Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.30 no.1 Bogotá ene./mar. 2015
A Review of Dilutional Hyponatremia and Liver Transplantation
Edilberto E. Núñez C. MD. (1), Juan C. Restrepo G. MD. (2), Rodrigo Castaño Llano MD. (3)
(1) Internist from the Universidad Pontificia Bolivariana and Gastroenterology Fellow at the Pontificia Universidad Javeriana in Bogotá, Colombia.
(2) Gastrohepatology Group and Hepatology and Liver Transplantation Unit at the Universidad de Antioquia and at the Hospital Pablo Tobón Uribe in Medellin, Colombia.
(3) Gastrohepatology Group at the Universidad de Antioquia, Head of Postgraduate General Surgery at the Universidad Pontificia Bolivariana. Instituto de cancerología Clínica las Américas in Medellin, Colombia.
Received: 26-03-14 Accepted: 02-02-15
Abstract
Patients with advanced liver disease may present volume overloads that can lead to the development of dilutional hyponatremia primarily as the result of persistently high levels of antidiuretic hormone. Liver transplantation is the therapy of choice for patients with terminal liver disease. Patient survival time following transplantation is excellent. Dilutional hyponatremia has proven to be a prognostic factor for patients with cirrhosis, and it can even lead to mortality in patients on the waiting list for liver transplantation. Consequently, it is currently part of the scoring system for classifying patients with advanced liver disease who are on the waiting list for liver transplantation. The role of pretransplant hyponatremia in the prognoses of patients scheduled for liver transplantation is the subject of renewed interest, but the data in the literature is rather contradictory. Until recently, the only treatment for dilutional hyponatremia was the restriction of water intake, but now new and encouraging treatments are available that are directed against dilutional hyponatremia in patients with cirrhosis. Nevertheless, little is known about their effects in patients with cirrhosis who are about to undergo liver transplantation.
Keywords
Hyponatremia, cirrhosis, liver transplantation, prognosis
INTRODUCTION
Hyponatremia in cirrhosis, arbitrarily defined as a low serum plasma sodium concentration of 130 mEq/L, is a fairly common complication (1). Hypovolemic hyponatremia is associated with significant loss of extracellular fluid. Hypervolemic hyponatremia, also known as dilutional hyponatremia, occur when there is disproportionate retention of water compared to sodium. Patients develop ascite s but no clinical signs of dehydration (2). It has been reported dilutional hyponatremia may be present in up to 21.6% of patients with cirrhosis at diagnosis (3). For patients with cirrhosis, the risk of dilutional hyponatremia is 14% per year and 37% at 5 years (4).
ORIGIN OF DILUTIONAL HYPONATREMIA
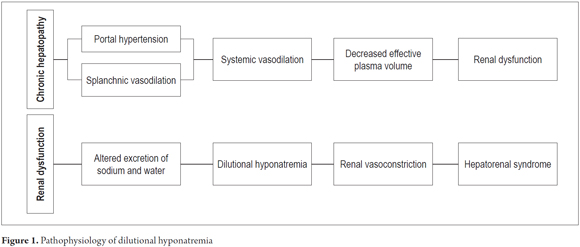
Hyponatremia develops in cirrhotic patients secondary to renal and severe circulatory dysfunction (1, 2). Initially, the kidneys of patients with advanced liver disease lose the ability to excrete sodium. Subsequently, this is followed by decreasing ability to remove solute free water (1, 5, 6). This leads to a state of hypervolemia with dilutional hyponatremia and hypoosmolarity which manifests clinically as ascites and peripheral edema (5). In more advanced stages, renal vasoconstriction develops and may lead to hepatorenal syndrome (HRS) (Figure 1) (2).
Although the pathogenesis of water retention in cirrhosis is complex, it is believed that the action of antidiuretic hormone, also known as arginine vasopressin (AVP), plays a key role in its genesis (7). Patients with cirrhosis have been documented to have increased serum levels of AVP due to non-osmotic secretion secondary to splanchnic vasodilation which occurs in order to maintain systemic perfusion (5, 8). AVP plays pathogenic roles in two ways. First it stimulates cells located in the convoluted distal duct and the collecting duct through the V2 receptors to reabsorb free water. Second, it stimulates vasoconstriction through the V1 receptors in the smooth muscle of blood vessels which affect renal perfusion (1). The perpetuation of AVP stimuli favors hypervolemia and therefore dilutional hyponatremia (8).
