Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.30 no.1 Bogotá Jan./Mar. 2015
Diagnosis and Differential Diagnosis Problems with Histological Variants of Benign Liver Neoplasms
Rocío del Pilar López Panqueva MD. (1)
(1) Pathologist at the Hospital Universitario Fundación Santa Fe de Bogotá and the Universidad de Los Andes in Bogotá, Colombia.
Received: 19-02-15 Accepted: 26-02-15
Abstract
A basic principle of pathology is that neoplasms differ according to their cells of origin. Neoplasms of the liver resemble its constituent liver, biliary, epithelial, endothelial, mesenchymal cells or some combination of these different types of cells.
It is important to remember here that metastases are the most frequent malignant liver tumor occurring at ratio of 30: 1 in patients without underlying chronic liver disease or cirrhosis. Metastases are rare in cirrhotic livers. The most common primary sites are the colon, pancreas, common bile duct, stomach, neuroendocrine tumors and GIST, or extraintestinal tumors from the lung, breast, head, neck and skin (1).
In this article we review only the most frequent benign neoplasms and tumor-like lesions with particular emphasis on diagnostic difficulties, special studies, and the usefulness of immunohistochemical or molecular studies for proper classification and differential diagnosis.
Keywords
Benign liver tumors, adenoma, focal nodular hyperplasia, nodular regenerative hyperplasia, hemangioma, angiomyolipoma, hamartoma, biliary hamartoma, mesenchymal hamartoma, von Meyenberg complexes, simple cysts, mucinous neoplasms, biliary cystadenoma, intraductal papillary neoplasms bile.
A simple classification of benign and malignant primary tumors and tumor-like lesions is summarized in Table 1.
HEPATOCELLULAR ADENOMAS
Hepatocellular adenomas (HCAs) are benign hepatocellular neoplasias which most often occur in young women of childbearing age who have histories of oral contraceptive, estrogen or exogenous androgen use. The annual incidence among women who take oral contraceptives is 4.3 per 100,000 in the USA and Europe. Other risk factors that have been described include deposit type diseases such as tyrosinemia, galactosemia, and familial diabetes. Among male patients glycogen storage disease type I, III or IV. Malignant transformations occur in 10% of cases (2).
HCAs are most often found incidental to MRIs or CT scans. When the adenoma is large, abdominal pain, a palpable mass and intra-abdominal or intramural bleeding may be the main clinical manifestations. The risk of rupture is greater in masses that are larger than 7 cm and when a patient has a history of using oral contraceptives.
These tumors are well defined individual masses that are not when the liver is not cirrhotic. When there are more than 10 nodes, the condition is called hepatic adenomatosis. Masses can vary in size from very small to masses than 10 cm. HCAs are monoclonal neoplasms which are associated with several recurrent mutations in genes encoding nuclear factor-1a (HNF1a), CTNNB1 which codes for B-catenin, and the APC gene (adenomatous polyposis). In 20% of cases there are alterations in the methylation of p14 (ARF) and p16 (INK4a). Familial forms of adenomatosis have heterozygous germline mutations in the HNF1A gene which are associated with MODY3. Sporadic adenomas with somatic mutations account for 85% of all cases (2-4).
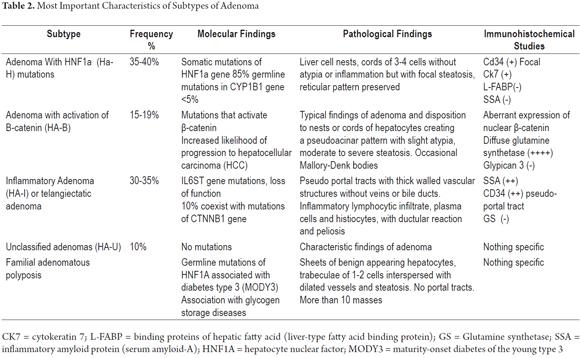
Table 2 summarizes the characteristic morphological, phenotypic and molecular findings for adenoma variants. Molecular characteristics are closely related to clinic characteristics, and pathological features. One of the most critical is the increased risk of malignant transformation of adenomas associated with activation of β-catenin (HA-B). Occasionally, it is absolutely impossible to diagnose adenoma with a trucut or wedge biopsy. In these cases, complete resection of the lesion followed by multiple additional samples, histochemical studies (reticulum) and immunohistochemistry is required. Molecular studies have led to a reclassification of this neoplasm which is of great prognostic importance (5-7).
