Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.30 no.2 Bogotá abr./jun. 2015
The Current State of Biodegradable Self-expanding Stents in Interventional Gastrointestinal and Pancreatobiliary Endoscopy
Óscar A. Álvarez B. MD. (1), Rodrigo Castaño Llano MD. (2), David Restrepo (3)
(1) Radiologist, Internist, Gastroenterologist and Professor at the University of Texas, San Antonio. Director of Gastroenterology at the Texas Valley Coastal Bend Veterans' Administration Hospital in Harlingen, Texas.
(2) Gastrointestinal and Endoscopic Surgeon and Head of the Postgraduate General Surgery program U.P.B., Gastro Group at the University of Antioquia, Institute of Cancer Clinic of the Americas in Medellin, Colombia.
(3) Medical Student at CES in Medellin, Colombia.
Received: 08-07-14 Accepted: 06-04-15
Abstract
Biodegradable stents are a very attractive option for use in patients with benign but recurrent and recalcitrant digestive tract and biliary strictures. In theory, use of these biodegradable stents mitigates the need for repetitive expansion of digestive or biliary strictures which are refractory to conventional management and avoids the need of surgical resection. This is especially true for patients at high surgical risk. Stents can also minimize the number of interventional endoscopic procedures performed on a patient.
Keywords
Reabsorbable stent, benign biliary stenoses, benign colon stenoses, benign gastroduodenal stenoses.
INTRODUCTION
The use of self-expanding metal stents has proven to be an effective treatment for managing refractory benign and malignant stenoses of the digestive tract. Nevertheless, these stents which are mostly used for treating benign pathologies, have been associated with various complications including difficult extraction, migration and hyperplasia. The costs inherent in procedures that must be repeated must also be taken into account (1-3). Plastic stents have higher migration rates, less flexibility and less expansive radial force (4, 5).
In the last two decades there have been significant advances in the development of biocompatible and biodegradable materials for medical applications. These materials have been used to develop biodegradable stents to remedy the problems associated with metal and plastic stents (6). This article reviews the scant and little known experience with biodegradable stents that has been accumulated to date. The emphasis is on their biomechanical aspects with the intention of consideration of these stents for future local use.
BIOMATERIALS
A biomaterial is an inert compound designed to be implanted or incorporated within a living biological system. Biomaterials are subject to adverse situations since they are temporarily or permanently exposed to body fluids which corrode elements of implants. Biomaterials can restore functions of living tissues and organs in the body, but the properties required of a material vary according to the particular application. For this reason it is essential to understand the relationships between the properties, functions and structures of biological materials. Biomaterials can be inert, bioactive, or biodegradable. Inert materials do not trigger any reaction in the host, bioactive materials can ensure stable and lasting performance, and biodegradable materials can decompose as the product of natural factors such as bacteria or can be chemically degraded. It is vital that biodegradable materials for medical use have no risks of carcinogenesis, immunogenicity, teratogenicity or toxicity (7).
Synthetic polymers and magnesium-based materials are the materials most commonly used for biodegradable applications. The synthetic polymers include polylactic acid, polyglycolic acid, polycaprolactone, polydioxanone and poly (lactic-co-glycolic acid) (8-10). Biomaterials made of magnesium-based alloys are very biocompatible and have the property of dissolving in the body during degradation. However, due to rapid corrosion, degradation can occur before the therapeutic objective is reached.
Polymeric biomaterials have several advantages: they are elastic, have low densities and relatively easy to manufacture. The polymers used in medical devices degrade more slowly than magnesium alloys, and the process is hydrolytic degradation. This hydrolysis process degrades the polymers into molecules with little molecular weight so that they can be metabolized by the body.
The following six qualities characterize the ideal polymer for use in biodegradable stents:
1. The ability to maintain sufficient expansive force until the stenosis is resolved.
2. It must not be toxic.
3. It must not induce an inflammatory response in the surrounding tissue.
4. It must be easily metabolizable in the body after it fulfills its function.
5. It must be easily processed and never leave traces.
6. It must be easy to sterilize.
Given these characteristics, the main advantages of a synthetic polymer are:
1. Adequate biocompatibility
2. The potential to change composition and physical-mechanical properties
3. Low friction coefficients
4. Viability and potential for being processed.
5. Alternatives for surface chemical and physical changes.
6. Ability to immobilize cells or biomolecules within cells or on the surfaces of cells.
This ability to immobilize cells and/or biomolecules led to the development of drug-eluting stents (DES) such as those used for some coronary applications. These biodegradable polymers used for drug delivery are a major technological advance which, until now, have primarily been used in DES for the vascular system (11).
