizations, Highly Active Antiretroviral therapy (HAART), treatment time, time between doses of vaccine, temporal relationships between measurements of levels of specific antibodies, and measurements after each of three vaccination doses, starting date of HAART, and date of HIV diagnosis. Paraclinical variables included viral load, CD4 percentage, direct bilirubin, and AST/ALT. Environmental variables included sexual activity, intravenous drug use, overcrowding, abandonment, breastfeeding and whether the mother and/or parents were alive or dead,. The outcome variable was the determination of SP for HBV defined as quantitative titers of antibodies to surface antigen (anti-HBsAg) greater than 10 IU/L as measured by the chemiluminescence method (microELISA) (7).
This work was approved by the Ethics Committee of the Universidad del Vale and the Pediatric HIV/AIDS Clinic of Cali, Colombia. Parents and/or guardians of children who agreed to participate in the study signed an informed consent form and answered a structured survey provided by researchers. After the survey, five ml samples of peripheral blood were taken from each child for analysis of Anti-HBsAg in an automated system (Team Elysis One) for microElisa tests for determining antibodies (7).
A database was created in EpiInfo 6.04 and statistical analysis was performed with Stata 10. To assess data entry typing errors, 10% of the database was reviewed and compared with the original to make any needed corrections. An exploratory analysis of all variables was performed to describe the distribution of the variables. Measures of central tendency and dispersion as well as histograms, box graphs, whisker plots, and symmetry graphs were calculated to describe the distribution of continuous variables. Normal analysis was performed and, if necessary, transformation of data developed for standardization. Frequencies and proportions were obtained for categorical variables, HIV viral loads were defined according to four categories: less than 400 copies/ml; between 401 and 29,999 copies/ml; between 30,000 and 99,999 copies/ml; and more than 100,000 copies/ml. The percentages of CD4 were divided into three categories: less than 15%, 15% to 24%, and greater than 25%. Three time variables were categorized. Time from the date of the third dose of vaccine to the dates samples used to evaluate SP were taken was categorized into 0-3 years, 3.1-6 years, and more than 6 years. Seroprotection was measured at different times following HIV diagnosis and categorized as follows: 0 -3 years, 3.1 to 6 years, 6.1 to 9 years and more than 9 years. Serum protection was categorized in relation to duration of treatment as follows: 0 to 3.3 years, 3.4 to 6.6 years, 6.7 to 10 years, and more than 10 years. The temporal relationship between the third dose of vaccine, the initiation of HAART, and measurement of specific antibody levels was categorized as follows: less than zero years, zero to three years, and more than three years.
The nutritional status of children in the study was established by referencing the tables of the WHO for Body Mass Index (BMI) (normal weight, risk of being underweight or overweight, overweight or thinness, and obesity or extreme thinness) and the WHO Length/height-for-age index (normal height for age, risk of stunting, stunted, and severely stunted).
The proportion of infected children was determined with the 95% corresponding confidence interval. Descriptive measures were determined with their corresponding standard deviations (SD) and ranges. Univariate analysis of each of the exposure variables of interest was done to assess potential risk factors, and the variable effects were analyzed. Besides possible associations among variables including viral load, CD4 percentage, treatment time in months, Association between the SP and other covariates were explored with the aim of identifying any confounders. Odds ratios (OR) were estimated with their respective 95% confidence intervals, and statistical significance was evaluated with Fisher's exact test. Multiple logistic regression was used to assess confounding and interaction. The Backward method (backward elimination) was used for the construction of the multivariate model. All variables that showed a significant statistical association at the 0.20 level of significance the in univariate analysis were included. The likelihood ratio test was used to eliminate variables from the model test when the p value was greater than 0.10. The Hosmer-Lemeshow (HL) test for logistic regression was used to test the model's goodness of fit.
RESULTS
Between September and December 2010 there were 122 children who were initially eligible. Thirty seven of these children were excluded. One child died, one had a transfusion, one had Guillán Barré syndrome, three 3 children developed serious illnesses and were hospitalized, and 31 children had incomplete vaccination cards. Eighty-five children were finally included in the study. Some data for some children was missing in regard to direct bilirubin (n = 83), ALT/AST (n = 84), breastfeeding (n = 80), and living parents (n = 79).