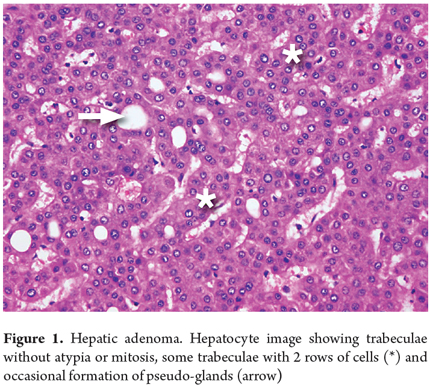
A liver biopsy is considered to be the gold standard for diagnosis, but diagnosis from a biopsy can be extremely difficult from a morphological point of view. The sample may look like a normal liver or appear to have well differentiated hepatocellular carcinoma. In fact, this is the main diagnostic difficulty with trucut biopsies (Figure 1).
The differential diagnosis must consider well-differentiated hepatocellular carcinoma, focal nodular hyperplasia and nodular regenerative hyperplasia.
FOCAL NODULAR HYPERPLASIA (FNH)
Focal Nodular Hyperplasia (FNH) is considered to be benign reactive nodular lesion rather than a true neoplasm. They result from alterations in blood flow or arterial malformation and have no malignant potential although they have been described in the vicinity of hepatocellular carcinomas. They are the second most frequently occurring liver hemangiomas after nodular lesions. They occur in 8% of children treated with chemotherapy several years after treatment. Occurrences in young and middle-aged women (20 to 50 years old) account for 75% of all cases with a female to male ratio of between five and ten to one. Usually they are individual masses: less than 20% to 30% are multiple (4).
In histological studies, FNH appears as nodules of no more than two or three cells of cytologically benign hepatocytes in trabeculae. They are separated by thin fibrous septa, with proliferation of cholangioles or with ductal metaplasia that coalesces in a radiating central lesion which has enlarged vascular wall structures of various shapes and sizes (Figure 2). Twenty-five percent of these cases are accompanied by steatosis and xanthomas of the septal hepatocytes. Real portal tracts are not seen, nor is there mitosis. The reticular pattern is preserved. Twenty percent of FNH cases are atypical, telangiectasia, or mixed forms (hyperplastic or adenomatous). Most FNH are polyclonal, but the molecular pathway and its mechanisms have not yet been elucidated.
They may be clinically silent or may present abdominal pain, weight loss, general malaise or masses. There is rarely any bleeding.
Diagnosis on the basis of a biopsy is difficult, is only possible in twenty-five to fifty percent of cases, and requires correlation with radiological images. The expression of glutamine synthetase (GS) through immunohistochemistry creates an irregular pattern resembling a geographical map which can help in the differential diagnosis. This must consider nodular regenerative hyperplasia and adenomas, especially inflammatory variants and telangiectatic adenoma. Staining for CD34 shows prominent sinusoidal capillarization in the vicinity of fibrous septa (4, 6, 8).