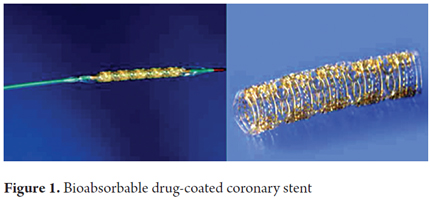
The development of drugs that cannot be administered through traditional intramuscular, subcutaneous or intravenous routes together with the convenience of delivering a drug in a very localized and controlled mannered has led to research into biomaterials for use in the field of pharmacy. One result has been the development of devices that incorporate a drug into a bioabsorbable matrix in which the release and subsequent availability of the drug is determined by the speed at which the polymer containing the drug degrades (12). Coronary stents are a great example of these devices. Different compounds such as tacrolimus, sirolimus, paclitaxel, aspirin, dexamethasone, and radioactive materials have been used in the matrixes of biodegradable stents in order to prevent restenosis and inflammation while avoiding intimal reactions (13-15). Until now, no similar applications have been developed in the gastroenterological area for humans (Figure 1).
Polymers, whether or not they are biodegradable, have different mechanisms of action. Although all matter degrades over time, the term biodegradable is only applied to polymers term that complete degrade within a short time. Polyesters are synthetic biodegradable polymers that have many commercial applications in the medical field. They are characterized by ester bonds within the main chain which permit them to be degraded hydrolytically. Processes of hydrolysis degrade polymers into low molecular weight molecules which can be metabolized by the body. The human physiological environment provides the appropriate conditions for these processes to occur under normal conditions between pH 7 and pH 7.4 (16).
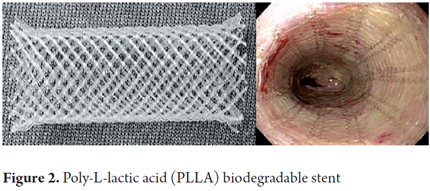
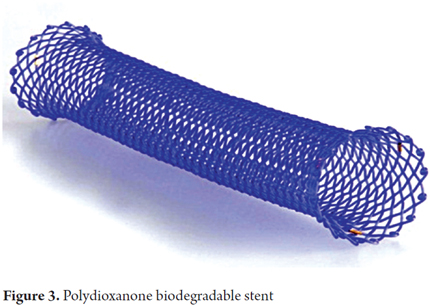
As mentioned, biodegradable stents are made from various synthetic polymers such as polylactic acid, polyglycolic acid, polyglyconate, poly-L-lactic (Figure 2) and copolymers of polylactic-co-glycolic acid and polydioxanone (Figure 3) (17). Biodegradable stents have been used for treatment of refractory benign stenoses including those in the ureters, urethra, trachea, bile ducts, pancreas, small intestine, colon and esophagus (18).
CLINICAL EXPERIENCE WITH GASTROINTESTINAL AND PANCREATOBILIARY BIODEGRADABLE STENTS
The idea of using biodegradable stents is not new. In fact there were there were experimental prototypes in 1991 and 1992. Nevertheless, only in recent years have models safely passed the experimental stage in animals and begun to be tested in humans. In 1993 Kemppainen published the first study of biodegradable stents. The study used an experimental model of treatment of urethral stenosis in rabbits with a biodegradable stent made of poly-L-lactic acid. The study concluded that this type of stent had a great future for preventing restenosis of the narrow urethra (19).
