Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.30 no.1 Bogotá Jan./Mar. 2015
How to Perform and Interpret High-resolution Esophageal Manometry
Albis Hani MD. (1), Ana María Leguízamo MD. (1), Jhon Jaime Carvajal MD. (1), Gabriel Mosquera-Klinger MD. (1), Valeria Atenea Costa MD. (1)
(1) Department of Gastroenterology at the Hospital Universitario San Ignacio of the Pontificia Universidad Javeriana in Bogotá, Colombia.
Received: 03-07-14 Accepted: 02-02-15
Abstract
The introduction of high-resolution esophageal manometry allowed physicians to identify both previously unidentified normal esophageal functions and patterns and various abnormalities. The creation of new charts of pressure patterns and the topography of esophageal pressure has led to the development of new tools for analysis and classification of esophageal motor disorders. At present, the Chicago classification is the diagnostic method of analysis used for various esophageal motor disorders. In Colombia, we see the use of high-resolution esophageal manometry growing on a daily basis. In this article we review how to perform and interpret high-resolution esophageal manometry.
Keywords
Esophageal motility disorders, esophageal manometry high resolution, achalasia.
OVERVIEW
For decades, esophageal manometry has been the method of choice for evaluating esophageal motor disorders. The introduction of high-resolution esophageal manometry simplifies study of the motor function of the esophagus (1).
Reliable evaluation of esophageal and gastrointestinal motility with manometric techniques became possible in 1970 when Jerry Dodds and Ron Wyle Arndorfer developed the first manometry system manometry. Except for a few technical changes, their approach has continued to be state of the art for two decades. In the 1990s, Ray Clouse and colleagues developed high-resolution manometry by increasing the number of sensors and decreasing the space between the sensors along the pressure catheter from the 5 cm intervals of conventional manometry to just 1 cm (1).
Conceptually, high-resolution manometry uses a catheter with multiple high fidelity pressure sensors that capture manometric data in a spatial continuum without the substantial gaps between pressure sensors that are typical of conventional manometry (2).
Some authors have used the term "high resolution esophageal pressure topography" for high-resolution manometry (3).
Whereas it is not possible to simultaneously watch the motor function of the upper esophageal sphincter (UES), the esophageal body and the lower esophageal sphincter (LES) with each swallow with conventional manometry, high-resolution manometry provides us with the possibility of complete representation in time and space of the motor function of the esophagus (1).
High-resolution manometry is useful because it overcomes the limitations of conventional esophageal manometry through advanced electronic technologies. The key to this development the increase in the number of hi-resolution pressure sensors along the catheter. There are 36 in total, and they are placed at intervals of less than 2 cm. This allows assessment of the intraluminal pressure across the entire length of the esophagus and the esophageal sphincter. In addition, each sensor has circumferential sensitivity (3).
High-resolution manometry has also improved our ability to predict failure or success in the movement of the bolus through the esophagus compared to what is possible with conventional manometry. Even the occurrence of reflux events can be predicted. The components of the anti-reflux barrier and its dynamic interaction can be very distinct. Records from high-resolution manometry reveal the complex functional anatomy of the esophagus and sphincter. Monitoring the pressure of the lower esophageal sphincter and recognizing when it spontaneously and momentarily relaxes can be evaluated more accurately than with conventional manometry equipment (4).
The presentation of the pressure data with color contours and esophageal pressures through topography has led to the development of new tools for analyzing and classifying esophageal motor patterns. The current standard approach to this is the Chicago classification (1).
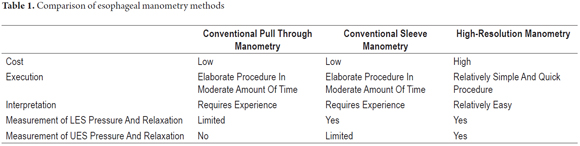
Differences with conventional manometry
High resolution esophageal manometry reveals the dynamic action of the upper esophageal sphincter, the segmental nature of esophageal peristalsis and the functional anatomy of the gastroesophageal junction. Space-time graphs constructed from the data obtained by the pressure sensor provide an accurate representation of the relationship between the clamping force (contractive force), clearance force and the flow resistance (pressure nadir and pressure gradient across the gastroesophageal junction). All these factors are necessary for full appreciation of the biomechanics of bolus transport (4).