NODULAR REGENERATIVE HYPERPLASIA
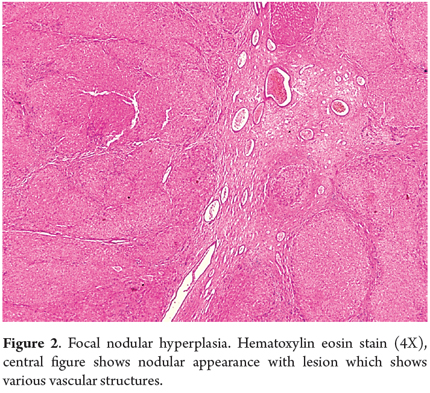
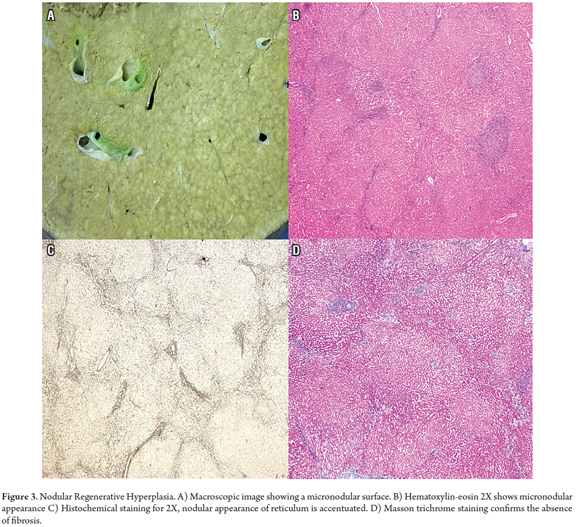
Nodular Regenerative Hyperplasia (NRH) develops in the context of several systemic diseases and after administration of some drugs. Associated disease include rheumatoid arthritis, systemic lupus erythematosus, celiac disease, polycythemia vera, chronic lymphocytic leukemia, Hodgkin's and non-Hodgkin's lymphomas, multiple myeloma, neuroendocrine tumors, and aplastic anemia. Associated medications include azathioprine, didanosine and oxaliplatin. Small regenerative nodules measuring between one and three mm form in a diffusely distributed pattern in the parenchyma. Unlike in cirrhosis, little or no fibrosis occurs. This nodular transformation is secondary to altered blood flow due to obliteration of small portal veins or damage to the sinusoids. It presents as non-cirrhotic portal hypertension. Macroscopically, it can mimic cirrhosis, but histological study will show no evidence of cirrhotic nodules or significant fibrosis. The coloring of the reticulum will show atrophy of the hepatocytes' trabeculae in zone 3, something that is normally observed in zone 1 with mild dilation of the sinusoids (Figure 3) (2, 9, 10).
In the differential diagnosis, all types of cirrhosis and focal nodular hyperplasia must be considered.
HEMANGIOMA
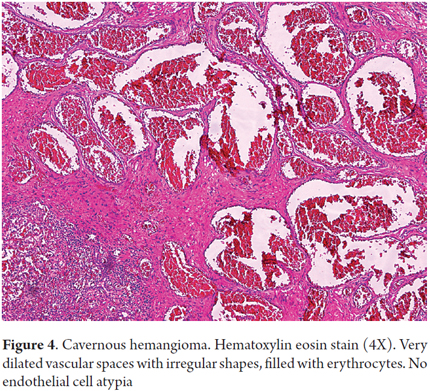
Cavernous hemangioma accounts for 73% of benign liver tumors of vascular origin. Its pathogenesis is not well understood, but it has been speculated that there may be abnormal vasculogenesis and/or abnormal angiogenesis resulting in proliferation of thin walled, dilated vascular structures which are filled with erythrocytes coated by typical endothelial cells with prominent fibrous septa (Figure 4). They are usually found incidentally, and in 90% of cases they are individual masses measuring less than 4 cm. Prevalence varies between 1% and 20%, they can occur at any age, but they occur five times more commonly in women than in men. They may grow enlarge during pregnancy or estrogen therapy. They are rarely biopsied and are usually diagnosed through imaging. Sclerosing hemangioma and capillary hemangiomas can sometimes require immunohistochemical studies to determine their vascular phenotypes given the tiny vascular openings and lobular growth patterns. In these cases CD34, CD31 and Factor VIII are useful markers (11).
Infant hemangioma is a variant of hemangioma that presents in the first six months of life. It is associated with multiple congenital malformations, and which compromises extrahepatic organs in 10% of the cases. Since infant hemangioma carries a risk of malignancy, the histological study should look for atypical cytology and architecture. These cases must be differentiated from vascular malformations for which the study of immunohistochemistry for GLUT-1 helps: in infantile hemangioma it is positive, while in vascular malformations, it is negative (12).
Differential diagnosis must consider peliosis, angiosarcoma and sclerotic bile duct hamartoma.
ANGIOMYOLIPOMA
Hepatic angiomyolipomas are rare mesenchymal tumors that belong to the group of tumors derived from perivascular epithelioid cells. Most are benign, although there have been reports of extrahepatic angiomyolipoma metastases. This tumor appears as a solitary mass in 90% of sporadic cases but occasionally appears associated with tuberous sclerosis. In these cases renal angiomyolipoma is the most common finding. Usually they are diagnosed incidental to an imaging study. They may present with abdominal pain, bloating, rupture or bleeding when the mass is large. They mostly affect women, can occur at any age, but are most common among young and middle aged people (13).