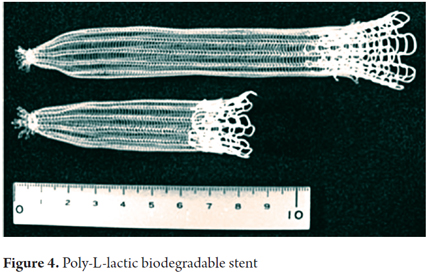
The first biodegradable stents for the gastrointestinal tract were made from polylactic acid and were developed by Goldin (20). He reported the experience of using these stents on five patients with benign esophageal stenoses that had been refractory to endoscopic treatment (Figure 4). The authors concluded that this prototype stent was unable to maintain significant sustained radial force for more than three weeks. The stent disintegrated within six weeks of placement and induced obstruction of the esophageal lumen. These findings were confirmed in another study by Fry & Fleisher (21).
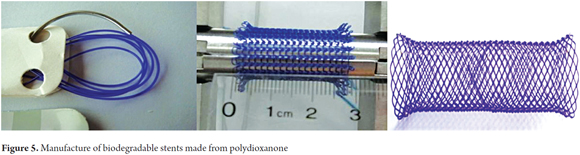
Results have improved significantly with biodegradable stents made from polydioxanone (Figure 5).
This type of stent has greater integrity and retains its radial force for 6 to 8 weeks after placement as has been demonstrated by Rejchrt (22). This pilot study of three patients with benign stenoses of the small intestine and colon confirmed that degradation and fragmentation of the stent occur between the 11th and 12th weeks after stent placement. It seems that the degradation of the stent is pH dependent: degradation is faster when the pH is lower. It seems that these preliminary observations of prolonged dilation before degradation and absorption of the stent may offer a solution for patients who are refractory to conventional dilatation of benign stenoses. This new type of biodegradable stent allows for constant radial expansion similar to that achieved with expandable metal stents. The only difference is that biodegradable stents need not be removed. Polydioxanone biodegradable stents may become an alternative treatment for refractory benign stenoses of the digestive tract.
Parviainen has treated two pancreaticojejunal anastomoses with biodegradable polylactide stents. Neither patient developed post-procedure complications. These stents degrade more easily in this environment than in the pancreatic biliary duct environment (23).
Esophagus
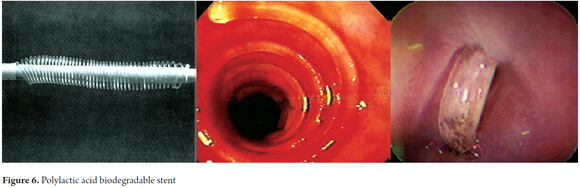
The placement of biodegradable stents is an alternative for treating benign esophageal stenoses that are refractory to conventional treatment with dilation. It should also be considered for patients with achalasia. These esophageal stenoses are generally related to gastroesophageal reflux, caustic ingestion or develop after esophageal surgery or are secondary to radiation therapy (18). Conventional dilation treatment of these stenoses uses plug dilators or hydrostatic balloons. Immediate clinical improvement of dysphagia occurs in 80% to 90% of cases. However, 30% to 60% of benign stenoses recur either in the short or long term. These patients with recurrent benign stenoses that are recalcitrant to conventional treatment are those who can benefit from an alternative treatment. Biodegradable stents were developed for this special group of patients (24). Saito has reported the results of two studies of patients treated with stents made of polylactic acid (Figure 6). Even though 77% of these stents had migrated over a period of 10 to 21 days after placement, clinical success was observed in all patients for a period of 7 months to two years (10).
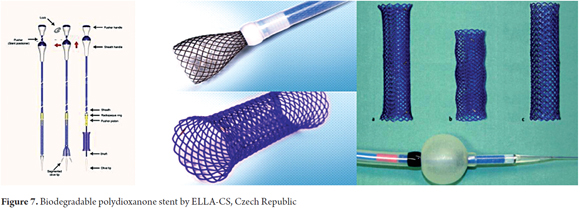
In 2007 the ELLA-CS stents (Hradec Kralove, Czech Republic) began to be used. This biodegradable stent is made of polydioxanone polymer and is currently the only biodegradable stent used in the digestive tract. This stent is placed onto a 28F delivery system and comes in sizes between 18 mm and 25 mm. This stent is manufactured for commercial use and is made of polydioxanone, a semicrystalline biodegradable polymer that degrades through hydrolysis. Biodegradation products are not toxic. The stent is transparent and has radiopaque markings on the proximal and distal ends (Figure 7) (25).
