Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.30 no.2 Bogotá Apr./June 2015
A Case Report of Circumferential Presentation with Stricture of Heterotopic Gastric Mucosa in the Cervical Esophagus
Camilo Blanco A. MD (1), Esperanza Teusabá T. MD (2), Karen Russi MD. (3)
(1) Gastrointestinal Surgeon and Digestive Endoscopist, Master's Degree in Education, Associate Instructor in the Faculty of Education at the Universidad El Bosque, Director of the Videoendoscopy Unit of Restrepo Ltda. in Bogotá, Colombia. Mail: camiloblancoagmail.com
(2) Medical Pathologist at Patolab Ltda attached to the Videoendscopy Unit of Restrepo Ltda. in Bogotá, Colombia.
(3) Medical Anesthesiologist in the Videoendscopy Unit of Restrepo Ltda. in Bogotá, Colombia.
Received: 10-11-14 Accepted: 06-04-15
Abstract
Heterotopic gastric mucosa in the cervical esophagus is a condition that is probably underdiagnosed. The vast majority of patients are asymptomatic, and detection is an incidental finding. In symptomatic patients, manifestations are associated with non-neoplastic or neoplastic changes that allow categorization into five types. The case presented here is a patient who had Type III with dysphagia and pharyngeal globus due to heterotopic gastric mucosa in the cervical esophagus with circumferential presentation with stenosis. At the time of publication, only seven similar cases could be found in the literature. Detection, supported by new imaging technologies such as chromoendoscopy, may be an indicator of the quality of endoscopic performance in a manner that is similar to detection of adenomas in colonoscopy.
Keywords
Patches in the cervical esophagus, gastric heterotopic mucosa in the cervical esophagus, esophageal patches, globus pharyngeal, cervical esophagus, endoscopic diagnosis.
INTRODUCTION
Heterotopic gastric mucosa of the proximal esophagus (HGMPE - Mucosa gástrica heterotópica en el esófago cervical" -MGHEC) is the name we prefer to endoscopically describe the presence of salmon colored islets in the gastric mucosa of the proximal esophagus (1, 2). This entity was first described by Schmidt in 1805 and is also called "islands of gastric esophageal patches", "inlet patch" and "cervical inlet patch" (3-5). Its origin and pathophysiology are not understood, nor is need of treatment understood. It has been accepted that it is a congenital condition, but this has never been proven (1, 5), and recent studies suggest that is has an acquired origin (6, 7). The prevalence reported by standard white light endoscopic studies varies from 1% to 10% in adults (8) and to 5.9% in children (2, 9). Electronic chromoendoscopy using narrowband imaging (NBI) may be able to increase the detection rate to 13.8% (10), although studies to validate the superiority of this diagnostic technique are ongoing.
The clinical significance of HGMPE is unclear, since most patients do not report associated symptoms (11, 12), however some studies consider that HGMPE is a risk factor for symptoms in the throat or cervical esophagus such as the feeling of globus pharyngis, hoarseness, dysphagia and chronic coughing which are sometimes misinterpreted as extraesophageal manifestations of gastroesophageal reflux disease (1, 9, 10).
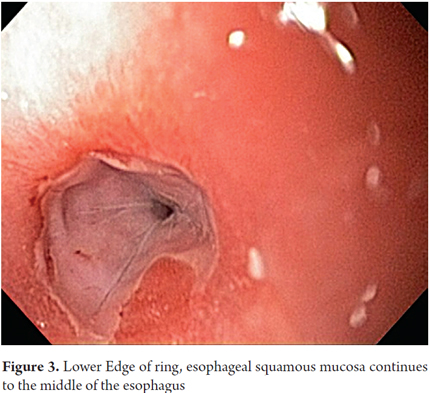
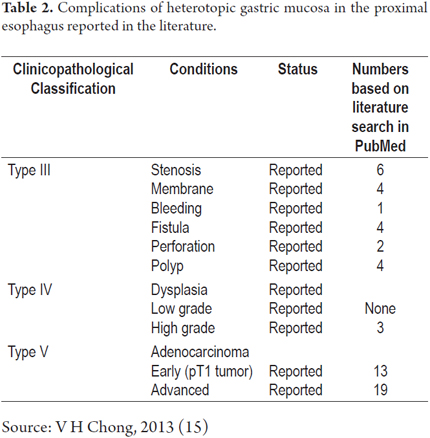
The clinicopathological classification proposed by von Rahden et al. (4) has of five categories. It divides clinical manifestations into those associated with non-neoplastic changes and those associated with neoplastic changes (Table 1). It allows better clinical understanding of the very small certain number of patients with complications generated by HGMPE such as ulcers, stenoses, perforations, fistulas (1) and even the extremely rare progression to cancer (4, 13).