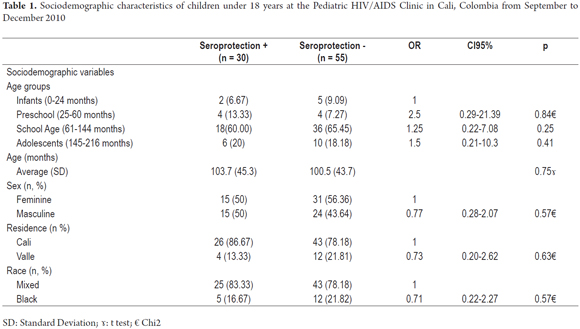
Among the 85 children, 30 had HBV seroprotection which was defined as SP prevalence of 35% (95% CI: 25.2 to 46.4%). These patients were defined as the study group, while children who did not have SP (n = 55) were considered to be controls. Table 1 describes the main characteristics of the study group. The female: male ratio was 1.1: 1.0, and the mean age was 44.07 ± 101.63 months. The patients were mainly mixed race (n = 58, 80%) school aged children (n = 54, 63.5%) from Cali (n = 69, 81.2%) with stage C HIV (n = 31, 50.6%) Most of them (54.1%) had been hospitalized at least once after being diagnosed with HIV/AIDS. A very high proportion of the (89.4%) had been receiving Highly Active Antiretroviral Therapy (HAART) for a median of 60 months treatment time (range 0 to 157 months) (Tables 1 and 2).
None of the children had histories of transfusions or hepatitis B. Only two children had liver and/or spleen enlargement.
Seventy percent of those treated had developed SP from 3.1 to 6 years after vaccination. SP increased through the years up to 53.3% measured both from the time of HIV diagnosis as well as from the beginning of HAART. Finally, in assessing the starting time of HAART and timing the third dose of vaccine, it was found that children who had undergone more than 3 years of treatment had an SP of 62% whereas untreated children developed an SP of 27.6%.
Association analysis
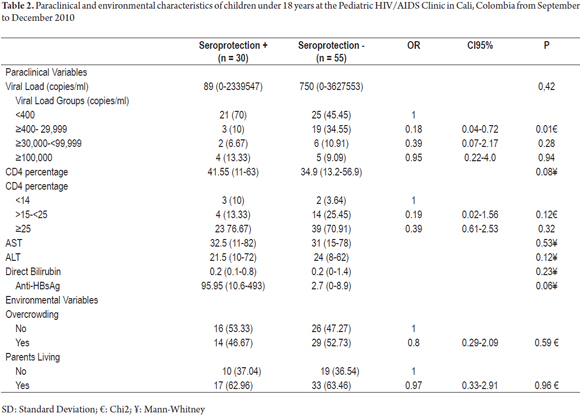
There were no significant differences between sociodemographic and environmental variables. Seroprotection was 0.2 times more likely to develop in the group with viral loads ≥ 400 to <29,999 copies/ml than in the control group (95% CI: 0.04 to 0.72, p = 0.01), 1.6 times more likely in the group who had received the final last dose of vaccine between 3.1 and 6 years previously (95% CI 0.42 to 5.8, p = 0.49); 0.7 times more likely in the group of 3.1 to 6 years after diagnosis of HIV infection (0.13 to 4.0, p = 0.12), and four times more likely in the group of 6.7 to 10 years after the start of HAART (95% CI 1.14-13.99, p = 0.03) (Table 2).
After controlling for potential confounders, the variables that best explained seroprotection were viral load, CD4 percentage, time from HIV diagnosis and start of HAART. The variable that best explains the presence of SP over time is the measurement of the relationship between start of treatment and timing of the last dose of vaccine between 0 and 3 years (OR = 4.3, 95% CI 0.96 to 19, 23; p = 0.05) or more than three years (OR = 9.69, 95% CI 2.37 to 39.5; p = 0.00). The estimations that 68.4% of classifications were good and that the area under the curve was 0.791 were good (Table 3).
DISCUSSION
Commonly HBV vaccine induces levels of Anti-HBsAg between 95% and 99% in healthy infants, children and young adults (8). In comparison, HIV-infected children have poor responses as a result of immunodeficiency (9). The study by Simani et al. in Sub-Saharan Africa that compared the prevalence of Anti-HBsAg in children 5-24 months of age stratified by HIV status and vaccinated against HBV found marked differences between a two selected groups. The group that received an expanded plan of immunization (EPI) showed high levels of seroprotection (89.3% vs. 81.7%; OR = 1.87, 95% CI: 0.87-4.29; p = 0.10) and SP (86% vs. 75%; 2.05, 95% CI; 0.97- 4.29; p = 0.03). When stratified by HIV status, the SP was 85.7% for HIV- negative and 78.1% for HIV-positive (p = 0.12) (3). Finally, we report that the SP was comparable among HIV-positive (84.3%; n = 51) and HIV-negative (86.5%; n = 192), (OR = 0.84 95% CI 0.33-2.10; p = 0.695) in the EPI group. In contrast, in the external group the SP observed among both HIV positive and negative patients decreased. For HIV positive patients it was 63.3% (n = 22), and for HIV negative patients it was 81.6% (n = 38) (OR = 0.4, 95% CI: 0.1-1.52, p = 0.12).
