Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.30 no.3 Bogotá jul./sep. 2015
Development and Implementation of a New Nitinol Stent Design for Managing Benign Stenoses and Fistulas of the Digestive Tract
Rodrigo Castaño, MD (1), Oscar Álvarez MD (2), Jorge Lopera (3), Mario H. Ruiz MD (4), Andrés Rojas MD (5), Alejandra Álvarez (6), Luis Miguel Ruiz (6), David Restrepo (7)
(1) Gastrointestinal Surgery and Endoscopy. Chief of Postgraduate General Surgery (UPB), Gastrohepatology Group at the University of Antioquia, Institute of Clinical Oncology of the Americas in Medellin, Colombia. rcastanoll@hotmail.com
(2) Gastroenterologist at Texas Valley Coastal Bend Veterans Administration Hospital and Clinical Assistant Professor at the University of Texas Health Science Center at San Antonio in San Antonio, Texas USA.
(3) Interventional Radiologist at the University of Texas in the United States.
(4) General Surgeon at the Hospital Pablo Tobón Uribe in Medellin, Colombia.
(5) General Surgeon, Clinic Cancerology Institute Las Americas. Medellin, Colombia.
(5) Undergraduate Students in the Faculty of Medicine at the Universidad Pontificia Bolivariana in Medellin, Colombia.
(6) Undergraduate Student in the Faculty of Medicine at CES in Medellin, Colombia.
Received: 19-08-14 Accepted: 21-07-15
Abstract
Background: Benign stenoses, digestive tract ruptures and fistulas are conditions that endanger life and are often treated surgically. Recently, the placement of partially or fully covered metal stents has emerged as a minimally invasive treatment option. This article looks at a new design for stents to determine its clinical effectiveness. The new stent is a completely covered nitinol stent for treatment of gastrointestinal perforations and anastomotic leaks. This article places special emphasis on evaluating reactive hyperplasia.
Methods: Fifteen had the new completely covered self-expanding nitinol stent placed for treatment of benign esophageal perforations, anastomotic leaks, and stenoses following upper or lower gastrointestinal surgery during 2012 and 2013. The stents are 20 mm in diameter in the middle and 28 mm in diameter at the proximal end. Information about patient demographics, type of lesion, lesion locations, stent removal, clinical success and complications was collected.
Results: A total of 15 stents were placed in 15 patients to treat anastomotic leaks (n = 8), esophageal stenoses (n = 2), colorectal stenoses (n = 2), a gastrojejunostomy stenosis (n = 1), an esophageal iatrogenic rupture (n = 1), and a pyloric stenosis (n = 1). Endoscopic removal of the stent was successful in all patients. Although it was particularly difficult in one case because of reactive hyperplasia. Clinical success was achieved in nine patients (73%). Average duration of time between stent placement and removal was 10 weeks with a range of 7 to 12 weeks. In total, seven complications occurred in 15 patients (47%): reactive hyperplasia (n = 1), migration (n = 3) severe pain (n = 2) esophageal ulceration (n = 1) only one patient required surgery after stent failure. No patients died as the result of stenting.
Conclusions: A redesigned completely covered stent kept in place for 10 weeks may be an alternative to surgery for treating gastrointestinal perforations, anastomotic stenoses and fistulas. The efficacy of the new stent differs from that of conventional stents. The choice depends on the expected risks of stent migration and reactive hyperplasia.
Keywords
Metal stent, nitinol, benign stenosis, gastrointestinal fistula, esophageal perforation.
INTRODUCTION
Stents have been used for many decades for palliation of obstruction in different segments of the biliary tract and gastrointestinal (GI) tract. Their usage has boomed since the recent introduction of self-expanding metal stents (SEMS) which have largely eliminated the discomforts and risks inherent in the use of rigid plastic stents in the GI and biliary tracts (1, 2).
Today, there is no doubt about the benefits of stents for palliative treatment of malignant strictures of the GI tract including in critical situations with high risks of morbidity and mortality such as esophageal-respiratory fistulas (3-5). Self-expanding metal stents have even replaced various surgical procedures that had been regarded as standard palliation for these patients (6, 7). In addition, their use now extends to other benign diseases both in the biliary tree and in the GI tract (8-11). Surgical interventions had been considered the "gold standard" of treatment of these lesions (12-14), but while surgery improves survival rates, it is still associated with high mortality rates (30%) mainly related to late diagnosis (15).