The patient in the case reported here had a Category III complication. This category consists of non-neoplastic benign complications in asymptomatic individuals. It is as unusual as all of the others.
CASE REPORT
The patient was a 55 year old woman who was referred for the first time to the Videoendoscopy Unit of Restrepo Ltda. (Uniendoscopia.com) in Bogota Colombia for upper gastrointestinal endoscopy after experiencing 6 months of persistent regurgitation, burping, heartburn, feeling of a lump in her throat. All symptoms had progressively worsened in the three months immediately before referral. Patient had lost 3 kg of weight, but had no other symptoms. She had no other relevant medical history.
Upon physical examination, she showed no abnormalities. Special attention was paid to her oral cavity, pharynx and neck.
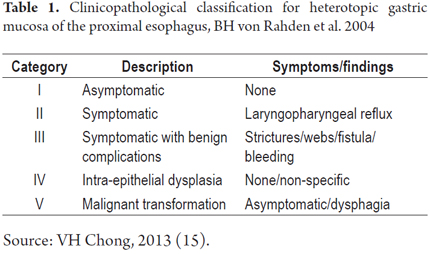
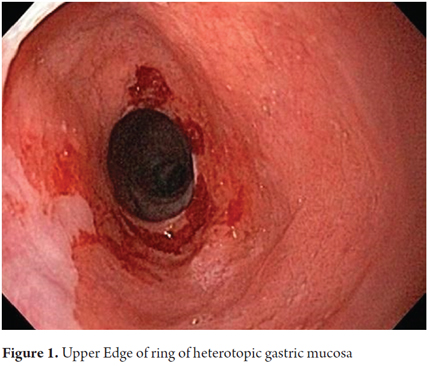
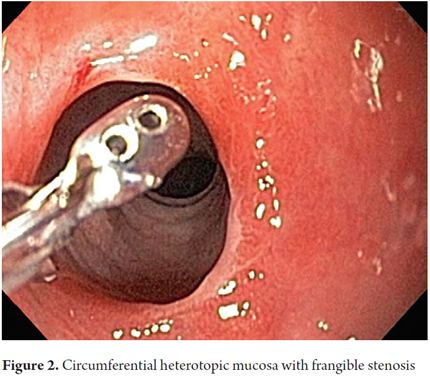
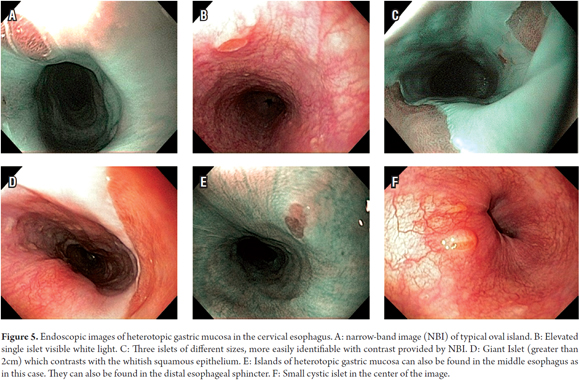
The procedure was performed with the patient under conscious sedation with a balanced scheme of propofol and remifentanil. It became evident that there was a complete circumferential ring of columnar heterotopic gastric mucosa 16 cm from the dental ridge in the cervical esophagus just below the upper esophageal sphincter. It had produced a 13 mm stenosis that was easily passable. It was 2 cm long, and was soft under pressure from biopsy forceps. There was continuous normal esophageal squamous mucosa for 18 cm from its distal edge to line Z at 37 cm (Figures 1, 2 and 3).
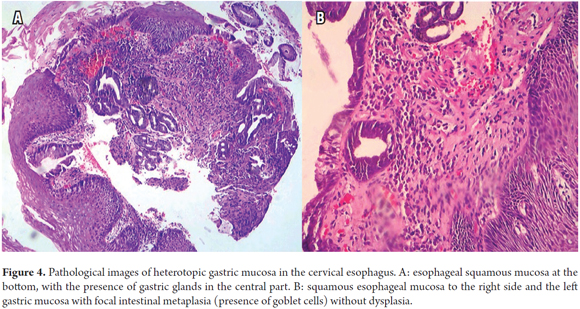
Biopsies taken from the columnar ring showed oxyntic gastric columnar glandular mucosa with parietal cells and cells of the type seen in the fundus and gastric corpus with foci of intestinal metaplasia without dysplasia (Figures 4A and B).