One of its advantages is reduction of the time needed for the study because catheter placement is quick and easy (average 8.2 minutes vs. 24.4 minutes for conventional manometry, p <0.0001) (3, 4).
Another clear advantage of high-resolution manometry is the elimination of the need to reposition the catheter during the course of the study ("pull through" technique of conventional manometry) to determine the characteristics of the lower esophageal sphincter and gastroesophageal junction. This has resulted in a significant improvement in the objectivity of evaluations of the gastroesophageal junction at rest and in response to swallowing (Table 1) (2, 3).
The disadvantages of high-resolution manometry lie in the high cost of equipment and lack of experience interpreting spatial records which can create a risk of over-diagnosis of insignificant records of esophageal dysmotility (Table 1) (4).
INDICATIONS FOR HIGH RESOLUTION ESOPHAGEAL MANOMETRY
Indications for high resolution esophageal manometry are the same as those for conventional manometry except that high resolution manometry has some advantages including provision of more detail for assessing issues such as upper and lower esophageal sphincters and bolus transport (5).
The most important reasons to use high resolution esophageal manometry is to study dysphagia whose cause has not been established by endoscopic or imaging studies and to investigate suspected esophageal motor impairment (5, 6).
Another indication for esophageal manometry is the study of non-cardiac chest pain. Hypertensive peristalsis, also known as nutcracker esophagus, hypercontractile esophagus, also known as jackhammer esophagus, or diffuse esophageal spasms may explain this type of pain (5, 7).
Esophageal manometry is the gold standard for the diagnosis of achalasia, so when it is suspected, it is imperative to conduct this study. With the advent of high-resolution esophageal manometry this disease classification was divided into sub-classifications which has led to demonstration of which subtypes may respond better to treatment (6, 7).
The usefulness of high resolution esophageal manometry for the study of esophageal motility disorders in diseases involving connective tissue such as systemic sclerosis has also been described. Esophageal involvement can be as high as 90% in patients with this condition. Findings from esophageal manometry indicating this condition include hypotonia of the lower esophageal sphincter, infective peristalsis and aperistalsis (5-7).
Prior to anti-reflux surgery, it is important to rule out motor disorders such as achalasia. Manometry is also useful for deciding the type of surgery performed, for example choosing between 180 degree and 360 degree fundoplication. Similarly, a patient with postoperative dysphagia should be examined with high-resolution esophageal manometry in order to establish a plan for further treatment (5, 7).
Positioning of the esophageal pH monitoring catheter requires prior location of the lower esophageal sphincter by esophageal manometry. This is used by various groups of gastroenterologists who study esophageal physiology (5).
HOW TO PERFORM HIGH RESOLUTION ESOPHAGEAL MANOMETRY
When performing high-resolution esophageal manometry, it is important to keep in mind several points.
1. Patient preparation
Ideally, most patient should be instructed to fast for six hours, but this should be increased to twelve hours if achalasia is suspected because of the risk of aspiration given the possibility of food content in the esophageal lumen due to motor alterations (5, 6).
2. Suspension of drugs that can alter esophageal motility on the day of the examination
This includes drugs such as calcium channel blockers, nitrates, prokinetics, loperamide, beta-receptor antagonists, opiates, and anticholinergics. These should only be used if suspension might lead to an alteration in the patient's welfare (5).
3. Explanation of procedure and signed informed consent form
Because this is an invasive study, the patient should be given a detailed explanation of the technique to be used in the procedure and any possible discomfort that might be experienced. Once the patient has approved the procedure, she or he should sign the informed consent form (6).
4. Preparation of equipment
Prior to esophageal intubation the equipment must be calibrated and cleaned. Calibration will vary according to the type of probe to be used, and cleaning must be done according to internationally accepted standards to ensure prevention of the spread of infection through the device (5, 7).