Metal stents have not been widely used to treat benign strictures of the GI tract for several reasons. First, most metallic stents cannot be removed without surgery and therefore cannot be used temporarily. Second, the long-term implications of a permanent metallic stent in the GI tract is unknown. Third, experiences in animal models and in some of our patients with benign obstructions have shown that in some cases mucosal hyperplasia may eventually occlude the stent. Fourth, for patients who are in good general condition, surgery is justified (16). Finally, there is no design or model of stent proposed for the management of benign situations.
To date, there have been few reports about the use of metallic stents for treatment of benign conditions, and most of those have described significant rates of complications resulting from the stent itself and from removal of the stent. The most frequent complication is migration, next frequent is formation of a new stenosis, followed by pain at the site of stent placement and then by specific situations such as facilitation of GERD. Proximal and distal migrations are more common with fully covered stents while stenoses are most frequently observed stents that are uncovered either at the ends or in the center to allow for tissue growth between the interstices of the fabric of stent (17).
Nevertheless, various reports suggest that the temporary use of self-expanding metal stents may be a reasonable option for patients with benign GI tract stenoses, fistulas or perforations (18-20). Song has found that esophageal stenoses recurred in three of four patients in whom stents migrated within the first two months after placement, but that there were no recurrences of stenoses among patients whose stents migrated or were removed more than two months after placement (21). For this reason, and because formation of a distal or proximal stenosis is rare during this period, Song has proposed that stents be kept in place for no less than eight weeks for management of benign strictures in not less than eight weeks esophagus.
The same stents that have traditionally been used for management of malignant strictures have been used for benign strictures even though they do not necessarily offer the tightest fit or the least likelihood of migration. They are also used for less common situations which have low rates of morbidity and mortality and which are not necessarily stenoses. These include situations such as gastrointestinal fistulas, endoscopic perforations and spontaneous perforations (22, 23). The mechanism of action of these stents is to seal and thereby close the fistula, but some studies and the guidelines of the American College of Gastroenterologists have concluded that the quality of evidence for the use of metallic stents for perforations and leakage is low and that the level of evidence for recommendation is weak (24, 25).
The purpose of this study is to show the development and clinical implementation of a new nitinol stent design. This new design is based on the original model of esophageal stent designed by Song but rather than being designed for conventional situations such as palliation of tumors, it is designed for benign pathologies such as cases of stenoses, leakage and perforations. The intention is to offer non-surgical management of these disorders since surgery in these cases may result in high risks of morbidity and mortality.
NEW CONCEPTS FOR BIOMECHANICAL NITINOL STENTS
The History of Nitinol
In 1959, William Beuhler discovered a 55% nickel (Ni) and 45% titanium (Ti) alloy while working in the laboratories of the US Naval Ordnance Laboratory. The new alloy was given the name Nitinol which is an acronym for its ingredients and the place where it was discovered (Nickel Titanium Naval Ordnance Laboratory) (26). This material is now known as the memory metal because two of its important properties are shape memory and superelasticity (27). Both of these properties are important for both gastrointestinal stents.
Fluoroscopic Features
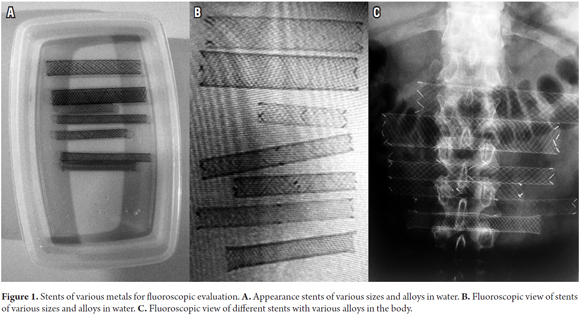
Nitinol has come to displace other metals such as steel and elgiloy that had been used in the manufacture of stents in part because it offers better contrast in fluoroscopic evaluations than do other materials. In tests run with the newly designed stent, it demonstrated contrast levels comparable to those of other stents (Figure 1).