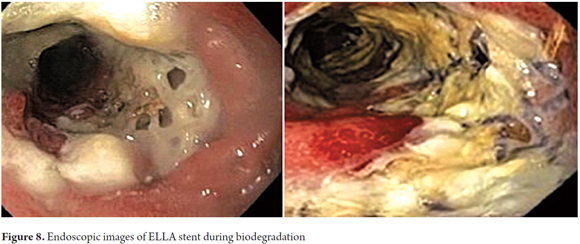
In 2009, a study published by Rejchrt of this polydioxanone stent showed that the radial force and integrity of the stent were continuously maintained for at least six weeks after placement (25). This is much better than previously published experience with other biodegradable stents. Sixty percent of stent degradation occurs 7 to 9 weeks after placement and 90% degradation occurs by 9 weeks. Acid suppression therapy is recommended as it has been shown that degradation of this type of stent is faster when exposed to acid. The average time of degradation of this type is between 11 and 12 weeks (Figure 8).
Several studies of experience with SX-ELLA polydioxanone biodegradable stents for treatment of refractory patients with benign stenoses of the esophagus and achalasia have been published (16, 18, 25-31, 32). Technical success, clinical responses and complications vary from study to study. Stenting was not a problem in any of the reported cases. Clinical success varies from 0% to 100% with an average of 40% (33-35). In 2010 Repici published a prospective study at two European centers (31). In this study biodegradable stents were used in 21 patients with esophageal stenoses that were refractory to endoscopic treatment. All patients were treated with ELLA-CS stents (Hradec Kralove, Czech Republic). The stenting was successful in 100% of the cases. Stents migrated in only two patients several weeks after placement. Stent biodegradation occurred between 3 and 6 months after placement. Follow-up endoscopy at three months showed that all stents had fragmented. Follow-up endoscopy at 6 months showed that all stents had dissolved. At the end of the follow-up, dysphagia had been resolved in nine patients (45%). The fact that the frequency of endoscopic dilatation was lower in patients who had been stented than among other patients should be taken into consideration. The study of this type of stent with the greatest long-term monitoring was reported by Hirdes. Clinical success was achieved in 25% of the 28 patients in this series (28).
Theoretically, biodegradable stents are ideal for benign stenoses that are refractory to endoscopic treatment. These stents can temporarily provide permeability together with remodeling the area of the stenosis. The effectiveness of biodegradable stents stems from a simple concept in which the repeated dilatation of the stenosis required for endoscopic treatment can be replaced by prolonged dilation through the use of a stent left in situ for several weeks or months. The basic principle is to remodel a fibrotic stenosis using a stent made of highly biocompatible materials that do not induce mechanical damage or irritation that could result in overgrowth of granulation tissue or in the formation of a new stricture or a fistula.
Three different kinds of expandable stents have been used to treat refractory esophageal stenoses: plastic, covered metal stents and biodegradable stents. So far only a single study has been published that compares the effectiveness of the three types of stents. Canena has shown that temporary placement of a self-expanding metal stent or a biodegradable stent has similar utility for treating refractory esophageal stenoses (36). In that study, long-term resolution of dysphagia was achieved in 40% of the patients treated with self-expanding metal stents and in 30% of those treated with biodegradable stents. Placement of self-expanding plastic stents had lowest clinical success rate of only about 10%. Self-expanding plastic stents were also associated with increased incidence of migration and repeated intervention. Thirty percent of the expandable metallic stents migrated while only 20% of the biodegradable stents migrated. Balloon dilation after placement of biodegradable stents decreased the incidence of migration.
Chest pain is the most common complication that has been reported in these studies. Tissue hyperplasia occurs in conjunction with stent degradation. Cases of severe hyperplasia with recurrence of dysphagia have also been reported (29, 32-35). Argon plasma treatment and balloon dilation have been used to solve this problem (29, 32, 35). Other potential complications of biodegradable stents are stent collapse in the esophageal lumen and tracheoesophageal fistulas (37).