Pippi et al. found an SP of 59.5% (50/84) five months after the third dose of HBV vaccine (10). A study by Pessoa et al. in Brazil of 40 adolescents with HIV and 23 healthy adolescents found an SP of 45% (18/40) in the group with HIV and an SP of 78.2% (18/23) in the control group (p = 0.01). A study by Wilson et al. of a cohort of 71 children reported that adolescents with HIV had an SP of 41.1% versus 70% in the HIV negative group (p = 0.05; 95% CI 0.36 to 0.96 RR 0.586) (11). We found an SP for HBV of 35.3% (30/85) in children with HIV infections which is lower than those reported by Simani et al. (3), Pippi et al. (10) and Wilson et al., (11) but slightly higher than that published by Abzug et al. (5) who found an SP of 24% in a study of 204 children. Our prevalence doubles that reported by Fernández-Ibieta et al. (6) in a study of 121 children with HIV. They observed a prevalence of HBsAg Anti-HBV of 16.5% (OR = 5.0 95% CI 10.4-24.3). Our findings are markedly higher than those of Siriakson et al. (12) who found a prevalence of only 1%. The unpublished study by Lopez P in Cali, Colombia reported an SP of 20%. The difference between the results in children with HIV and those in healthy children could be explained by the low SP at the time the vaccination scheme began (failure of primary vaccination) or a fast rate of decline of antibodies (second vaccination failed) (11). The comparison of our findings and those of other studies could be explained by a lack of response to vaccination, or by prolonged second determination of SP in relation to the timing of the last dose of vaccine. In other words, the time from completion of vaccination is an important factor that may influence whether these antibodies have declined below SP levels.
Risk Factors
Sociodemographic: Our group of patients was mostly school aged with an average age of 8.5 ± 3.8 years (range 1.1 ± 16.1 years). This is in contrast to the group of infants studied by Simani et al. (3), who studied a group of infants, and the adolescents studied by Pessoa et al. (4) and Siriakson et al. (12) who studied only adolescents. It also differs from the group described by Pippi et al. (10) which consisted of only school children. Our study group had 54.9% girls whereas the group described by Pessoa et al. (4) had similar numbers of girls and boys (p = 0.16). These data do not match the initial observations of Washburn et al. (13) who observed that refers morbidity in most of diseases, including infectious diseases, occurs more often among boys than among girls. Contrary to our findings, Pippi et al. (10) and Siriakson et al. (12) describe larger proportions of males: 59.5% and 52%, respectively.
Paraclinical: The univariate analysis of our data suggests that as the percentage of CD4 increases, the SP is likely to increase, too. This was observed for children with CD4 ≥ 15% and <25% (OR = 0.19, 95% CI: 0.02 to 1.56; p = 0.12) and for those with CD4 ≥ 25% (OR = 0.39, 95% CI: 0.61 to 2.53; p = 0.32). Our findings indicate that 72.94% had CD4 percentages higher than 25%. In contrast, Pessoa et al. (4) found a lower CD4 percentage (21% ± 10.8) in adolescents who did not develop SP than in those who did (30.2% ± 9.9, p = 0.00). Within the group that developed SP, 50% (9/18) had viral loads of more than 400 copies/ml (p = 0.775). In comparison, in our study 70% (21/30) had less than 400 copies/ml (p = 0.06) which indicates better immune statuses and better responses to vaccinations. The multivariate analysis in the study by Abzug et al. (5) shows that those with higher CD4 percentages after initiation of HAART were more likely to develop SP (OR = 1.27; p = 0.00). The response of antibody concentration is associated with good immune status (OR = 2.69, p = 0.00), viral loads of less than 400 copies/ml (OR = 2.03 p = 0.05) and CD4 percentages (OR = 1.23; p = 0.10). In contrast to the study by Abzug (5), our multivariate analysis does not show an association between SP and low viral loads (less than 400 copies/ml) or high CD4 percentages.