Biomedical Properties
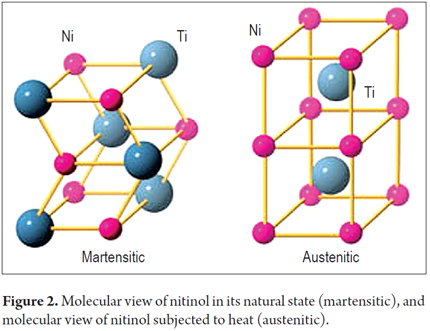
Like other metals, nitinol, undergoes phase transitions between the face-centered cubic (FCC) configuration of gamma iron crystal structures (Austenite, the most common form of stainless steel) and a body-centered tetragonal form (Martensite, alloys which have austenitic structure at high temperatures). At room temperature the nitinol is in its martensite phase which is soft and easily deformed. It has shape memory which is the effect of something that is plastically deformed returning to its original shape (Figure 2).
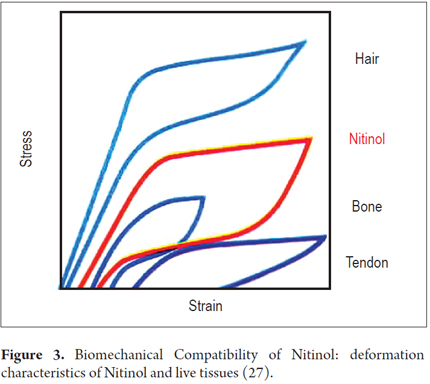
Conventional materials such as stainless steel and cobalt alloys exhibit very different elastic deformation behaviors from those of the structural elements of the human body. Elastic deformation occurs when there is no permanent or plastic deformation. In conventional materials, elastic deformation is limited to approximately 1% and elongation increases or decreases proportionally (linearly) to the stress applied. In contrast, natural structural materials such as hair, tendons and bones may deform elastically by as much as 10% (Figure 3). The graph of stress against strain shows nonlinear behavior and a charge/discharge cycle with significant hysteresis (28).
The similarity between the stress-strain behavior of nitinol and structural tissues is very important to note for biomechanical considerations. Figure 4 shows characteristic stress/strain behaviors of nitinol and steel at body temperature. Steel's elastic deformation has a nearly vertical slope (approximately 1% deformation), but nitinol's elastic deformation is about 10 times higher. In other words, nitinol recovers its shape. Nitinol has curve plateaus that can be observed for both loading and unloading of stress. These plateaus (horizontal areas of the curve) allow large deflections without increasing stress. A more than 10% ability to recover shape is called superelasticity.
In summary, the properties that make nitinol a suitable material for gastrointestinal stents are shape memory and superelasticity. Nitinol's shape memory means that at body temperature the stent's radial force is reduced which facilitates insertion into the guide catheter. Its superelasticity allows almost constant radial force to be exerted while the diameter changes from a small diameter to the stent's maximum diameter. This behavior is natural and contrasts with that of stainless steel in which radial force is inversely proportional to the diameter of the stenosis.
A new model of stent
Bearing in mind that acceptance of the use of metallic stents in various situations for palliation of malignant biliary digestive tract stenoses is increasing, it is understandable that a single model of stent may not be the solution for every type of benign and malignant gastrointestinal disease. Therefore, it is necessary to develop and implement a new model of stent that conforms to new needs such as the management of gastrointestinal fistulas and benign strictures.
Based on these requirements, it is necessary to create a stent that can remain in position in situations such as the fistulas of gastric bypass when there is no stenosis. Together with this feature, the new stent should be able to seal the fistula, keep it sealed, while correcting local stenosis. While stent diameter must be increased, it cannot be increased too much because it could induce necrosis of the anastomosis or the suture line of a gastric bypass or gastric sleeve. Also, a large diameter stent used to treat a benign disease could eventually induce a complication inherent in the use of stents such as the appearance of reactive hyperplasia in the surrounding tissue. This occurs most often with the use of completely covered or partially covered stents (29, 30). This hyperplastic reaction can occur even in reaction to a short period of stenting and may even occlude the ends the stent making removal very difficult (24).
The probability that a conventional fully covered stent will migrate is between 31% and 37% according to recent reports (31, 32), and no reduction in these rates has been achieved with the development of internally covered stents (33). In response to this problem, the new stent and its implementation were modified. The conventional stent diameter of 18 mm to 20 mm has been maintained in the central portion of the new stent, but the diameter of the proximal end has been increased to 28 mm to impede migration. In addition, the stent has been coated with silicone to reduce the risk of hyperplasia at the ends of the stent.