5. Perform the examination
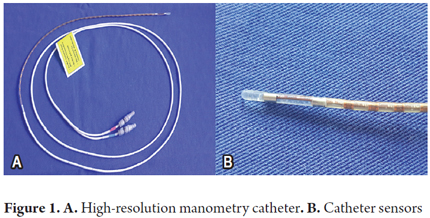
The probe must be positioned through a nostril. Once cannulation is achieved, the catheter is positioned according to the type of equipment used. In the case of the Sierra Scientific Instruments® high resolution manometer that we used, it is positioned according to height. For other makes of equipment, the catheter is positioned when the lower and upper esophageal sphincters are identified on the screen (Figure 1) (1, 5, 6).
Once in starting position, measurement of basal sphincter pressure can begin. For this, the patient is requested to refrain from swallowing for 30 seconds. Once this measurement can be taken, the study continues with the patient taking ten swallows of 5 cc of water. It has recently been proposed that once ten swallows have been completed, multiple swallows should be added to the study to assess neuromuscular integrity. This is not yet standard, so implementation depends on the preference of the group conducting the study (8).
HOW TO READ HIGH-RESOLUTION MANOMETRY
High-resolution manometry demonstrates the resting pressure of the sphincter and esophageal motor activity triggered by swallowing. Although most analyses are generated by computer software, the algorithms are not perfect, so it is imperative that each swallow is reviewed to ensure that the parameters and measurements are appropriate.
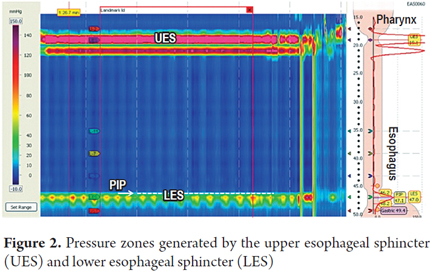
The study begins with the evaluation of resting pressures in the UES and LES. These are identified as two areas with increased pressure that are easily identified by color changes in the esophageal topography (Figure 2).
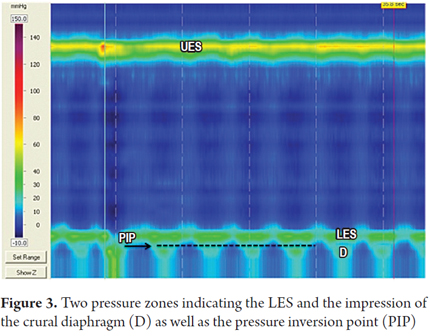
High resolution manometry differentiates the basal pressure of the lower esophageal sphincter from contractions of the crural diaphragm. Under normal conditions these two pressure zones should coincide. Separation of the two pressure zones indicates the presence of a herniated diaphragm (7). Similarly, the pressure inversion point (PIP) can be identified. This is the point where the negative pressure generated by intrathoracic pressure changes to positive pressure generated by intra-gastric pressure. This point indicates the division produced by the diaphragm between the chest and abdomen (Figure 3).
It is convenient to analyze high-resolution esophageal manometry in stages. Initially, the LES and relaxation of the LES are evaluated. Then the topography of esophageal pressure is evaluated during each swallow to define the different esophageal motility disorders (duration, speed and amplitude) (9).
1. Identify basal LES pressure and whether or not there is a hiatal hernia. This is achieved by finding the pressure inversion point (PIP) which is normally located immediately above the proximal edge of the lower esophageal sphincter. This means that pressure generated by the LES and the pressure generated by the crural diaphragm match (Figure 2).
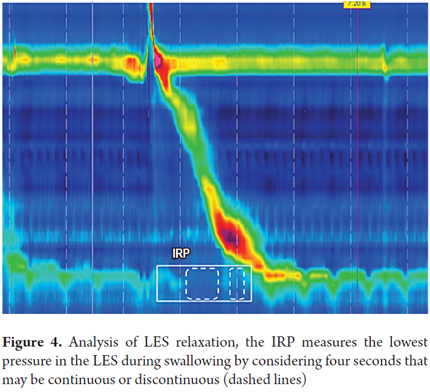
2. Define whether LES relaxation is normal or not during swallowing: Assessing LES relaxation with high-resolution manometry is achieved by measuring integrated relaxation pressure (IRP). The equipment takes 10 seconds after the beginning of swallowing (this is when UES relaxation begins) to measure the IRP. The IRP is the lowest average pressure in the esophagogastric junction for four of those ten seconds. Swallowing can be continuous or discontinuous (Figure 4).