Angiomyolipomas are mixtures of various components in varying proportions of smooth muscle, myoid differentiation, fat, epithelioid cells and vascular structures. Atypical cellular structures with nuclear pleomorphism are possible (Figure 5A).
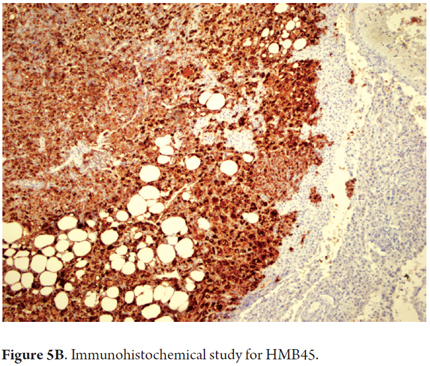
Immunohistochemical study is very useful for diagnosis since the diversity of patterns hinders morphological interpretation. This type of tumor is reactive to HMB45 (Figure 5B) and Melan A (melanocyte markers). Other markers such as S100, AML and CD117 may also show reactivity. They are negative for cytokeratin and hepatocyte.
The differential diagnosis must consider lipomas, muscle tumors such as leiomyomas, or angiomas, pleomorphic epithelial tumors such as metastatic melanoma, pleomorphic spindle cell sarcoma and gastrointestinal stromal tumors (GIST). The epithelioid variant resembles hepatocellular carcinoma (14).
BILIARY HAMARTOMAS AND MICROHAMARTOMAS (VON MEYENBERG COMPLEX)
Biliary hamartomas and microhamartomas are birth defects of the formation of the ductal plate. They are part of the spectrum that includes polycystic diseases in other organs and biliary cysts. There are no specific symptoms so hamartomas are usually incidental findings which cause difficulty in frozen sections of intraoperative biopsies. They are small, white, single or multiple, subcapsular lesions that suggest metastasizing disease. They are usually found during abdominal surgery conducted because of suspected tumors.
Biliary hamartomas appear as irregular biliary structures with some dilated ducts measuring less than 0.5 cm. They are delineated by simple cubic or squamous epithelium and may contain bile or proteinaceous detritus. They are surrounded by abundant stroma which may be myxoid or fibrous and are located in the periportal area. Immunohistochemical studies are not very useful and are even unnecessary for diagnosis since biliary hamartomas have the same phenotype as normal bile ducts. Contrary to what is observed in malignant lesions, hamartomas exhibit little or no cell proliferation when Ki67 is measured (15).
The differential diagnosis must consider metastatic adenocarcinoma and bile duct adenomas.
MESENCHYMAL HAMARTOMAS
Mesenchymal hamartomas are benign lesions that are most common in boys under the age of five. In fact, 85% of the cases occur before age three including prenatal diagnoses. There are elevated levels of alpha fetoprotein (AFP) and a single mass with heterologous mesenchymal components is usually observed. They rarely present multifocal masses and rarely occur in adults. Patients usually have abdominal distension and a palpable mass.
Mesenchymal hamartomas can be very large and are composed of multiple cystic spaces filled with gelatinous material. Under a microscope this material can be seen to be primitive mesenchymal tissue with trapped bile ducts, vascular spaces that resemble sinusoids, and spindle cells in a myxoid stroma. They can be very loose and edematous but do not have atypical cellular structures.
Immunohistochemical study shows reactivity for cytokeratin 7 and 19 in the epithelium of the bile ducts. The stroma tests positive for vimentin and smooth muscle actin. Islands of entrapped hepatocytes test positive for AFP (16, 17).
The differential diagnosis must consider lymphangiomas and infantile hemangioendothelioma.