The release of other vasoactive substances such as norepinephrine and renin-angiotensin-aldosterone has also been demonstrated to occur concomitantly with the secretion of AVP (8). In addition, the use of diuretics may contribute significantly to the development of dilutional hyponatremia in patients with cirrhosis and advanced liver failure (9).
CLINICAL MANIFESTATIONS OF DILUTIONAL HYPONATREMIA IN CIRRHOSIS
Data on the clinical manifestations of patients with dilutional hyponatremia during cirrhosis are limited (2). Although the symptoms of hyponatremia are predominantly neurological, a wide spectrum of symptoms of all sorts have been reported. They range from nonspecific findings such as anorexia, lethargy, nausea and difficulty concentrating to severe focal neurological deficits, seizures and even coma (although this is quite rare) (10). It is often difficult to differentiate whether patients' symptoms are secondary to their hyponatremia or if these are symptoms of hepatic encephalopathy (2). It is known that patients with hyponatremia, generally those in advanced stages of liver disease, may have a greater predisposition to hepatic encephalopathy (11).
Dilutional hyponatremia in pre-liver transplant patients
For several decades liver transplantation has been the ideal treatment for patients with advanced liver disease, acute liver failure with poor prognostic indicators, some metabolic diseases affecting the liver, and some primary tumors of the liver (12). In our environment the most common cause of liver transplantation is alcoholic liver disease (12). In 2002, the MELD score (Model for End-Stage Liver Disease) began to be used in the United States to designate distribution of organs from deceased patients. This score is based on a logarithmic calculation that takes into account bilirubin, creatinine and INR that was initially designed to predict survival in patients with transjugular intrahepatic portosystemic shunts (TIPS) (13).
Recently the prognostic role of hyponatremia for liver transplant candidates has been revisited (1, 2). A study by Kim et al. tried to predict mortality of patients registered on the waiting list for liver transplantation in the United States between 2005 and 2006 during their first 90 days on the list (14). This study looked at all patients on the list. The hazard ratio (HR) for death of patients on the waiting list for 2005 was 1.05 for each decrease by 1 mmol/L sodium values between 125 and 140 mmol/L with a 95% confidence interval of 1.03 to 1.08. For 2006, the combined measurement of MELD and sodium (MELD-Na) (see below) proved to be a better predictor of adverse outcomes than the conventional MELD alone (14). A study by Moini et al. also reported that patients on the waiting list for liver transplantation who had hyponatremia had higher risks of dying than did patients who had normal sodium levels (15). Sodium levels below 130 mEq/L increased mortality rates at 90 and 180 days with odds ratios (OR) of 4.519 [95% CI, 1,956 to 10,439] and 2.667 [95% CI, 1,554 to 4,579] respectively. Measured by the MELD score, a decrease of 1 mEq/L meant an increased risk of death of 6% at 90 days and 7% at 180 days (15).
Among pediatric patients on the transplant waiting list, low levels of sodium, the presence of ascites, increased bilirubin and age (0 to 1 years vs. older than 1 year) have also been demonstrated to be independent predictors of 90-day mortality (16).
The role of MELD-Na for pre-liver transplant patients
Since hyponatremia has been demonstrated to independently predict adverse outcomes in patients in patients with cirrhosis on the waiting list for liver transplantation (17), it was logical to think that adding serum sodium to the MELD equation could improve its predictive power regarding survival of patients on the waiting list for liver transplantation. The addition of serum sodium to the MELD equation is now called the MELD-Na (18). The possibility has even been raised of using the MELD-Na equation to determine the best candidates for transplantation from among patients (18, 19). Nevertheless, there is still controversy regarding the generalized use of MELD-Na since serum sodium may be an easily modifiable variable which might only be a manifestation of advanced liver disease (1, 2).