After reviewing the studies published to date it can be concluded that a subgroup of patients with esophageal stenoses refractory to endoscopic treatment seem destined to prolonged treatment with dilations which increases the risk of complications, increase the costs involved in treatment, and significantly reduce the quality of life. These patients are candidates for treatment with biodegradable stents. Despite design limitations of the studies that have been published, they have shown that biodegradable stents are effective, safe and represent a very attractive option for this subgroup of patients.
Small Intestine and Colon
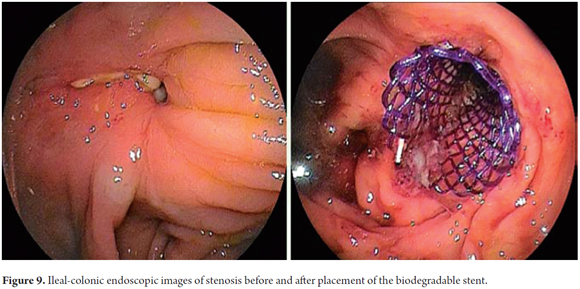
Biodegradable stents may be a promising therapeutic option for patients with stenoses of the small intestine and colon. The first description of the use of a biodegradable stent at this location was by the Czech group led by Rejchrt (22). Stenoses after colon and rectum surgery are the most common of these stenoses. They occur in between 1.5% and 8% of patients who undergo this type of surgery. A stenosis may also occur after resection of colon cancer, resection of colonic fistulas, and stenosis associated with Crohn's disease (22, 35, 38-41, 42). Placement in the intestine is possible and relatively simple except in cases of proximal stenosis and when there is a marked deformity or angulation. One limitation is 75 cm length of the delivery system. In most cases it is difficult to place a stent more than 30 cm from the anus. To address this problem an over tube extension has been suggested (22). Stent migration, one of the major limitations, is the main reason for failure of clinical response. Different techniques have been used to prevent migration. Mucosal hyperplasia has not been documented to date in cases of intestinal stenosis treated with biodegradable stents (Figure 9).
One can say biodegradable stents are a new option for treating stenosis of the postoperative small bowel and colon stenoses and stenoses secondary to Crohn's disease. Using this type of stents reduces the need for repeat endoscopy and dilatation. Consequently, they reduce the risk of perforation and surgical procedures. The high incidence of early migration can be solved with improvements in stent design. Further studies are needed to determine the efficacy and safety of these stents over the long term.
Biliary Tract and Pancreas
Endoscopy is the treatment of choice for benign biliary stenoses. The role of biodegradable stents in clinical practice is not well established and is one of the areas of current research in animal models (43, 44). There are many causes of biliary stenoses, but the two most common are postsurgical stenoses and stenoses secondary to chronic pancreatitis (45). Stents currently available in the market for bile ducts and pancreatic ducts are made of plastic or metal. These stents have limitations even in published studies of animal models (46, 47, 48). Laukkarinen has published various animal model studies using biodegradable stents in the bile ducts and pancreatic ducts. This group of authors has investigated the degradation, permeability and toxicity of biodegradable stents made of polylactic acid. In studies of stent placement pigs, no histological or anatomical changes were observed six months after placement.
Petrtyl and Mauri have published their experiences using biodegradable polydioxanone stents placed percutaneously into the bile duct. In these two studies twelve patients with postoperative biliary stenoses were treated with good clinical success after two years of monitoring (49, 50).
CONCLUSIONS
Although there are no domestically produced biodegradable stents in the market, and their costs are currently prohibitive, the experience accumulated using the only biodegradable polydioxanone polymer stents available for endoscopic use has shown promising results. This literature review allows us to say that biodegradable stents are a new option for the treatment of refractory stenoses in the esophagus, biliary ducts, small intestine and colon as well as stenoses secondary to Crohn's disease. Using this type of stents reduces the need for repeated endoscopy and dilatation. Consequently, it reduces the risks of perforation and surgical procedures. The high incidence of early migration can be solved with improvements in stent design. Further studies are needed to determine the efficacy and safety of these stents long term, and the loss of radial force over time due to degradation needs to be overcome or mitigated.