The patient's physician has treated her with a 40 mg twice daily dose of a proton pump inhibitors for 5 months (as of publication). Her symptoms of globus pharyngis, and regurgitation have improved. She continues to have a sensation of choking that has required dilation although it has not worsened. For now, the patient has ruled out other therapeutic alternatives such as argon plasma coagulation and radiofrequency ablation.
DISCUSSION
HGMPE is a common entity, but relatively less has been published about it compared to other esophageal disorders (14). Reports of its incidence vary according to the types of studies. Endoscopic studies report incidences ranging from less than 1% to 13.5% (13, 15). Autopsy studies report incidences above 70% (4, 13).
The low level of interest in this entity may be related to dismay at the lack of clarity regarding several of its aspects including its pathogenesis, its correlation with clinical, non-neoplastic complications, its progression to neoplastic transformations, its association with extraesophageal neoplasms, as well as lack of clarity about important issues such as the best way to make an endoscopic diagnosis and therapeutic alternatives.
There are three theories about its origin: congenital (13), chronic mucosal lesions resulting from acid similar to Barrett's esophagus (8), and as the result of rupture of cystic glands in the cervical esophagus (7). The congenital hypothesis is best accepted. It suggests that by 24 weeks of fetal gestation when the embryo is about 90 mm, the columnar epithelium of the esophagus initially starts to be replaced by squamous epithelium. This process begins in the middle part of the esophagus and progresses toward the proximal and distal ends. Since the proximal esophagus is the last part to achieve squamous stratification, it may not occur completely. This could be the reason for the increased frequency of heterotopic mucosa in the proximal esophagus (14). Nevertheless, although it is less common, heterotopic gastric mucosa is sometimes found in the middle and distal esophagus as in the case described here (Figure 5E). The second theory is that this condition arises from irritation and injury due to gastric acid in a way similar to Barrett's esophagus. According to this theory, the acid inhibits the proliferation of stem cells that enable the final metaplastic squamous epithelium transformation into a columnar line (8).
The most common histologic type reported is gastric cardia type HGMPE or oxyntic gastric mucosa (with mucous glands containing parietal cells). Other histological types include gastric fundus type, antral type, and gastric corpus type identified by the gastric gland mucosal cells or mixed mucosal cells and parietal cells (14). The histological type is associated with the possibility of acid production (7, 16, 17). Given the proximity of the location to laryngopharyngeal mucosa complex because it is highly sensitive to acid injury, this production is presented as one of the mechanisms for the generation of symptoms (14). Nevertheless, it has been reported that the non-acidic mucus production can also cause symptoms (4, 5).
Helicobacter pylori (H. pylori) can colonize HGMPE as reported by Gutierrez et al. (18) in this magazine. The prevalence can be as high as 82% although this could be related to the general prevalence of H. pylori infections in the population. This infection could eventually cause the same inflammatory changes described in stomach atrophy, metaplasia, dysplasia and cancer (9, 13, 19). The patient reported here had the histological type with oxyntic gastric mucosa with parietal and principal cells corresponding to the corpus or fundus with foci of intestinal metaplasia, goblet cells without dysplasia, but without colonization by H. pylori (Figures 4 A and B).
Although few studies compare symptoms between patients who have HGMPE and patients who do not, some studies show that patients who have gastric heterotopia in the cervical esophagus have significantly more laryngopharyngeal symptoms (9, 12, 19, 20, 21). Those which have been studied the most studied include dysphagia, odynophagia, globus pharyngis, regurgitation, chronic coughing, hoarseness and chronic heartburn.
The large range of prevalences reported, from 20% to 73.1% (13), may be due to the difficulty in comparing studies in terms of small signs, symptoms considered or methodologies used. However, for clinical practice, it is proposed that HGMPE should be considered as a separate entity for patients with laryngopharyngeal symptoms who do not have any manifestation of gastroesophageal reflux disease rather than as an "extraesophageal manifestation" of gastroesophageal reflux disease as is usually the case now (14).