SIMPLE CYSTS: BILE, MESOTHELIAL AND FOREGUT
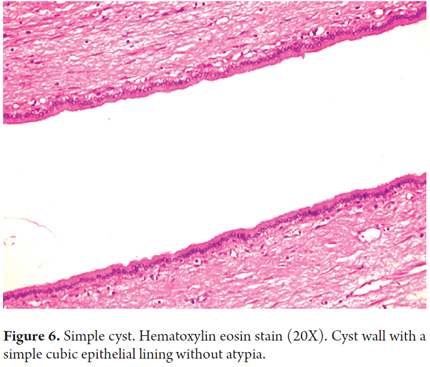
Cystic neoplasm of the liver accounts for less than 10% of all liver tumors. Solitary or simple cysts occur most frequently among women in the sixth decade of life and are rarely observed in biopsies. Fifty percent of them are asymptomatic. Simple cysts have a single layer of cuboidal epithelium with no evidence of atypical cells or ovarian stroma (Figure 6). Biliary cysts with columnar cells similar to epithelial cells can be found close to biliary glands. Mesothelial cysts are small and subcapsular and are covered by mesothelium.
The differential diagnosis should include immunohistochemical studies to determine whether mesothelial cells are positive for WT1, calretinin, CAM 5.2, epithelial membrane antigen (EMA) and cytokeratins AE1 and AE3.
Foregut cysts occur equally frequently among men and women. They have a single locus and ciliated columnar cells with a wall of smooth muscle (18, 19).
MUCINOUS CYSTIC NEOPLASMS
Within this group are the hepatic (biliary) cystadenomas and cystadenocarcinoma which are mucinous cystic neoplasms with invasive carcinoma. The World Health Organization classification of 2010 says that they are very similar to pancreatic neoplasms such as low, medium and high grade and mucinous neoplasms.
They occur twenty times more often women than men, especially in the fourth and fifth decades of life. They are almost always solitary and multilocular biliary epithelial stroma on the ovarian wall.
BILIARY CYSTADENOMA
Biliary cystadenoma are rare lesions in the ductal epithelium. They are usually asymptomatic but may produce mild abdominal pain. Ninety percent originate from the intrahepatic bile ducts, especially those in the right lobe. After a diagnosis is made with imaging, surgical resection is the treatment of choice because, despite some risk of invasive carcinoma, biliary cystadenoma's malignant counterpart cystadenocarcinoma will have already been discarded after the imaging (18).
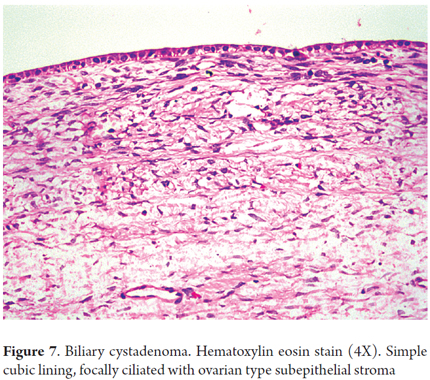
Histologically, biliary cystadenoma is characterized by cystic spaces coated with biliary and stroma cells in the cyst wall (Figure 7). Sometimes, there is low or even high grade dysplastic epithelium. There is no communication with the bile ducts which helps separate mucinous cystic neoplasms from intraductal papillary neoplasms (20).
Immunophenotyping shows no expression of cytokeratin 7, 19, 8, 20 except in the areas of dysplasia which is important as these lesions are potentially malignant. The ovarian stroma express estrogen, progesterone, inhibin-α, vimentin, actin and desmin receptors (18, 21).
The differential diagnosis must consider simple cysts, intraductal papillary cysts, mucinous cystic neoplasms, cystadenocarcinoma, polycystic disease, and parasitic cysts especially hydatid cysts.
Intraductal papillary neoplasm of the bile duct (IPNB)
These neoplasms include biliary papillomatosis. They grow within the biliary tract and half of the patients have histories of gallstones with no significant differences between men and women. They are multilocular masses connected to the bile ducts with a high risk of invasive carcinoma.
Histologically the ductal lumen is occupied by papillary proliferation of neoplastic biliary epithelial cells with fine stalks. A third of them produce mucin. The cells of the epithelial lining vary but may be biliary, intestinal, oncocytic, or gastric.
Differentiation of mucinous neoplasms requires thorough histopathology with multiple slices. At times it is necessary to process the entire mass of the specimen to find the connection with the bile ducts. This is especially true for large masses with marked cystic changes and when no ovarian stroma are found to help in the differential diagnosis (22, 23).
The lists below summarize the "Diagnostic keys" for those liver tumors that pose the greatest diagnostic difficulty.