Without doubt studies that compare biodegradable stents with fully covered expandable stents are needed. Studies with long term follow-up are needed to assess remission of symptoms with the use of these stents. Evaluation of the use of these biodegradable stents before chemotherapy or radiotherapy in patients with esophageal cancer or malignant dysphagia would also be of great significance. Finally, the ideal stent for benign stenoses should have a large diameter and be very expandable and highly flexible. This would maintain luminal integrity, avoid epithelial hyperplasia and tissue damage and would not require repeat endoscopy for removal of the stent. It is very likely that biodegradable stents are ideal for achieving these goals.
REFERENCES
1. Tringali A, Blero D, Boskoski I, et al. Difficult removal of fully covered self expandable metal stents (SEMS) for benign biliary strictures: The "SEMS in SEMS" technique. Dig Liver Dis. 2014;46(6):568-71. [ Links ]
2. Sharaiha RZ, Kim KJ, Singh VK, et al. Endoscopic stenting for benign upper gastrointestinal strictures and leaks. Surg Endosc. 2014;28:178-84. [ Links ]
3. Almadi MA, Azzam N, Alharbi O, Mohammed AH, Sadaf N, Aljebreen AM. Complications and survival in patients undergoing colonic stenting for malignant obstruction. World J Gastroenterol. 2013;19:7138-45. [ Links ]
4. van Boeckel PG, Dua KS, Weusten BL, et al. Fully covered self-expandable metal stents (SEMS), partially covered SEMS and self-expandable plastic stents for the treatment of benign esophageal ruptures and anastomotic leaks. BMC Gastroenterology. 2012;12:19. [ Links ]
5. Gutierrez-Salmean G, Pelaez-Luna M, Gonzalez-Galeote E, Lozoya-Gonzalez D, Fuchs-Tarlovsky V, Farca-Belsaguy A. Outcomes of temporary self-expanding plastic stents (SEPS) use for gastrointestinal leaks. A case series. Rev Gastroenterol Méx. 2009;74:181-6. [ Links ]
6. Committee AT, Tokar JL, Banerjee S, et al. Drug-eluting/biodegradable stents. Gastrointest Endosc. 2011;74:954-8. [ Links ]
7. Lorenzo-Zuniga V, Moreno-de-Vega V, Marin I, Boix J. Biodegradable stents in gastrointestinal endoscopy. World J Gastroenter. 2014;20:2212-7. [ Links ]
8. Guo Q, Knight PT, Mather PT. Tailored drug release from biodegradable stent coatings based on hybrid polyurethanes. J Control Release. 2009;137:224-33. [ Links ]
9. Hermawan H, Dube D, Mantovani D. Degradable metallic biomaterials: design and development of Fe-Mn alloys for stents. J Biomed Mater Res A. 2010;93:1-11. [ Links ]
10. Saito Y, Tanaka T, Andoh A, et al. Usefulness of biodegradable stents constructed of poly-l-lactic acid monofilaments in patients with benign esophageal stenosis. World J Gastroenter. 2007;13:3977-80. [ Links ]
11. Neamtu I, Chiriac AP, Diaconu A, Nita LE, Balan V, Nistor MT. Current concepts on cardiovascular stent devices. Mini Rev Med Chem. 2014;14(6):505-36. [ Links ]
12. Austin AS, Khan Z, Cole AT, Freeman JG. Placement of esophageal self-expanding metallic stents without fluoroscopy. Gastrointest Endosc. 2001;54:357-9. [ Links ]
13. Han Y, Jing Q, Xu B, et al. Safety and efficacy of biodegradable polymer-coated sirolimus-eluting stents in «real-world» practice: 18-month clinical and 9-month angiographic outcomes. JACC. 2009;2:303-9. [ Links ]
14. Onuma Y, Serruys P, den Heijer P, et al. MAHOROBA, first-in-man study: 6-month results of a biodegradable polymer sustained release tacrolimus-eluting stent in de novo coronary stenoses. Eur Heart J. 2009;30:1477-85. [ Links ]