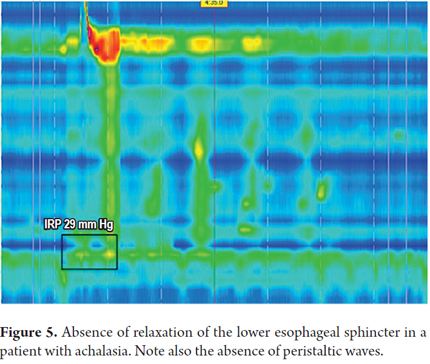
IRP greater than 15 mmHg means increased resistance to bolus transit in the esophagogastric junction and is considered to be pathological (10). Any mechanical or functional process that blocks the gastroesophageal junction can increase the IRP and is pathological (1). Some of these alterations may be due to achalasia and outflow tract obstructions such as neoplasms, benign strictures and complications of GERD (Figure 5).
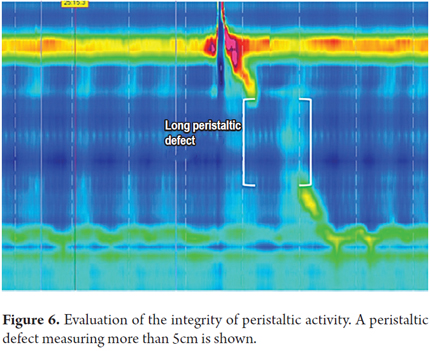
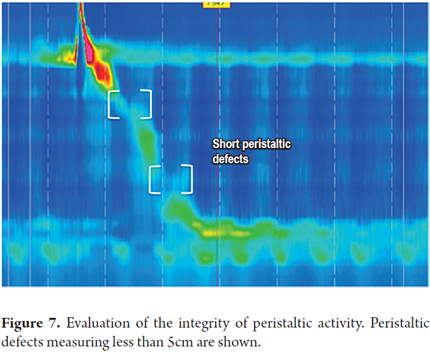
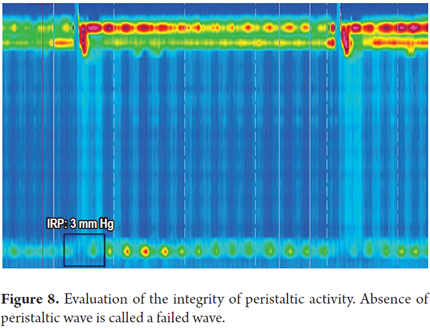
3. Determine the integrity of peristaltic activity. We define whether peristaltic activity is normal or has failed, or whether there are areas of pressure under 20 mmHg extending over 2-5 cm (short) or greater than 5 cm (long) on the isobaric contour. These areas are known as peristaltic defects. The minimum pressure limit required for transit of the bolus is 20 mmHg. This was chosen because it is the lowest pressure at which the esophagogastric junction works adequately (1).
Peristaltic defects are more significant if they occur in the distal esophagus because there is often a decrease in pressure at the junction of the proximal third and the middle third of the esophagus where there is a transition zone from striated muscle to smooth muscle. Nevertheless, this decrease in pressure should not be very long (Figures 6, 7 and 8).
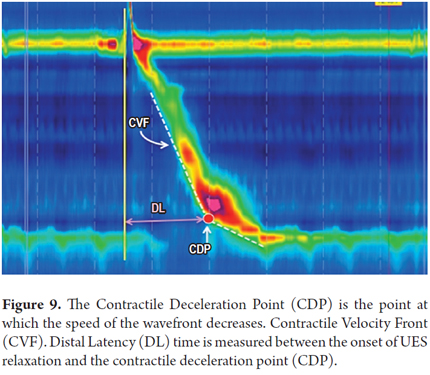
4. The contractile deceleration point (CDP) is where the speed of the peristaltic wave front slows. It is located in the distal third of the esophagus near the lower esophageal sphincter (11). It is usually associated with the point of greatest axial contraction of the esophagus. Functionally, the CDP is the point in time where the peristaltic wave ends and the LES initiates the descent into its position of repose (Figure 9) (10).