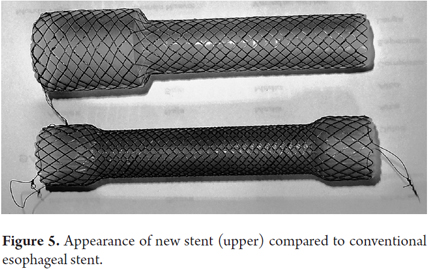
This means that not only the size of the stent has changed, but its appearance has changed as well. Rather than the appearance of a "dog bone" with larger diameters at both ends, the new stent has a single-shoulder look with a large diameter at the proximal end, but the diameter of the body at the distal end (Figure 5).
Implementation in Clinical Practice
The conditions required to release the stent and procedure for stent placement are as follows:
- A placement device to move the stent to the site of the lesion and introduce it into place is required.
- The diameter of the self-expanding stent must be reducible that it can be accommodated within the placement device. After being released, the stent must have sufficient radial force to dilate the area of the stricture. This requirement implies that the stent material and structure allow compression to 3mm or less (to accommodate the placement device) and must expand to a diameter up to 22mm once released while maintaining adequate radial strength.
- The placement device must be low profile so that it can be guided through the digestive tract to the site of the lesion. Generally, it should not exceed 10 Fr (3.3mm) in order to be introduced through the endoscope channel.
- The stent must be biocompatible with the area in which it will be placed.
- The stent has nylon threads at both ends (proximal and distal) for traction and removal.
- Nitinol has a combination of properties which makes it suitable for the manufacture of self-expanding stents.
METHODS
Between June 2012 and June 2013, all patients who were treated with the new model of stent for stenoses or benign fistulas were included in a prospective, nonrandomized, open-label study performed in various hospitals in Medellin.
We selected patients with benign strictures, fistulas secondary to gastric bypass or gastric sleeve surgery for obesity, patients with leaking anastomoses following esophageal-enteric gastrectomies (whether or not they had iatrogenic esophageal perforations). We excluded patients with an activity index of less than 40, patients with stenoses that were very close to the cricopharyngeal muscle, and patients whose conditions prevented them from tolerating endoscopy or sedation. All patients provided signed informed consent for both placement and removal of the new type of stent. All procedures were done under conscious sedation with midazolam, meperidine and ketamine. There were no adverse reactions to sedation.
The New Nitinol Stent
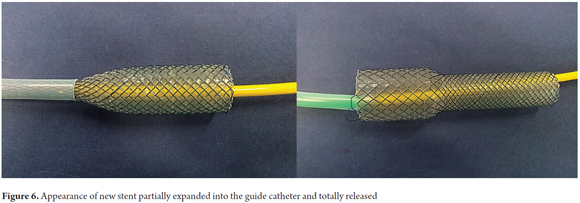
The new nitinol stent is constructed of multiple strands of interwoven nitinol and an outer coating of silicone. The diameter of the central portion of the stent is 20 mm with a 28 mm in diameter anchoring end. It comes in three lengths: 8 cm, 10 cm and 12 cm (Figure 6).
In all cases of benign strictures, the stent passed beyond the proximal and distal margins of the stricture by at least two centimeters. We did not dilate the areas of stent placement prior to the procedure in any of the patients. In all cases the procedure was done under conscious sedation, and there were no complications with sedation.
Objectives of the Study
The main objectives of the study were to seal fistulas and alleviate refractory benign strictures. In addition, the study measured rates of migration and occlusion of the new model stent. As secondary objectives, technical success and complications were considered. Technical success was defined as verification by fluoroscopy and/or endoscopy of the release and proper placement of the stent in the stricture or fistula to be treated. Major complications that merited hospitalization or the realization of a second endoscopy were considered. Minor complications were defined as those that could be managed conservatively without hospitalization. Evaluation of the treatment of benign lesions took into account the severity of local reactions (hyperplasia), the ease of stent removal, and either whether or not the fistula was successfully closed or whether or not the stenosis was successfully corrected.
Follow-up
During the week of stenting patients were followed up prospectively in person. After that they were monitored by telephone every two weeks until the removal of the stent. Activity The WHO patient index of activity was used to evaluate patient activity. This includes evaluation of the abilities to eat and swallow, rating of dysphagia, and assessment of specific symptoms such as chest pain and regurgitation (34). Stents were removed two months after placement except in one patient whose stent was removed at seven weeks because of chest pain.