15. Wykrzykowska JJ, Onuma Y, Serruys PW. Advances in stent drug delivery: the future is in bioabsorbable stents. Expert Opin Drug Deliv. 2009;6:113-26. [ Links ]
16. Basha J, Appasani S, Vaiphei K, Gupta V, Singh K, Kochhar R. Biodegradable stents: truly biodegradable with good tissue harmony. Endoscopy. 2013;45(Suppl 2):E116-7. [ Links ]
17. Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17:93-102. [ Links ]
18. Griffiths EA, Gregory CJ, Pursnani KG, Ward JB, Stockwell RC. The use of biodegradable (SX-ELLA) oesophageal stents to treat dysphagia due to benign and malignant oesophageal disease. Surg Endosc. 2012;26:2367-75. [ Links ]
19. Kemppainen E, Talja M, Riihela M, Pohjonen T, Tormala P, Alfthan O. A bioresorbable urethral stent. An experimental study. Urol Res. 1993;21:235-8. [ Links ]
20. Goldin E, Fiorini A, Ratan Y, et al. A new biodegradable and self-expandable stent for benign esophageal strictures. Gastrointest Endosc. 1996;43:294. [ Links ]
21. Fry SW, Fleischer DE. Management of a refractory benign esophageal stricture with a new biodegradable stent. Gastrointest Endosc. 1997;45:179-82. [ Links ]
22. Rejchrt S, Kopacova M, Brozik J, Bures J. Biodegradable stents for the treatment of benign stenoses of the small and large intestines. Endoscopy. 2011;43:911-7. [ Links ]
23. Parviainen M, Sand J, Harmoinen A, et al. A new biodegradable stent for the pancreaticojejunal anastomosis after pancreaticoduodenal resection: in vitro examination and pilot experiences in humans. Pancreas. 2000;21:14-21. [ Links ]
24. Tanaka T, Takahashi M, Nitta N, et al. Newly developed biodegradable stents for benign gastrointestinal tract stenoses: a preliminary clinical trial. Digestion. 2006;74:199-205. [ Links ]
25. Rejchrt S, Kopacova M, Bartova J, Bures J. Intestinal biodegradable stents. initial experience in the czech republic. Folia Gastroenterol Hepatol. 2009;7:7. [ Links ]
26. Vandenplas Y, Hauser B, Devreker T, Urbain D, Reynaert H. A biodegradable esophageal stent in the treatment of a corrosive esophageal stenosis in a child. J Pediatr Gastroenterol Nutr. 2009;49:254-7. [ Links ]
27. van Hooft JE, van Berge Henegouwen MI, Rauws EA, Bergman JJ, Busch OR, Fockens P. Endoscopic treatment of benign anastomotic esophagogastric strictures with a biodegradable stent. Gastrointest Endosc. 2011;73:1043-7. [ Links ]
28. Hirdes MM, Siersema PD, van Boeckel PG, Vleggaar FP. Single and sequential biodegradable stent placement for refractory benign esophageal strictures: a prospective follow-up study. Endoscopy. 2012;44:649-54. [ Links ]
29. Karakan T, Utku OG, Dorukoz O, et al. Biodegradable stents for caustic esophageal strictures: a new therapeutic approach. Dis Esophagus. 2013;26:319-22. [ Links ]
30. Stivaros SM, Williams LR, Senger C, Wilbraham L, Laasch HU. Woven polydioxanone biodegradable stents: a new treatment option for benign and malignant oesophageal strictures. Eur Radiol. 2010;20:1069-72. [ Links ]
31. Repici A, Vleggaar FP, Hassan C, et al. Efficacy and safety of biodegradable stents for refractory benign esophageal strictures: the BEST (Biodegradable Esophageal Stent) study. Gastrointest Endosc. 2010;72:927-34. [ Links ]
32. Hair CS, Devonshire DA. Severe hyperplastic tissue stenosis of a novel biodegradable esophageal stent and subsequent successful management with high-pressure balloon dilation. Endoscopy. 2010;(42 Suppl 2):E132-3. [ Links ]
33. Orive-Calzada A, Alvarez-Rubio M, Romero-Izquierdo S, et al. Severe epithelial hyperplasia as a complication of a novel biodegradable stent. Endoscopy. 2009;41(Suppl 2):E137-8. [ Links ]