Propagation of the peristaltic wave: The propagation time of the peristaltic wave is determined by the distal latency (DL) which indicates if the contraction is premature and whether there is any alteration in the normal inhibition of esophageal body that regulates the speed of wave propagation (12). The distal latency is obtained from the interval of time between the start of UES relaxation and the contractile deceleration point (CDP). Its lower limit is 4.5 seconds (Figure 9) (13). The speed of the peristaltic wave is measured as the contractile front velocity (CFV). The computer calculates the slope of the line between the transition zone and the CDP. Its normal value should not exceed 9 cm/second (Figure 9) (11).
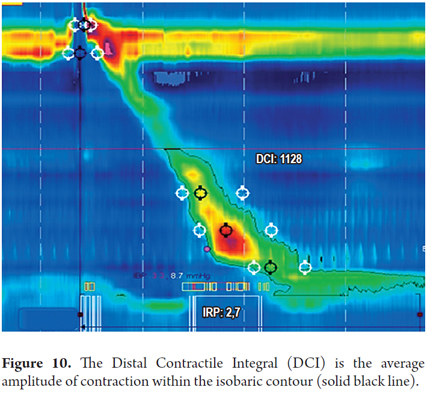
Contractile force: The richness of detail generated by high-resolution manometry favors better measurement of the contractile force of the esophagus. This allows generation of a defined value that takes into account the pressure, time and distance in the distal two thirds of the esophagus (14). This measurement, called the distal contractile integral (DCI), is generated on the basis of the average amplitude of smooth muscle contraction, contraction duration and the distance of wave propagation between the transition zone and the most proximal portion of the esophagogastric junction. Its normal value is <5,000 mmHg/cm/second (Figure 10) (15).
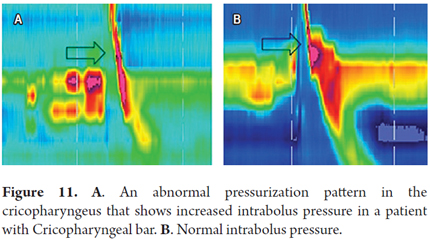
Determine abnormal pressurization patterns. Abnor-mally high intrabolus pressure (IBP) is a sign of impaired bolus transit mechanics which may be secondary to outflow obstruction or alteration of esophageal wall distensibility. Measurement makes pathologies that cause obstruction of bolus transit in the esophagogastric junction or in the upper esophageal sphincter evident. The former can include tumors, benign strictures, tight fundoplication, and some variants of achalasia while the latter includes cricopharyngeal achalasia and obstructive changes in the upper esophageal sphincter. Impairment of transit is identified by a zone of constant isobaric pressure of variable length in the distal esophagus or in the segment proximal to the cricopharyngeal area (Figure 11) (7, 10).
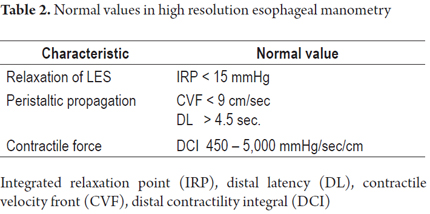
After each swallow has been analyzed, use the Chicago classification for the most appropriate diagnosis of esophageal motor disorders (Table 2) (15).
Manometric alterations rated according to the Chicago Classification of esophageal motility
The Chicago classification is used to classify esophageal motor disorders. The first step of the interpretation is to review the competence of the lower esophageal sphincter, followed by characterization of esophageal motor function. It is worth mentioning that the Chicago ranking only takes into account the motility of the distal esophagus and the lower esophageal sphincter.