Pretransplant Dilutional hyponatremia as a post-transplant prognostic indicator
Pretransplant hyponatremia has also been linked with adverse outcomes after transplantation (2), although this issue is still a matter of controversy in the literature.
Studies supporting dilutional hyponatremia as a prognostic factor
Londoño et al., published a study of 241 consecutive patients who underwent liver transplantation that retrospectively demonstrated that patients with serum sodium levels below 130 mEq/L (8% of patients) before transplantation had 3 month post-transplant a survival rate lower than that of patients without hyponatremia (84% vs. 95%, p <0.05) (20).
Karapanagiotou et al. published a study that evaluated outcomes following orthotropic liver transplants (21). They found that the rates of neurological complications, acute kidney damage and ICU mortality were lower for patients with hyponatremia than for those with eunatremia. The differences were statistically significant. The survival thirty day and one year survival rates were also lower for the group of patients with hyponatremia (62.5% vs. 88.5% and 60.9% vs. 88.5%, respectively). Multivariate logistic regression demonstrated that both acute kidney failure and hyponatremia were pretransplant factors that independently predicted survival at 30 days and one year after liver transplantation (21).
A cohort study by Boin et al. has also demonstrated that patients who had sodium levels lower than 130 mEq/L had worse prognoses than those of a control group. Those with lower sodium survived months less than those in the other group (mean survival 37.2 + 51.2 months versus 51.2 + 54.1 months respectively, p <0.05) (22).
A retrospective review by Li et al. of 207 patients who had received liver transplants from living donors showed that pretransplant hyponatremia, defined as serum levels less than 130 mEq/L, was an independent predictor of postoperative bacterial infections in the transplanted organ (23).
A Japanese retrospective review of 134 patients found that those with pretransplant levels of sodium less than 130 mEq/L had 3 months and 1 year survival rates that were much lower than those of patients whose sodium levels were higher (97 % vs. 74%, and 96% vs. 58%, respectively, p <0.001). In the multivariate analysis, sodium levels predicted graft survival after transplantation (p = 0.005) (24).
Finally the study of Abdalla et al. documented that graft loss occurred in the first months after transplantation more frequently in patients with serum sodium levels below 135 mEq/L than in patients without hyponatremia (p = 0.01) (25).
Studies that do not support dilutional hyponatremia as a prognostic factor
A study by Leise et al. have published their experience in the management of liver transplant patients with pretransplant hyponatremia (26). Their study involved 19,357 patients. It showed that the probability of survival was similar in groups of patients with hyponatremia and normal natremia (94.7% and 94.0 % respectively).
A Korean study sought to demonstrate the prognostic impact of pretransplant hyponatremia and of correction of pretransplant hyponatremia for outcomes of patients undergoing liver transplantation (27). That study included a new variable known as the delta of sodium which measures serum sodium in the first 48 hours after liver transplantation. The study included 512 orthotopic liver transplant patients. Patients with severe pretransplant hyponatremia, defined as serum sodium lower than 125 mmol/L, had higher in-hospital rates of mortality (9.6%), delirium (54.8%), neurological complications (24.7%) and acute kidney failure. All of these were statistically significant. Nevertheless, the multivariate logistic regression showed that neither the concentration of sodium and nor the delta of sodium were associated with in-hospital mortality.
Treatment of dilutional hyponatremia in patients who are candidates for liver transplantation
Little is known about whether treating patients with hyponatremia prior to liver transplantation improves their prognoses, and if it does whether or not it should become a therapeutic modality (1, 2). A study by Fukuhara et al. looked at 24 patients with hyponatremia whose sodium levels were corrected at speeds that would prevent the development of osmotic demyelination syndrome. The prognoses of these patients with corrected sodium levels were similar to those of patients whose levels could not be corrected (24).