Statistical analysis
The following patient characteristics were evaluated: sex, age, location of stenosis or leakage, stricture length, cause of leakage or stenosis, and previous treatments. Factors used to evaluate clinical and technical success included relief of dysphagia, cause of recurrencde of dysphagia, and any major or minor complications that developed. The results are expressed as means ± standard deviations or as medians plus ranges. Categorical variables were assessed using the X2 test or Fisher's exact test as appropriate. Variables that were not normally distributed were evaluated with the Mann-Whitney test. All analyses were done with the intention to treat. A value of p <0.05 was considered statistically significant. Analyses were done using SPSS version 20 (SPSS Inc., Chicago, IL).
RESULTS
Patient characteristics
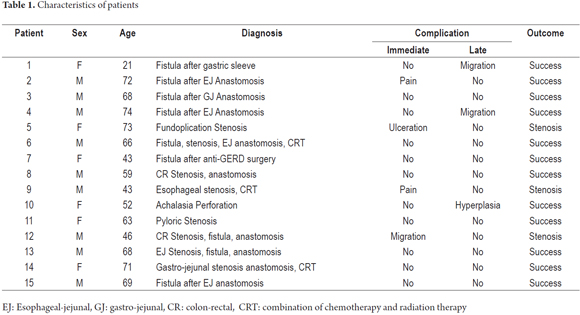
Self-expanding fully covered stents made of nitinol were placed in a total of 15 patients with various benign pathological conditions of the upper or lower digestive tract. Stent placement was successful in all patients, and strictures were not dilated prior to placement in any of the patients. There were no deaths associated with stent placement or patient outcomes (Table 1).
Complications and Stent Removal
Two patients with postoperative fistulas and stenoses were treated concomitantly with adjuvant CRT prior to stent removal. The stent migrated in one of these patients but only after the fistula and stenosis were resolved. The stent was recovered endoscopically without problems.
Stent removal was successful in all 12 of the patients in whom it was attempted. The first patient in the series vomited the stent the week prior to planned removal. The patient had had a fistula and stenosis secondary to gastric sleeve surgery which were both resolved through the stenting. Stent migration occurred the day after placement in another patient who had a postoperative stenosis and fistula at the ureter following colorectal anastomosis to treat diverticulosis.
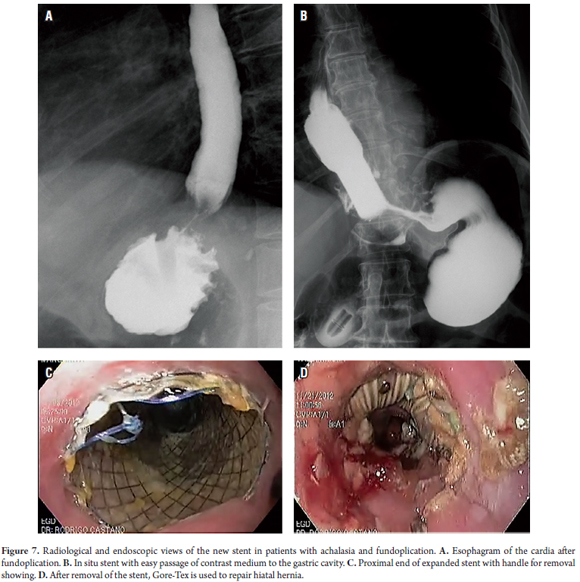
A particular problem with removal of the stent arose in another patient who had undergone fundoplication. The patient developed achalasia. A hiatal hernia had been corrected with Goretex mesh. Pressure from the stent against the tissue induced a large local ulcer The patient was managed with an advanced nasal-enteral tube. Later, the gastroesophageal junction was resected and an esophageal anastomosis was created. The patient's subsequent progress was good (Figure 7).
One patient who had a stenosis due to CRT for carcinoma in the mid to upper esophagus medium high and who refused surgery required removal of the stent after seven weeks because of chest pain. Stenosis reoccurred after one month.
Stent Induced Hyperplasia
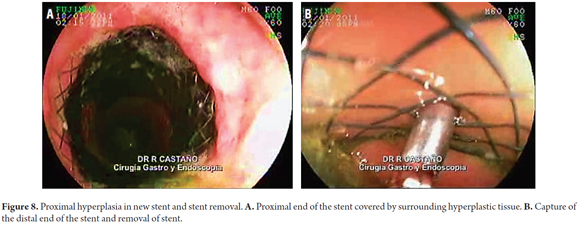
The removal of the stent was particularly difficult in patients with esophageal perforations that had occurred during pneumatic dilation for achalasia (Rigiflex 35 mm achalasia balloon). The proximal end of the stent became fully embedded in the surrounding tissue which required that the stent be removed from the distal end to the proximal end (Figure 8).