The five clinicopathological categories of von Rahden et al. (Table 1) divide the clinical manifestations of HGMPE into neoplastic and non-neoplastic (4). For the vast majority of patients who have asymptomatic HGMPE, HGMPE is an incidental finding in a review of other digestive symptoms. Most of these patients have Type I HGMPE. Patients who have Types II and III with non-neoplastic manifestations are the ones with laryngopharyngeal symptoms of strictures, bleeding, and irritation with acid production as postulated the causal agent. HGMPE Types IV and V are associated with non-neoplastic or neoplastic changes (4, 13, 14). It should be noted that all these symptoms have been reported in adult and pediatric patients with predominance of neoplastic changes in the adult population associated with (14, 22).
The patient discussed here clearly belongs in Type III with clinical manifestations associated with a non-neoplastic complication, passable circumferential stenosis, with histology reports of intestinal metaplasia that had not progressed to dysplasia. If it had, the patient would have been categorized as type IV.
A quick endoscopic assessment of the cricopharyngeal sphincter and of the first 3 cm of the cervical esophagus may not detect HGMPE. For this reason, we can assume that prevalences have been underreported (1). Careful, detailed assessment of this area, facilitated by conscious sedation/especially at the end of the examination (1), allows easy detection of HGMPE especially because of its salmon coloring that contrasts with the adjacent surface of the pale columnar mucosa which can be smooth or nodular, round or oval, flat, depressed or elevated, single or multiple, and smaller or larger than 2 cm (Figures 5A, 5B, 5C, 5D). Islets or patches can be found in the distal esophagus (Figure 5E) that may also be cystic (Figure 5F). In the rare type of case presented, a circumferential ring can produce stenosis (Figures 1, 2 and 3).
For clinical practice, there is evidence that the identification of HGMPE is most commonly performed by endoscopists who pay special attention to search and detection (23) Hence, three actions are proposed to achieve higher detection rates of HGMPE which is probably underdiagnosed:
1. Monitor the quality of each individual endoscopist similar to the way the detection rate for colonic adenomas is used (24). Diagnosis of only 5% to 10% incidence of HGMPE in diagnostic endoscopy should be considered a low score for endoscopic performance. The endoscopist should be trained, especially in the two issues mentioned below.
2. Emphasize the careful removal of the endoscope, especially in the final part of endoscopy when the effect of sedation is ending (1) and emphasize that the view of the laryngopharyngeal sector can be difficult.
3. Routinely use new imaging modalities such as electronic chromoendoscopy which increase differentiation of contrast between islands and the adjacent mucosa. (25, 26). This allows for identification of vascular disruption patterns for any neoplastic changes that cannot be viewed with white light (27).
Most patients with HGMPE are asymptomatic and require no treatment. Progression to severe disease (categories III to V) is extremely rare although there have been reports of ulceration, bleeding, fistulas, laryngospasms, adenocarcinoma, perforations and, as in the case presented here, frangible stenosis (1).
Persistent and chronic laryngopharyngeal symptoms that compromise the quality of life of patients should be managed. If parietal cells are detected through histology, acid suppression with proton pump inhibitors can improve symptoms (22, 28, 29). Although no conclusive studies have yet confirmed this, it is especially true for patients with laryngopharyngeal symptoms associated with gastroesophageal reflux disease (30).
Interventional treatment in symptomatic patients have included argon plasma ablation (APC) with positive and comprehensive response to symptoms in up to 74% of cases. The follow-up on patients with clinical relapse shows greater persistence of HGMPE. This can merit retreatment with APC (31-34). Posttreatment restenosis following APC has occurred when compromise with HGMPE covers more than 30% of the circumference of the esophagus (35, 36) hence authors have not recommended APC as therapy for this size of lesions (1).
The alternative for these cases is radiofrequency ablation with the BÂRRX HALO 90 device because stenoses appear to occur much less frequently than with APC (34).
The other treatment schemes for other complications mentioned above (primary adenocarcinoma, aspiration, esophageal-tracheal fistulas) are outside the scope of this presentation.
Our patient has Type III HGMPE with circumferential stenosis which is a highly unusual presentation. Table 2 shows that up to January 2013 only 6 cases had been published in the literature (14). In January 2014, another case was reported (2).
CONCLUSION
The presentation of this rare case allows us to:
Stimulate the detection of this entity. Potential for complications is low but real.