Currently, dilutional hyponatremia in patients with cirrhosis is handled with fluid restriction and diuretic therapy for patients with volume overload (28). The most successful therapeutic regimen is the combination of daily doses of 100 mg of spironolactone and 40 mg of furosemide daily. Doses are titrated gradually to maintain the same ratio of the dose in order to maintain normal levels. Nevertheless, in some cases the use of these diuretics subjects the patient to serious complications such as kidney injury and electrolyte disturbances which limits their usefulness (29).
Vaptans
Recently, a new group of drugs that antagonize the effect of AVP has come onto the market. These drugs, known as vaptans, include conivaptan, lixivaptan, satavaptan and tolvaptan. They have been shown to correct the sodium levels in patients with euvolemic hyponatremia or hypervolemic hyponatremia (28). Their use is restricted to patients with persistent secretion of antidiuretic hormone despite low plasma osmolality (28).
Some studies have shown that conivaptan and lixivaptan may be useful in patients with cirrhosis and dilutional hyponatremia (30-32). Some small clinical trials have shown that lixivaptan and satavaptan are more effective than placebos for correcting sodium levels, but they suggest that they have less effect on hyponatremia in patients with cirrhosis than on hyponatremia due to other causes (30, 33). The Study of Ascending Levels of Tolvaptan (SALT) which analyzed subgroups within the study population including a group of patients with cirrhosis supports this observation (34). Some authors propose that the limited response in these patients is the effect of proximal reabsorption of solutes leading to decrease in the amount of glomerular filtration in distal nephrons (35), although this possibility has not been studied. Others have proposed that regulation of aquaporin 2 in patients with cirrhosis is regulated independently by means of the AVP V2 receptors (36). Although initial studies showed that satavaptan could be useful, Phase 3 studies did not have results that were hoped-for. A satavaptan Phase 3 study demonstrated that mortality increased among patients with cirrhosis who used the drug (37). For this reason, research was suspended in 2008 (28).
Tolvaptan has the advantage that it does not require adjustment according to age, gender, or race, or to cardiovascular, hepatic or renal impairment (GFR> 10 mL/min). Nevertheless, it is contraindicated for patients with hypovolemic hyponatremia, those who have altered thirst centers, those who cannot access liquids orally if required, those with acute symptomatic hyponatremia, patients with anuria, and those undergoing treatment with potent inhibitors of isoenzyme CYP3A4 (28). Furthermore, although tolvaptan is taken orally, serum sodium levels and any changes in them must be monitored constantly while a patient is taking this medication which means that, ideally, the patient should be hospitalized. On the other hand, it is important to remember that evidence only supports the use of tolvaptan for a maximum for 30 days: there is no evidence to support prolonged use (38).
The treatment of patients with chronic liver disease with tolvaptan has generated safety concerns since a clinical trial found that episodes of gastrointestinal bleeding increased in patients managed with tolvaptan (34, 39). Nevertheless, Sakaida et al. recently demonstrated the usefulness of tolvaptan for management of edema in patients with cirrhosis (40). This study showed that doses of 7.5 mg daily of tolvaptan for 7 days may improve serum sodium levels at a rate of mmol/L without deviation from the normal range. Although tolvaptan may be useful for management of hyponatremia in patients with chronic liver disease (40), the study reported two episodes of severe complications. One was a case of bleeding gastrointestinal varices on the 14th day of treatment, and the other was a patient who developed hepatic encephalopathy in the third day of treatment (40).
Additional studies are needed to support the use of vaptans in patients with cirrhosis and advanced liver failure let alone their use for perioperative liver transplantation patients. In any case, vaptans are not yet available in Colombia.
CONCLUSION
Dilutional hyponatremia in patients with chronic liver disease is an indicator of severity and a marker of poor prognoses. It is a consequence of the pathophysiology of an advanced stage of renal dysfunction and a hemodynamic consequence of cirrhosis. The use of this prognostic marker for determining the list of candidates for liver transplantation has been helpful, however little is known about whether pretransplant hyponatremia may be an adverse indicator in patients who have had liver transplants. Our group is currently conducting a study here in Colombia to clarify that question.
To date, the only way to reverse the process that leads to hyponatremia is transplantation. Other non-pharmacological and pharmacological measures such as vaptans are being tried, and there are promising drugs, but there are still not enough studies supporting their use are lacking to allow us to recommend their use.