1. Adenoma
- Benign neoplasm in non-cirrhotic liver
- Young women of childbearing age
- Use of oral contraceptives
- Solitary mass or multiple solid masses
- Nests of liver cells, without atypical cells, arranged in trabeculae of 2-3 cells, in some cases accompanied by steatosis and Malloy-Denk bodies
- Molecular subtypes: HNF1A (HA-H) adenoma mutations, adenoma with activation of B-catenin (HA-B), Inflammatory adenoma (HA-I) or telangiectatic adenoma, unclassified adenoma
2. Focal Nodular Hyperplasia (FNH)
- Benign nodular lesion in non-cirrhotic liver
- Women in third to fourth decade of life
- Usually single mass with well circumscribed central lesion
- Hepatocyte nodules with incomplete fibrovascular septa arising from the central lesion, thick-walled vessels, and no portal tracts
- Immunohistochemical study for glutamine synthetase (GS) which characteristically has a map pattern
3. Nodular Regenerative Hyperplasia
- Transformation of parenchyma into small nodules with minimal or no evidence of fibrosis, focal sinusoidal dilation and trabecular atrophy
- Related to several systemic diseases. Produces non-cirrhotic portal hypertension
4. Hemangioma
- Most common benign liver tumor, vascular origin.
- Occurs at any age, slightly more frequent in women, can grow with estrogen or pregnancy.
- Well defined subcapsular mass in non-cirrhotic liver
- Cavernous hemangioma have dilated vascular spaces filled with erythrocytes coated by endothelial cells without atypical cells but with fibrous septa.
- Capillary and sclerotic forms may need immunohistochemical studies for CD31, CD34 and factor VIII to determine the phenotype
5. Angiomyolipomas
- Benign tumor originating in perivascular epithelioid cells with a mixture of mature adipose tissue, smooth muscle, blood vessels and epithelioid cells
- Most common in women in the fourth decade of life
- Usually large solitary masses
- Immunohistochemical study of reactivity for HMB45, MELAN-A, plus muscle markers and CD117 is important.
6. Mucinous neoplasms
Biliary cystadenoma or Mucinous cystic neoplasms
- Women
- Absence of Hepatolithiasis
- Multiple cysts
- Ovarian stroma with expression of hormone receptors
- No connection with bile ducts
- Low risk of invasive carcinoma
Intraductal papillary mucinous neoplasm
- Men = Women
- Frequent Hepatolithiasis
- Multilocular
- Absence of ovarian stroma
- Connection with bile ducts with expansion of intraductal papillary tumor
- High risk of invasive carcinoma
Benign liver tumors are a large group of neoplasms which have various epithelial origins from mesenchymal to ductal. They are difficult to diagnosis clinically as well as through imaging and often require histopathology for differentiation. Molecular and phenotypic studies are very useful for proper diagnosis of these neoplasms.
REFERENCES
1. Goodman ZD. Neoplasms of the liver. Mod Pathol Off J U S Can Acad Pathol Inc 2007;20(Suppl 1):S49-60. [ Links ]
2. Paradis V. Benign liver tumors: an update. Clin Liver Dis 2010;14(4):719-29. [ Links ]
3. Greaves WOC, Bhattacharya B. Hepatic adenomatosis. Arch Pathol Lab Med 2008;132(12):1951-5. [ Links ]
4. Lizardi-Cervera J, Cuéllar-Gamboa L, Motola-Kuba D. Focal nodular hyperplasia and hepatic adenoma: a review. Ann Hepatol 2006;5(3):206-11. [ Links ]
5. Dhingra S, Fiel MI. Update on the new classification of hepatic adenomas: clinical, molecular, and pathologic characteristics. Arch Pathol Lab Med 2014;138(8):1090-7. [ Links ]