Final Considerations
Clinical success was achieved in 9 out of 15 patients (75%). All fistulas were closed, but results with stenoses were less encouraging. There was no need to place more than one stent in any of these patients. Only one patient required surgery to correct a large ulcer at the gastroesophageal junction due to the local presence of Gore-Tex. There were no complications related to placement or removal of stents.
DISCUSSION
The benefits of palliative stenting at various locations in the gastrointestinal and biliary tract are not in doubt in the published local and world literature (5-7, 22, 23, 34, 35). Nevertheless, we have reached a new stage in the usage of stents which includes endoscopic management of benign conditions such as stenoses and fistulas, the simultaneous presence of both, and even management of iatrogenic perforations. Most of these esophageal ruptures occur after endoscopic surgery in the vicinity but may also occur "spontaneously" after vomiting (Boerhaave syndrome). The role of stents exclusively used to treat fistulas secondary to bariatric surgery has been assessed in a meta-analysis of seven studies with 67 patients. The fistula closure rate reported was 87.7% while the rate of migration reported was 16.9% (36). Surgical interventions in these situations have rates of morbidity and mortality (37).
The same stents have been used for both malignant and benign situations with or without stenoses. A recent study has shown that, while conventional stents improves malignant dysphagia, they only improve benign dysphagia temporarily. On top of this, dysphagia recurred in all patients and there was a high rate of migration (33).
This study presents the development and implementation of a new nitinol stent whose design features are intended to reduce the rate of migration and to relieve benign stenoses and fistulas. It may potentially have a higher rate of hyperplasia of reactive tissue in the vicinity of the new stent. We did not perform follow-up endoscopy in asymptomatic patients who had stents in situ to verify whether or not hyperplasia had developed, but animal studies suggest that a 30 day stent placement results in scarring of the tissue (38). Based on those results and our own results, we have suggested that stents be removed eight weeks after placement although there are studies that suggest removing them six weeks after placement (39). Average amount of time between stent placement and removal varies in published reports from 20 days to 135 days (40-45). Although more studies to determine the safest stent removal time are warranted, the issue of time must be looked at in combination with other variables such as the type of stent used, associated pathologies , and the presence of mediastinal and/or pleural extraesophageal collections. Resolution of extraesophageal mediastinum and pleural collections is an absolute prerequisite for complete healing of any rupture or fistula (44).
In this study, seven complications occurred in 15 patients (47%). This is comparable to complication rates reported in other series which range from 20% to 72% (39). Major complications that have been reported include stent migration, growth within the stent (primarily in uncovered stents), and induced hyperplasia (18, 19, 40, 41, 45-49). In this study, stent migration occurred in three of the 15 patients (20%). The most likely explanations are that two of these three patients did not clearly have stenoses and that the stents used were fully covered. The use of endoscopic clips to prevent this type of migration has been described, but this technique was not implemented in this study (50).
Stent occlusion resulting from colonization of tissue within the stent and by hyperplasia of the tissue at the ends of the stent occurs most frequently in the partially covered stents. Nevertheless, partially covered stents are preferred by many endoscopists because of their lower rates of migration and because the reactive tissue induced projects into areas of leakage which improves rates of sealing. It has been shown that reactive hyperplastic tissue is the result of local fibrotic reactions and/or proliferation of granulation tissue. This has been observed starting from 14 days after stent placement onward (51). Nitinol stents have a higher reactive tissue growth rates than do plastic stents. In addition, the stent's radial force must be taken into account because the pressure induced by radial force induces reactive hyperplastic tissue. Another factor is the amount of time the stent remains in place. The longer it stays in place, the greater the amount of hyperplasia which complicates stent removal. It is suggested that stents be changed every two to four weeks during management of anastomotic leaks and perforations. Another alternative is to use the stent-in-stent- technique in which a SEMS is left in place until 10 to 14 days prior to planned removal at which point a totally coated stent is placed inside of the first stent to facilitate removal of both the initial stent and the recently placed stent (52).