REFERENCES
1. Guevara M, Gines P. Hyponatremia in liver cirrhosis: pathogenesis and treatment. Endocrinol Nutr 2010;57(Suppl 2):15-21. [ Links ]
2. Cardenas A, Gines P. Dilutional hyponatremia, hepatorenal syndrome and liver transplantation. Gastroenterol Hepatol 2008;31:29-36. [ Links ]
3. Angeli P, Wong F, Watson H, Gines P. Hyponatremia in cirrhosis: results of a patient population survey. Hepatology 2006;44:1535-42. [ Links ]
4. Planas R, Montoliu S, Balleste B, et al. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol 2006;4:1385-94. [ Links ]
5. Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodes J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology 1988;8:1151-7. [ Links ]
6. Epstein M. Deranged sodium homeostasis in cirrhosis. Gastroenterology 1979;76:622-35. [ Links ]
7. Arroyo V, Claria J, Salo J, Jimenez W. Antidiuretic hormone and the pathogenesis of water retention in cirrhosis with ascites. Semin Liver Dis 1994;14:44-58. [ Links ]
8. Schrier RW. Water and sodium retention in edematous disorders: role of vasopressin and aldosterone. Am J Med 2006;119:S47-53. [ Links ]
9. Bichet DG, Van Putten VJ, Schrier RW. Potential role of increased sympathetic activity in impaired sodium and water excretion in cirrhosis. N Engl J Med 1982;307:1552-7. [ Links ]
10. Gines P, Berl T, Bernardi M, et al. Hyponatremia in cirrhosis: from pathogenesis to treatment. Hepatology 1998;28:851-64. [ Links ]
11. Haussinger D, Kircheis G, Fischer R, Schliess F, vom Dahl S. Hepatic encephalopathy in chronic liver disease: a clinical manifestation of astrocyte swelling and low-grade cerebral edema? J Hepatol 2000;32:1035-8. [ Links ]
12. Santos O, Marín J, Muñoz O, et al. Trasplante hepático en adultos: Estado del arte. Rev Col Gastroenterol 2012;27: 21-31. [ Links ]
13. Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000;31:864-71. [ Links ]
14. Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med 2008;359:1018-26. [ Links ]
15. Moini M, Hoseini-Asl MK, Taghavi SA, et al. Hyponatremia a valuable predictor of early mortality in patients with cirrhosis listed for liver transplantation. Clin Transplant 2011;25:638-45. [ Links ]
16. Pugliese R, Fonseca EA, Porta G, et al. Ascites and serum sodium are markers of increased waiting list mortality in children with chronic liver failure. Hepatology 2013. [ Links ]
17. Londoño MC, Cárdenas A, Guevara M, et al. MELD score and serum sodium in the prediction of survival of patients with cirrhosis awaiting liver transplantation. Gut 2007;56:1283-90. [ Links ]
18. Ruf AE, Kremers WK, Chavez LL, Descalzi VI, Podesta LG, Villamil FG. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl 2005;11:336-43. [ Links ]
19. Biggins SW, Rodriguez HJ, Bacchetti P, Bass NM, Roberts JP, Terrault NA. Serum sodium predicts mortality in patients listed for liver transplantation. Hepatology 2005;41:32-9. [ Links ]
20. Londoño MC, Guevara M, Rimola A, et al. Hyponatremia impairs early posttransplantation outcome in patients with cirrhosis undergoing liver transplantation. Gastroenterology 2006;130:1135-43. [ Links ]
21. Karapanagiotou A, Kydona C, Papadopoulos S, et al. The effect of hyponatremia on the outcome of patients after orthotopic liver transplantation. Transplant Proc 2012;44:2724-6. [ Links ]
22. Boin IF, Capel C, Jr., Ataide EC, Cardoso AR, Caruy CA, Stucchi RS. Pretransplant hyponatremia could be associated with a poor prognosis after liver transplantation. Transplant Proc 2010;42:4119-22. [ Links ]