6. Joseph NM, Ferrell LD, Jain D, Torbenson MS, Wu T-T, Yeh MM, et al. Diagnostic utility and limitations of glutamine synthetase and serum amyloid-associated protein immunohistochemistry in the distinction of focal nodular hyperplasia and inflammatory hepatocellular adenoma. Mod Pathol Off J U S Can Acad Pathol Inc 2014;27(1):62-72. [ Links ]
7. Rebouissou S, Bioulac-Sage P, Zucman-Rossi J. Molecular pathogenesis of focal nodular hyperplasia and hepatocellular adenoma. J Hepatol 2008;48(1):163-70. [ Links ]
8. Makhlouf HR, Abdul-Al HM, Goodman ZD. Diagnosis of focal nodular hyperplasia of the liver by needle biopsy. Hum Pathol 2005;36(11):1210-6. [ Links ]
9. Al-Mukhaizeem KA, Rosenberg A, Sherker AH. Nodular regenerative hyperplasia of the liver: an under-recognized cause of portal hypertension in hematological disorders. Am J Hematol 2004;75(4):225-30. [ Links ]
10. López R, Barrera L, Vera A, Andrade R. Concurrent liver hodgkin lymphoma and nodular regenerative hyperplasia on an explanted liver with clinical diagnosis of alcoholic cirrhosis at University Hospital Fundación Santa Fe de Bogotá. Case Rep Pathol 2014;2014:193802. [ Links ]
11. Hsi Dickie B, Fishman SJ, Azizkhan RG. Hepatic vascular tumors. Semin Pediatr Surg 2014;23(4):168-72. [ Links ]
12. Mo JQ, Dimashkieh HH, Bove KE. GLUT1 endothelial reactivity distinguishes hepatic infantile hemangioma from congenital hepatic vascular malformation with associated capillary proliferation. Hum Pathol 2004;35(2):200-9. [ Links ]
13. Lo RC. Epithelioid angiomyolipoma of the liver: a clinicopathologic study of 5 cases. Ann Diagn Pathol 2013;17(5):412-5. [ Links ]
14. Nonomura A, Enomoto Y, Takeda M, Takano M, Morita K, Kasai T. Angiomyolipoma of the liver: a reappraisal of morphological features and delineation of new characteristic histological features from the clinicopathological findings of 55 tumours in 47 patients. Histopathology 2012;61(5):863-80. [ Links ]
15. Bailador Andrés MC, Vivas Alegre S, Rueda Castañón R. Multiple bile-duct hamartomas (Von Meyemburg complexes). Rev Esp Enfermedades Dig Organo Of Soc Esp Patol Dig 2006;98(4):306-7. [ Links ]
16. Stringer MD, Alizai NK. Mesenchymal hamartoma of the liver: a systematic review. J Pediatr Surg 2005;40(11):1681-90. [ Links ]
17. Fretzayas A, Moustaki M, Kitsiou S, Nychtari G, Alexopoulou E. Long-term follow-up of a multifocal hepatic mesenchymal hamartoma producing a-fetoprotein. Pediatr Surg Int 2009;25(4):381-4. [ Links ]
18. Soares KC, Arnaoutakis DJ, Kamel I, Anders R, Adams RB, Bauer TW, et al. Cystic neoplasms of the liver: biliary cystadenoma and cystadenocarcinoma. J Am Coll Surg 2014;218(1):119-28. [ Links ]
19. Michael Torbenson. Biopsy Interpretation of the liver. 2014. [ Links ]
20. Hernandez Bartolome MA, Fuerte Ruiz S, Manzanedo Romero I, Ramos Lojo B, Rodriguez Prieto I, Gimenez Alvira L, et al. Biliary cystadenoma. World J Gastroenterol WJG 2009;15(28):3573-5. [ Links ]
21. Tsepelaki Α, Kirkilesis I, Katsiva V, Triantafillidis JK, Vagianos C. Biliary cystadenoma of the liver: case report and systematic review of the literature. Ann Gastroenterol 2009;22(4):278-83. [ Links ]
22. Zen Y, Pedica F, Patcha VR, Capelli P, Zamboni G, Casaril A, et al. Mucinous cystic neoplasms of the liver: a clinicopathological study and comparison with intraductal papillary neoplasms of the bile duct. Mod Pathol Off J U S Can Acad Pathol Inc 2011;24(8):1079-89. [ Links ]
23. Li T, Ji Y, Zhi X-T, Wang L, Yang X-R, Shi G-M, et al. A comparison of hepatic mucinous cystic neoplasms with biliary intraductal papillary neoplasms. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 2009;7(5):586-93. [ Links ]











 text in
text in