The traditional dog bone design of the stent has been replaced by a single-shoulder stent in order to reduce the pressure exerted by the distal end of the stent, reduce pain after its release, and reduce the chance of distal hyperplasia and ulceration. In fact, fistulas that were induced by the large diameter of the distal end of the stent have been described. In addition to reducing these risks, the smaller distal diameter facilitates stent removal.
In this study, there were no cases of mortality although other studies have reported mortality rates ranging from 10% to 28% which is comparable to rates described for surgery which range from 12% to 50% (19, 20, 25, 26, 40-42, 46, 47, 53-56). Studies suggest that the time between the occurrence of a rupture or fistula and stenting is the most important determinant of success (14, 15, 20). Higher success rates result from earlier stent placement because of the high rate of septic complications which otherwise occur.
To date, it is unclear which types of intestinal leakage should be treated with stenting and which types of intestinal leakage should be treated with surgery. It has been proposed that stenting is preferable for minor disruptions of up to 70% of the circumference while surgery is preferable for ruptures of larger diameters (44).
We are aware of the limitations of this study which include the small number of patients and the absence of a randomized comparison of the new stent with other stents. Nevertheless, the results of this short series suggest that use of this new stent is feasible. Only the rather unlikely possibility of randomized testing of stents might provide more reliable information about this stent's usefulness.
CONCLUSIONS
These first results demonstrate the feasibility of using a new nitinol stent for managing fistulas and benign strictures. The new stent had a lower rate of stent migration than those found for stents studied in other reports, and the new stent also has a lower rate of complications. The answer will only be given by a randomized study with special emphasis on assessing the causes, extent and timing of intestinal leakage and severity of contamination outside of the viscera. The limited number of patients and the promising results with stents have made it very difficult, if not impossible, to conduct this type of study.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgements
This study was conducted with the support of the Sustaina-bility Project of the office of the Vice Rector of Research at the University of Antioquia.
REFERENCES
1. Castaño R. Prótesis metálicas autoexpansibles gastroduodenales, intestinales y colónicas. En: Landazábal G (editores). Endoscopia y patología biliodigestiva. Bogotá: Ediciones Médicas Latinoamericanas S.A.; 2011. p. 389-408. [ Links ]
2. Castaño R, Álvarez O, García A, et al. Stent metálico versus plástico en la obstrucción biliar maligna distal. Rev Col Gastroenterol. 2009;24:248-55. [ Links ]
3. Castaño R, Álvarez O, Lopera J. Endoprótesis metálicas autoexpandibles en la obstrucción maligna esofágica y gastroduodenal. Rev Col Cirugía. 2005;20:33-48. [ Links ]
4. Castaño R. Stents en el tracto gastrointestinal y biliar. En: Feris J (editor). Tópicos selectos en gastroenterología. Bogotá: Sociedad Colombiana de Gastroenterología; 2007. p. 309-29. [ Links ]
5. Castaño R, Ruiz MH, Sanín E. Stent esofágico de nitinol en el manejo de las fístulas esofagorrespiratorias malignas. Revista Col de Gastroenterol. 2003;18:78-82. [ Links ]
6. Castaño R, Álvarez O, Ruiz MH, Cárdenas A, Sanín E, Erebrie F. Comparación entre la gastroyeyunostomía y el stent metálico autoexpandible para la paliación de la obstrucción maligna gastroduodenal. Rev Col Gastroenterol. 2006;21:62-7. [ Links ]
7. Castaño R, Lopes TL, Álvarez O, Calvo V, Luz LP, Artifon EL. Nitinol biliary stent versus surgery for palliation of distal malignant biliary obstruction. Surg Endosc. 2010;24:2092-8. [ Links ]
8. Artifon EL, Castaño R, Álvarez O, Aparicio DP, Sakai C, Paione JB. Fully covered self-expandable Metal Stent versus plastic stent in the treatment of biliary leaks after cholecystectomy: A multicenter study. Gastroint Endosc. 2010;71:AB 310. [ Links ]