Clinical: Our patients were predominantly in stage C HIV (56.67%, p = 0.674) which is similar to the patients in the study by Siriakson et al. (12) who reported that half of the patients (51%) were in stage C. This is contrary to the study of Pessoa et al. (4) which showed a higher proportion in stage B (50%) followed by 33.3% in stage C and 16.7% in stage A (p = 0.718). Similarly, the study population of Fernández-Ibieta et al. (6) included 20% in stage A, 16% in stage, and 9.4% in stage C (p = 0.19). The study by Pippi et al. (10) had 75.8% in stage A (25/33), 50% in stage B (23/46, p = 0.03) and 40% in stage C (2/5, p = 0.13).
We found clearly compromised nutritional statuses in terms of malnutrition for all of the children: 27.1% had stunting and 29.7% were at risk of stunting. In 2005, a study of this same group of children by Velasco et al. (14) found that 72% of the children suffered from malnutrition: 67% suffered from chronic malnutrition and 35% from acute malnutrition. In 2009, Velasco et al. (15) found improved nutritional status with only 13% suffering from severe chronic malnutrition. Our results are not comparable because different nutritional assessment tables were used (National Center for Health Statistics of the United States vs. WHO). Good nutritional status is probably secondary to the efficiency and effectiveness of HAART and to the program monitoring and control that allow greater adherence and decreased morbidity and mortality in this group of children (16). We found a protective effect in our patients between nutritional status and development of SP. The stunting we found may have multifactorial causes including malnutrition, constitutionally short stature, shortness within families, constitutional developmental delays, and slow maturation. In these cases case stunting has been reported in children with HIV/AIDS (15, 17-20).
Some studies have suggested that the rate of SP improves in adults receiving HAART and then remains in ranges from 32% to 59% (13, 21). The effect that antiretroviral therapy has on the response to the vaccine in children has not been sufficiently studied. The highest levels of SP in children in our study were in those who had received HAART for more than 10 years) (OR = 6.0; 95% CI: 1.27 to 28.25, p = 0.023). This finding from our univariate analysis is consistent with the findings of Pippi et al. who found a positive correlation between the response to the vaccine and HAART with high levels of SP in 70.8% of patients receiving HAART (34/48) vs. only 44.4% (16/36) among those without antiretroviral therapy (p = 0.03) (10).
Nevertheless, in contrast to our results, they found that the association disappeared after adjusting for CD4 cell counts. In their final model, the variable of CD4 cell counts following vaccination remained significantly associated to SP (OR = 0.05; 95% CI: 0.002 to 0.096; p = 0.04). Fernández-Ibieta et al. (6) observed a 16.5% prevalence of Anti-HBsAg compared to healthy children which decreased over time following the vaccination. Within 3 years of vaccination it was 36.8%, but it decreased to 7.4% over 12 years. Our univariate analysis found a prevalence of 35.3%. When we assessed the temporal relationship of the timing of the last dose of vaccine and detection of SP our findings matched those of Fernández (6). We found that this percentage decreased as the time from vaccination decreased. The prevalence of SP was 70% (21/30) within 6 years of vaccination but decreased to 16.47% past 6 years of vaccination. Abzug et al. (5) found an association between SP and prior administration of HBV vaccine for not more than four years (OR = 4.57; p <0.001). Our multivariate analysis included all variables in the model. In doing the logistic regression, the only significant variable possibly associated with development of SP was the time between the start of treatment and development of SP. For category 2 (0 to 3 years), the OR was 4.3 (95% CI: 0.96 to 19.23; p = 0.05) and for category 3 (more than 3 years), the OR was 9.69 (95% CI: 2.37 to 39.5; p = 0, 00). These findings are not comparable with other studies found in the literature since they do not study this relationship between time of treatment start and completion of the vaccination scheme.
In conclusion, the prevalence of SP in 85 children with HIV at the Pediatric HIV/AIDS Clinic in Cali, Colombia was 35.3% with possible risk factors associated with the temporal relationship between start of treatment and timing of the last dose of vaccine.