23. Li C, Wen TF, Mi K, Wang C, Yan LN, Li B. Analysis of infections in the first 3-month after living donor liver transplantation. World J Gastroenterol 2012;18:1975-80. [ Links ]
24. Fukuhara T, Ikegami T, Morita K, et al. Impact of preoperative serum sodium concentration in living donor liver transplantation. J Gastroenterol Hepatol. 2010;25: 978-984. [ Links ]
25. Abdalla S, Alves RCP, Fonseca EA, Vicenzi R, Gonçalves JE, Waisberg J. A hiponatremia como fator preditivo da perda precoce do transplante hepático na modalidade intervivos. Arq Bras Ciênc Saúde 2012;37:76-80. [ Links ]
26. Leise MD, Yun BC, Larson JJ, et al. The effect of pretransplant serum sodium concentration on outcome following liver transplantation. Liver Transpl 2014. [ Links ]
27. Lee J, Kim DK, Lee JW, et al. Rapid correction rate of hyponatremia as an independent risk factor for neurological complication following liver transplantation. Tohoku J Exp Med 2013;229:97-105. [ Links ]
28. Gaglio P, Marfo K, Chiodo J, 3rd. Hyponatremia in cirrhosis and end-stage liver disease: treatment with the vasopressin V(2)-receptor antagonist tolvaptan. Dig Dis Sci 2012;57:2774-85. [ Links ]
29. Ishikawa T. Novel additional indication of tolvaptan: Can tolvaptan provide a beneficial therapeutic option in cirrhotic patients with ascites? Hepatol Res 2014;44:70-2. [ Links ]
30. Gerbes AL, Gulberg V, Gines P, et al. Therapy of hyponatremia in cirrhosis with a vasopressin receptor antagonist: a randomized double-blind multicenter trial. Gastroenterology 2003;124: 933-9. [ Links ]
31. Guyader D, Patat A, Ellis-Grosse EJ, Orczyk GP. Pharmacodynamic effects of a nonpeptide antidiuretic hormone V2 antagonist in cirrhotic patients with ascites. Hepatology 2002;36:1197-205. [ Links ]
32. OLeary JG, Davis GL. Conivaptan increases serum sodium in hyponatremic patients with end-stage liver disease. Liver Transpl 2009;15:1325-9. [ Links ]
33. Gines P, Wong F, Watson H, Milutinovic S, del Arbol LR, Olteanu D. Effects of satavaptan, a selective vasopressin V(2) receptor antagonist, on ascites and serum sodium in cirrhosis with hyponatremia: a randomized trial. Hepatology 2008;48:204-13. [ Links ]
34. Cárdenas A, Gines P, Marotta P, et al. Tolvaptan, an oral vasopressin antagonist, in the treatment of hyponatremia in cirrhosis. J Hepatol 2012;56:571-8. [ Links ]
35. Lehrich RW, Ortiz-Melo DI, Patel MB, Greenberg A. Role of vaptans in the management of hyponatremia. Am J Kidney Dis 2013;62:364-76. [ Links ]
36. Krag A, Moller S, Pedersen EB, Henriksen JH, Holstein-Rathlou NH, Bendtsen F. Impaired free water excretion in child C cirrhosis and ascites: relations to distal tubular function and the vasopressin system. Liver Int 2010;30:1364-70. [ Links ]
37. Wong F, Gines P, Watson H, et al. Effects of a selective vasopressin V2 receptor antagonist, satavaptan, on ascites recurrence after paracentesis in patients with cirrhosis. J Hepatol 2010;53: 283-90. [ Links ]
38. Boyer TD. Tolvaptan and hyponatremia in a patient with cirrhosis. Hepatology 2010;51:699-702. [ Links ]
39. Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 2006;355:2099-112. [ Links ]
40. Sakaida I, Kawazoe S, Kajimura K, et al. Tolvaptan for improvement of hepatic edema: A phase 3, multicenter, randomized, double-blind, placebo-controlled trial. Hepatol Res 2014;44:73-82. [ Links ]











 texto en
texto en