34. Fischer A, Bausch D, Baier P, Braun A, Richter-Schrag H. Risk of biodegradable stent-induced hypergranulation causing re-stenosis of a gastric conduit after esophageal resection. Endoscopy. 2012;44(Suppl 2):E125-6. [ Links ]
35. Dumoulin FL, Plassmann D. Tissue hyperplasia following placement of a biodegradable stent for a refractory esophageal stricture: treatment with argon plasma coagulation. Endoscopy. 2012;44(Suppl 2):E356-7. [ Links ]
36. Canena JM, Liberato MJ, Rio-Tinto RA, et al. A comparison of the temporary placement of 3 different self-expanding stents for the treatment of refractory benign esophageal strictures: a prospective multicentre study. BMC. 2012;12:70. [ Links ]
37. Jung GE, Sauer P, Schaible A. Tracheoesophageal fistula following implantation of a biodegradable stent for a refractory benign esophageal stricture. Endoscopy. 2010;42(Suppl 2):E338-9. [ Links ]
38. Repici A, Pagano N, Rando G, et al. A retrospective analysis of early and late outcome of biodegradable stent placement in the management of refractory anastomotic colorectal strictures. Surg Endosc. 2013;27:2487-91. [ Links ]
39. Perez Roldan F, Gonzalez Carro P, Villafanez Garcia MC, et al. Usefulness of biodegradable polydioxanone stents in the treatment of postsurgical colorectal strictures and fistulas. Endoscopy. 2012;44:297-300. [ Links ]
40. Toth E, Nielsen J, Nemeth A, et al. Treatment of a benign colorectal anastomotic stricture with a biodegradable stent. Endoscopy. 2011;43(Suppl 2):E252-3. [ Links ]
41. Janik V, Horak L, Hnanicek J, Malek J, Laasch HU. Biodegradable polydioxanone stents: a new option for therapy-resistant anastomotic strictures of the colon. Eur Radiol. 2011;21:1956-61. [ Links ]
42. Rodrigues C, Oliveira A, Santos L, Pires E, Deus J. Biodegradable stent for the treatment of a colonic stricture in Crohns disease. World J Gastrointest Endosc. 2013;5:265-9. [ Links ]
43. Ginsberg G, Cope C, Shah J, et al. In vivo evaluation of a new bioabsorbable self-expanding biliary stent. Gastrointest Endosc. 2003;58:777-84. [ Links ]
44. Meng B, Wang J, Zhu N, Meng QY, Cui FZ, Xu YX. Study of biodegradable and self-expandable PLLA helical biliary stent in vivo and in vitro. J Mater Sci Mater Med. 2006;17:611-7. [ Links ]
45. Kaffes AJ, Liu K. Fully covered self-expandable metal stents for treatment of benign biliary strictures. Gastrointest Endosc. 2013;78:13-21. [ Links ]
46. Itoi T, Kasuya K, Abe Y, Isayama H. Endoscopic placement of a new short-term biodegradable pancreatic and biliary stent in an animal model: a preliminary feasibility study (with videos). J Hepatobiliary Pancreat Sci. 2011;18:463-7. [ Links ]
47. Lamsa T, Jin H, Mikkonen J, Laukkarinen J, Sand J, Nordback I. Biocompatibility of a new bioabsorbable radiopaque stent material (BaSO4 containing poly-L,D-lactide) in the rat pancreas. Pancreatology. 2006;6:301-5. [ Links ]
48. Laukkarinen JM, Sand JA, Chow P, et al. A novel biodegradable biliary stent in the normal duct hepaticojejunal anastomosis: an 18-month follow-up in a large animal model. J Gastrointest Surgery. 2007;11:750-7. [ Links ]
49. Petrtyl J, Bruha R, Horak L, Zadorova Z, Dosedel J, Laasch HU. Management of benign intrahepatic bile duct strictures: initial experience with polydioxanone biodegradable stents. Endoscopy. 2010;42(Suppl 2):E89-90. [ Links ]
50. Mauri G, Michelozzi C, Melchiorre F, et al. Biodegradable biliary stent implantation in the treatment of benign bilioplastic-refractory biliary strictures: preliminary experience. Eur Radiol. 2013;23:3304-10. [ Links ]











 texto en
texto en