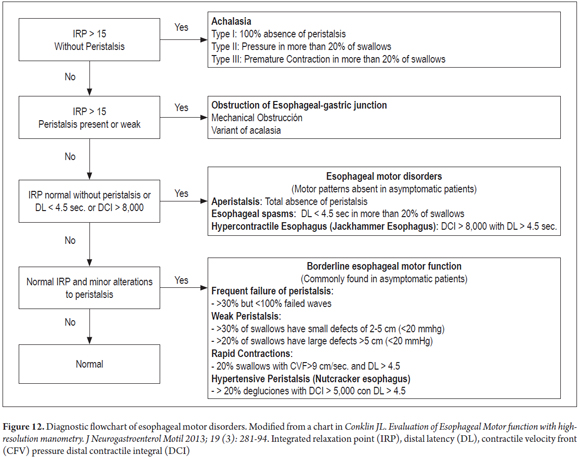
The Chicago Classification divides esophageal motor abnormalities into four large groups: achalasia, esophageal obstruction, abnormal esophageal motor function, and borderline esophageal motor function (which can be seen in asymptomatic patients) (Figure 12).
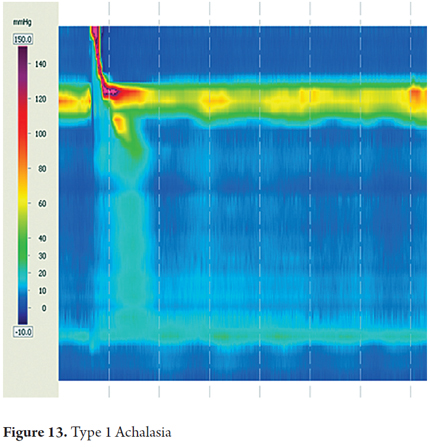
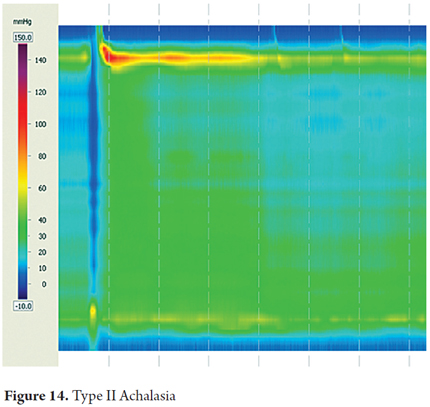
The most important pattern and esophageal motor disorders are types of achalasia, which are the disorders that are clinically and pathophysiologically best understood. For many years achalasia has been diagnosed by conventional manometry as the failure of the lower esophageal sphincter to relax and the absence of peristalsis in the smooth esophageal muscle. With the introduction of high-resolution esophageal manometry, the diagnosis has been divided into three subtypes characterized by failure of relaxation of the lower esophageal sphincter but with different esophageal motor patterns (7). Esophageal motor activity is not noticeable in Type I achalasia which is the previously described classic case with simultaneous contractions of low amplitude. Type II achalasia is characterized by pan-esophageal pressurization in more than 20% of swallows. Type III achalasia is characterized by premature spastic contractions in over 20% of the swallows.
A review of the evidence regarding treatment outcomes based on the type of achalasia has shown that Type I achalasia (Figure 13) responds best to treatment with Heller myotomy or balloon dilation. Type II is very sensitive to any treatment chosen (Figure 14), and type III has the worst therapeutic prognosis (16, 17).
The classification abnormal esophageal motor function includes a diverse group of esophageal motor abnormalities that do not occur in asymptomatic individuals. These disorders are nutcracker esophagus, jackhammer esophagus, aperistalsis, and distal esophageal spasms (DES) (1).
Hypertensive peristalsis is defined as esophageal peristaltic waves in the smooth muscle whose DCI is between 5000 and 8000 mmHg/cm/sec but with normal IRP. These patients may have dysphagia and/or non-cardiac chest pain. This includes nutcracker esophagus and jackhammer esophagus.