REFERENCES
1. Broderick AL, Jonas MM. Hepatitis B in children. Semin Liver Dis 2003;23:59-68. [ Links ]
2. World Health Organization. Hepatitis B. 2010. Disponible en: URL http://www.who.int/mediacentre/factsheets/fs204/en/ Consultada enero 31 de 2014. [ Links ]
3. Simani OE, Leroux-Roels G, Francois G, Burnett RJ, Meheus A, Mphahlele MJ. Reduced detection and levels of protective antibodies to hepatitis B vaccine in under 2-year-old HIV positive South African children at a paediatric outpatient clinic. Vaccine 2009;27:146-51. [ Links ]
4. Pessoa SD, Miyamoto M, Ono E, Gouvea A, De Moraes-Pinto MI, Succi R. Persistence of vaccine immunity against hepatitis B virus and response to revaccination in vertically HIV-infected adolescents on HAART. Vaccine 2010;28:1606-12. [ Links ]
5. Abzug MJ, Warshaw M, Rosenbllatt HM, Levin MJ, Nachman SA, Pelton SI, et al. Immunogenicity and immunologic memory after hepatitis B virus booster vaccination in HIV-infected children receiving highly active antiretroviral therapy. J Infect Dis 2009;200:935-54. [ Links ]
6. Fernández-Ibieta M, Ramos JT, González-Tome MI, Guillén S, Navarro M, Cilleruelo MJ. Anticuerpos anti-VHB y VHA en niños y adolescentes con VIH. Enferm Infecc Microbiol Clin 2009;27:449-52. [ Links ]
7. Elisys Uno. Fully Automated ELISA Analizer. Microelisa. En: http://www.trendsandcompetence.com/userDownloads/en/981230.pdf Consultada enero 31 de 2014. [ Links ]
8. Hoofnagle JH. Hepatitis B - preventable and now treatable. N Engl J Med 2006;354:1074-6. [ Links ]
9. Laurence JC. Hepatitis A and B immunizations of individuals infected with human immunodeficiency virus. Am J Med 2005;118(10A):75S-83S. [ Links ]
10. Pippi F, Bracciale L, Stolzuoli L, Giaccherini R, Montomoli E, Gentile C, et al. Serological response to hepatitis B virus vaccine in HIV-infected children in Tanzania. HIV Med 2008;9:519-25. [ Links ]
11. Wilson CM, Ellenberg JH, Sawyer MK, Belzer M, Crowley-Nowick PA, Puga A, et al. Serologic response to hepatitis B vaccine in HIV infected and high risk HIV uninfected adolescents in the REACH cohort. Reaching for Excellence in Adolescent Care and Health. J Adolesc Health 2001;29(3 Suppl):123-9. [ Links ]
12. Siriaksorn S, Puthanakit T, Sirisanthana T, Sirisanthana V. Prevalence of protective antibody against hepatitis B virus in HIV-infected children with immune recovery after highly active antiretroviral therapy. Vaccine 2006;24:3095-9. [ Links ]
13. Washburn TC, Medearis DN, Childs B. Sex differences in susceptibility to infections. Pediatrics. 1965;35:57-64. [ Links ]
14. Velasco CA, López P, Contreras LJ. Anthropometric nutritional status in children HIV positive with vertical transmission. J Pediatr Gastroenterol Nutr 2005;41:508-9 (Abstract). [ Links ]
15. Velasco CA, Méndez F, López P. Cryptosporidiosis in Colombian children with HIV/AIDS infection. Rev Colomb Med 2011;42:418-29. [ Links ]
16. Peters D. Diagnosis and management of hepatitis B virus and HIV coinfection top. HIV Med 2007;15:163-6. [ Links ]
17. Padmapriyadarsini C, Pooranagangadevi N, Chandrasekaran K, Subramanyan S, Thiruvalluvan C, Bhavani PK, et al. Prevalence of underweight, stunting, and wasting among children infected with human immunodeficiency virus in South India. Internat J Pediatr 2009 Article ID 837627, 5 pages doi:10.1155/2009/837627. [ Links ]
18. Macallan DE. Wasting in HIV infection and AIDS. J Nutr 1999;129(Suppl 1):238-42. [ Links ]
19. Lepage P, Msellati P, Hitmana DG, Bazubagira A, van Goethem C, Simonon A, et al. Growth of human immunodeficiency type I-infected and uninfected children: A prospective cohort study in Kigali, Rwanda, 1988 to 1993. Pediatr Infect Dis J 1996;15:479-85. [ Links ]
20. Myhre JA, Chadwick EG, Yogev R. Failure to thrive in HIV infected children: Incidence, prevalence, and clinical correlates. Pediatr AIDS HIV Infect 1997;7:83-90. [ Links ]
21. Pasricha N, Datta U, Chawla Y, et al. Immune responses in patients with HIV infection after vaccination with recombinantihepatitis B vaccine. BMC Infect Dis 2006;6:65. [ Links ]











 texto en
texto en