Propose using detection of HGMPE as a measure of quality of endoscopic performance similar to the use of the rate of detection of colon polyps.
Identify HGMPE as a potential cause of difficult to manage laryngeal and pharyngeal symptoms transcending the group of extra-esophageal symptoms associated with gastroesophageal reflux disease.
Continue medical treatment of this patient with PPIs, endoscopic and histopathological follow-up of reported intestinal metaplasia and eventually manage her condition with radiofrequency ablation.
REFERENCES
1. Bajbout M, Meining A, Schmid R. Endoscopic diagnosis and treatment of intel patch: Justification, techniques, and results. Tech Gastrointest Endosc. 2014;16(1):49-52. [ Links ]
2. Cheng CL, Lin CH. Endoscopic diagnosis of cervical esophageal heterotopic gastric mucosa with conventional and narrow-band images. World J Gastroenterol. 2014;20(1):242-9. [ Links ]
3. Schmidt FA. De Mammalium Oesophage Atque Ventriculo. Inaugural Dissertation. Halle: Bethenea; 1805. [ Links ]
4. Von Rahden BH, Stein HJ, Becker K, et al. Heterotopic gastric mucosa of the esophagus: literature-review and proposal of a clinicopathologic classification. Am J Gastroenterol. 2004;99:543-51. [ Links ]
5. Borhan-Manesh F, Farnum JB. Incidence of heterotopic gastric mucosa in the upper oesophagus. Gut. 1991;32:968-72. [ Links ]
6. Willis RA. Some unusual developmental heterotopias. Br Med J. 1968;3:267-72. [ Links ]
7. Meining A, Bajbouj M. Erupted cysts in the cervical esophagus result in gastric inlet patches. Gastrointest Endosc. 2010;72:603-5. [ Links ]
8. Avidan B, Sonnenberg A, Chejfec G, et al. Is there a link between cervical inlet patch and Barrett´s esophagus? Gastrointest Endosc. 2001;53:717-21. [ Links ]
9. Alagozlu H, Simsek Z, Unal S, Cindoruk M, Dumlu S, Dursun A. Is there an association between Helicobacter pylori in the inlet patch and globus sensation? World J Gastroenterol. 2010;16:42-7. [ Links ]
10. Macha S, Reddy S, Rabah R, Thomas R, Tolia V. Inlet patch: heterotopic gastric mucosa--another contributor to supraesophageal symptoms? J Pediatr. 2005;147:379-82. [ Links ]
11. Hori K, Kim Y, Sakurai J, Watari J, Tomita T, Oshima T, et al. Non-erosive reflux disease rather than cervical inlet patch involves globus. J Gastroenterol. 2010;45:1138-45. [ Links ]
12. Weickert U, Wolf A, Schröder C, et al. Frequency, histopathological findings, and clinical significance of cervical heterotopic gastric mucosa (gastric inlet patch): a prospective study in 300 patients. Dis Esophagus. 2011;24:63-8. [ Links ]
13. Maconi G, Pace F, Vago L, Carsana L, Bargiggia S, Bianchi Porro G. Prevalence and clinical features of heterotopic gastric mucosa in the upper oesophagus (inlet patch). Eur J Gastroenterol Hepatol. 2000;12:745-9. [ Links ]
14. Chong VH. Heterotopic gastric mucosal patch of the proximal esophagus. En: Pascu O, editor. Gastrointestinal endoscopy. Croatia: InTech Publishing; 2011. p. 125-48. [ Links ]
15. Ohara M. Incidence of heteroptopic gastric mucosa in the upper esophagus in first time narrow banding image endoscopy of consecutive 900 patients. Gastrointest Endosc. 2010;71:AB316-7. [ Links ]
16. Korkut E, Bektaş M, Alkan M, Ustün Y, Meco C, Ozden A, et al. Esophageal motility and 24-h pH profiles of patients with heterotopic gastric mucosa in the cervical esophagus. Eur J Intern Med. 2010;21:21-4. [ Links ]
17. Galan AR, Katzka DA, Castell DO. Acid secretion from an esophageal inlet patch demonstrated by ambulatory pH monitoring. Gastroenterology. 1998;115:1574-6. [ Links ]
18. Gutierrez O, Akamatsu T, Cardona H, Graham DY, El- Zimaity HM. Helicobacter pylori and heterotopic gastric mucosa in the upper esophagus (the inlet patch). Am J Gastroenterol. 2003;98:1266-70. [ Links ]