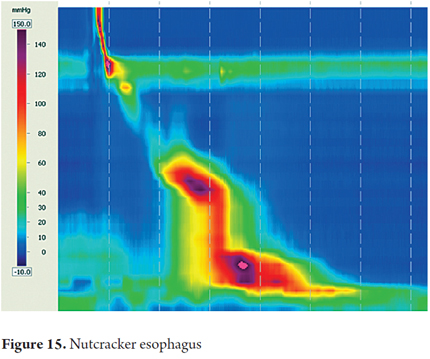
Nutcracker esophagus is defined as two or more swallows that produce a contraction in the smooth muscle with DCI greater than 5000 mmHg/cm/sec with a normal DL and IRP (Figure 15).
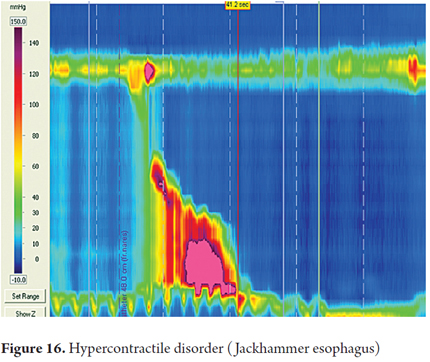
Jackhammer esophagus is a relatively rare pattern which is defined as one or more swallows that produce a contraction in the smooth muscle with DCI greater than 8000 mmHg/cm/sec with normal DL and IRP (Figure 16).
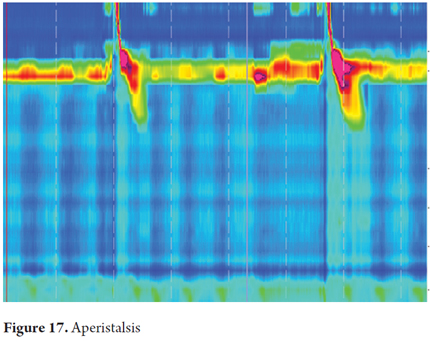
Aperistalsis is characterized by normal integrated relaxation pressure (IRP) without any peristaltic wave in the distal esophagus. Whenever we see this manometric pattern we must ask whether the patient has scleroderma, diabetes or hypothyroidism (Figure 17).
A distal esophageal spasm is a relatively rare topographic pattern characterized by short distal latency (DL) of less than 4.5 seconds in less than 20% of swallows with normal IRP.
Normally, these contractions are longer and multiphasic. This pattern is typically associated with dysphagia and/or non-cardiac chest pain and may be susceptible to management with visceral analgesics and/or smooth muscle relaxants/botulinum toxin. This combination of patterns may also be the early signs of an inhibitory myenteric neuropathy with a possibility that it could become achalasia in the future.
Borderline esophageal motor function comprises a heterogeneous group of motor disorders including weak peristalsis and rapid contractions. They probably account for most of the manometric abnormalities observed in most motility laboratories.
Weak peristalsis is characterized by defects in the isobaric contour of 20 mmHg, and by failed peristalsis. Frequent failures are similar to the ineffective peristalsis described in conventional manometry. These motor abnormalities are associated with poor bolus transit and proximal escape, especially when the opening is in the transition zone (1, 18). Rapid contraction is characterized by more than 20% of swallows with peristaltic contractions with speed increased to 9cm/sec with normal DL and IRP.
CONCLUSIONS
High-resolution esophageal manometry has for the first time provided complete spatial and temporal views of the motor function of the esophagus (1).
In fact, almost all esophageal motor disorders can produce different patterns of esophageal pressure topography that are easily recognized (1).
Serious efforts to distinguish different esophageal motor disorders led to the development of the Chicago Classification which remains the most currently used classification for the interpretation of these disorders (1).
One of the objectives of a diagnosis through the Chicago ranking scheme is the exact classification of patients for inclusion in various clinical trials. Ultimately it will help determine the specific treatments needed for each disorder (20).
All images were taken from the high-resolution manometry equipment of the Gastroenterology Unit of the Hospital Universitario San Ignacio.
REFERENCES
1. Conklin JL. Evaluation of Esophageal Motor function with high-resolution manometry. J Neurogastroenterol Motil 2013;19(3):281-94. [ Links ]
2. Bansala A, Kahrilas P. Has high-resolution manometry changed the approach to esophageal motility disorders? Curr Opin Gastroenterol 2010;26(4):344-51. [ Links ]
3. Roman P, Kahrilas P. Challenges in the swallowing mechanism; non-obstructive dysphagia in the era of high-resolution manometry and impedance. Gastroenterology Clin N Am 2011;40:823-35. [ Links ]
4. Fox MR, Bredenoord AJ. Oesophageal high-resolution manometry: Moving from research into clinical practice. Gut 2008;57:405-23. [ Links ]
5. Murray J, Clouse R, Conklin J. Components of the standard esophageal manometry. Neurogastroenterol Motil. 2003;15:591-606. [ Links ]
6. Kharilas P, Sifrim D. High-resolution manometry and impedance-pH/manometry: valuable tools in clinical and investigational esophagology. Gastroenterology 2008;135:756-69. [ Links ]
7. Conklin JL, Pimentel M, Soffer E. A color atlas of high-resolution manometry. Springer; 2009. [ Links ]
8. Fornari F, Bravi I. Multiple rapid swallowing: A complementary test during standard esophageal manometry. Neurogastroent Motil 2009;21:718. [ Links ]
9. Dustin A, Pandolfino J. High-resolution manometry and esophageal pressure topography. Filling the gaps of convention manometry. Gastroenterl Clin N Am 2013;62:1-15. [ Links ]
10. Ghosh SK, Pandolfino JE, Rice J, Clarke JO, Kwiatek M, Kahrilas PJ. Impaired deglutitive EGJ relaxation in clinical esophageal manometry: A quantitative analysis of 400 patients and 75 controls. Am J Physiol Gastrointest Liver Physiol 2007;293:878-85. [ Links ]
11. Pandolfino JE, Leslie E, Luger D, Mitchell B, Kwiatek MA, Kahrilas PJ. The contractile deceleration point: An important physiological landmark on oesophageal pressure topography. Neurogastrenterol Motil 2010;22:395-400. [ Links ]
12. Behar J, Biancani P. Pathogenesis of simultaneous esophageal contractions in patients with motility disorders. Gastroenterology 1993;105:111-8. [ Links ]
13. Roman S, Lin Z, Pandolfino JE, Kahrilas PJ. Distal contraction latency: A measure of propagation velocity optimized for esophageal pressure topography studies. Am J Gastroenterol 2011;106:443-51. [ Links ]
14. Pandolfino JE, et al. Distal esophageal spasm in high-resolution esophageal pressure topography: Defining clinical phenotypes. Gastroenterology 2011;141:469-75. [ Links ]
15. Gyawali CP, et al. Evaluation of esophageal motor function in clinical practice. Neurogastroenterol Motil 2013;25:99-133. [ Links ]
16. Pandolfino JE, Kwiatek MA, Nealis T, Bulsiewicz W, Post J, Kahrilas PJ. Achalasia: A new clinically relevant classification by high-resolution manometry. Gastroenterology 2008;135:1526-33. [ Links ]
17. Salvador R, et al. The preoperative manometric pattern predicts the outcome of surgical treatment for esophageal achalasia. J Gastrointest Surg 2010;14:1635-45. [ Links ]
18. Pandolfino JE, Zhang QG, Ghosh SK, Han A, Boniquit C, Kahrilas PJ. Transient lower esophageal sphincter relaxations and reflux: Mechanistic analysis using concurrent fluoroscopy and high-resolution manometry. Gastroenterology 2006;131:1725-33. [ Links ]
19. Ravi K, Friesen L, Issaka RB, Kahrilas PJ, Pandolfino JE. The natural history of patients with normal and borderline motor function on high-resolution manometry. Gastroenterology 2012;12(Suppl 1):S34. [ Links ]
20. Carlson D, Pandolfino J. The Chicago Criteria for esophageal motility disorders: What has changed in the past 5 years? Curr Opin Gastroenterol 2012;28(4):395-402. [ Links ]











 text in
text in