19. Akbayir N, Alkim C, Erdem L, Sökmen HM, Sungun A, Başak T, et al. Heterotopic gastric mucosa in the cervical esophagus (inlet patch): endoscopic prevalence, histological and clinical characteristics. J Gastroenterol Hepatol. 2004;19:891-6. [ Links ]
20. Chong VH, Jalihal A. Heterotopic gastric mucosal patch of the esophagus is associated with higher prevalence of laryngopharyngeal reflux symptoms. Eur Arch Otorhinolaryngol. 2010;267:1793-9. [ Links ]
21. Poyrazoglu OK, Bahcecioglu IH, Dagli AF, Ataseven H, Celebi S, Yalniz M. Heterotopic gastric mucosa (inlet patch): endoscopic prevalence, histopathological, demographical and clinical characteristics. Int J Clin Pract. 2009;63:287-91. [ Links ]
22. Di Silverio Carulli C, Lima M, Bergamaschi R, Bernardi F, Cicognani A. A rare association of inlet patch with laryngospasm: a report of two children and literature review. Pediatr Pulmonol. 2011;46:934-8. [ Links ]
23. Azar C, Jamali F, Tamim H, et al. Prevalence of endoscopically identified heterotopic gastric mucosa in the proximal esophagus: endoscopist dependent. J Clin Gastroenterol. 2007;41:468-71. [ Links ]
24. Coe SG, Crook JE, Diehl NN, et al. An endoscopic quality improvement program improves detection of colorectal adenomas. Am J Gastroenterol. 2013;108:219-26. [ Links ]
25. Kuznetsov K, Lambert R, Rey JF. Narrow-band imaging: potential and limitations. Endoscopy. 2006;38:76-81. [ Links ]
26. Yokoyama A, Ichimasa K, Ishiguro T, et al. Is it proper to use non-magnified narrow-band imaging for esophageal neoplasia screening? Japanese singlecenter, prospective study. Dig Endosc. 2012;24:412-8. [ Links ]
27. Sharma P, Hawes RH, Bansal A, et al. Standard endoscopy with random biopsies versus narrow band imaging targeted biopsies in Barrett´s oesophagus: a prospective, international, randomised controlled trial. Gut. 2013;62:15-21. [ Links ]
28. Silvers WS, Levine JS, Poole JA, et al. Inlet patch of gastric mucosa in upper esophagus causing chronic cough and vocal cord dysfunction. Ann Allergy Asthma Immunol. 2006;96:112-5. [ Links ]
29. Bataller R, Bordas JM, Ordi J, et al. Upper gastrointestinal bleeding: a complication of "inlet patch mucosa" in the upper esophagus. Endoscopy. 1995;27:282. [ Links ]
30. Bajbouj M, Becker V, Eckel F, et al. Argon plasma coagulation of cervical heterotopic gastric mucosa as an alternative treatment for globus sensations. Gastroenterology. 2009;137:440-4. [ Links ]
31. Meining A, Bajbouj M, Preeg M, et al. Argon plasma ablation of gastric inlet patches in the cervical esophagus may alleviate globus sensation: a pilot trial. Endoscopy. 2006;38:566-70. [ Links ]
32. McBride MA, Vanagunas AA, Breshnahan JP, et al. Combined endoscopic thermal electrocoagulation with high dose omeprazole therapy in complicated heterotopic gastric mucosa of the esophagus. Am J Gastroenterol. 1995;90:2029-31. [ Links ]
33. Klare P, Meining A, von Delius S, et al. Argon plasma coagulation of gastric inlet patches for the treatment of globus sensation: it is an effective therapy in the long term. Digestion. 2013;88(3):165-71. [ Links ]
34. Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett´s esophagus with dysplasia. Gastroenterology. 2011;141:460-8. [ Links ]
35. Bright T, Watson DI, Tam W, et al. Randomized trial of argon plasma coagulation versus endoscopic surveillance for Barrett esophagus after antireflux surgery: late results. Ann Surg. 2007;246:1016-20. [ Links ]
36. Sharma P, Wani S, Weston AP, et al. A randomised controlled trial of ablation of Barrett´s oesophagus with multipolar electrocoagulation versus argon plasma coagulation in combination with acid suppression: long term results. Gut. 2006;55:1233-9. [ Links ]











 text in
text in