9. Castaño R, Ruiz MH, Restrepo JC, et al. Manejo endoscópico de las complicaciones biliares después del trasplante ortotópico de hígado. Rev Col Gastroenterol. 2012;27:173-84. [ Links ]
10. Castaño R, Álvarez O, Lopera J, Sanín E, Nuñez E, García L. Expandable nitinol stent for treatment of malignant and benign esophagorespiratory fistulas. Gastrointest Endosc. 2008;67:AB 151-2. [ Links ]
11. Zeng Y, Dai YM, Wan XJ. Clinical remission following endoscopic placement of retrievable, fully covered metal stents in patients with esophageal achalasia. Dis Esophagus: Official journal of the International Society for Diseases of the Esophagus / ISDE 2013. [ Links ]
12. Jougon J, Delcambre F, MacBride T, Minniti A, Velly JF. Mortality from iatrogenic esophageal perforations is high: Experience of 54 treated cases. Ann Chir. 2002;127:26-31. [ Links ]
13. Siersema PD. Treatment of esophageal perforations and anastomotic leaks: The endoscopist is stepping into the arena. Gastrointest Endosc. 2005;61:897-900. [ Links ]
14. Sung SW, Park JJ, Kim YT, Kim JH. Surgery in thoracic esophageal perforation: Primary repair is feasible. Dis Esophagus: Official journal of the International Society for Diseases of the Esophagus / ISDE 2002;15:204-9. [ Links ]
15. Attar S, Hankins JR, Suter CM, Coughlin TR, Sequeira A, McLaughlin JS. Esophageal perforation: A therapeutic challenge. AnnThorac Surg. 1990;50:45-9; discussion 50-1. [ Links ]
16. Castaño R, Artifon El. Técnicas endoscópicas en próteses gastrointestinais: Como e quando utilizar, manejo de complicacoes, selecao e custos. En: Artifon EL, de Moura E, Sakai P (editores). Próteses endoscópicas no aparelho digestorio. Sao Paulo: Editora Atheneu; 2012. [ Links ]
17. Castaño R. Técnicas en stents gastrointestinales endoscópicos: cómo, cuándo, manejo de las complicaciones, selección del stent y costos. Rev Col Gastroenterol. 2012;27:32-45. [ Links ]
18. Freeman RK, Ascioti AJ, Wozniak TC. Postoperative esophageal leak management with the polyflex esophageal stent. J Thorac Cardiov Surg. 2007;133:333-8. [ Links ]
19. Kauer WK, Stein HJ, Dittler HJ, Siewert JR. Stent implantation as a treatment option in patients with thoracic anastomotic leaks after esophagectomy. Surg Endosc. 2008;22:50-3. [ Links ]
20. Fischer A, Thomusch O, Benz S, von Dobschuetz E, Baier P, Hopt UT. Nonoperative treatment of 15 benign esophageal perforations with self-expandable covered metal stents. Ann Thorac Surg. 2006;81:467-72. [ Links ]
21. Song HY, Park SI, Do YS, et al. Expandable metallic stent placement in patients with benign esophageal strictures: Results of long-term follow-up. Radiology. 1997;203:131-6. [ Links ]
22. Sharaiha RZ, Kim KJ, Singh VK, et al. Endoscopic stenting for benign upper gastrointestinal strictures and leaks. Surg Endosc. 2014;28(1):178-84. [ Links ]
23. Lamazza A, Fiori E, De Masi E, Scoglio D, Sterpetti AV, Lezoche E. Self-expanding metal stents for treatment of anastomotic complications after colorectal resection. Endoscopy. 2013;45:493-5. [ Links ]
24. Hirdes MM, Vleggaar FP, Van der Linde K, Willems M, Totte ER, Siersema PD. Esophageal perforation due to removal of partially covered self-expanding metal stents placed for a benign perforation or leak. Endoscopy. 2011;43:156-9. [ Links ]
25. Sharma P, Kozarek R. Role of esophageal stents in benign and malignant diseases. Am J Gastroenterol. 2010;105:258-73; quiz 74. [ Links ]
26. Kauffman GG, Mayo I. The story of nitinol: The serendipitous discovery of the memory metal and its applications. Chemical Educator. 1996;2:1-21. [ Links ]
27. Stoeckel D, Pelton A, Duerig T. Self-expanding nitinol stents: material and design considerations. Eur Radiol. 2004;14:292-301. [ Links ]
28. ShabaJovskaya SB. On the nature of the biocompatibility and medical applications of NiTi shape memory and superelastic alloys. BioMed Mat Eng. 1996;6:267. [ Links ]
29. Wadhwa RP, Kozarek RA, France RE, et al. Use of self-expandable metallic stents in benign GI diseases. Gastrointest Endosc. 2003;58:207-12. [ Links ]
30. Ackroyd R, Watson DI, Devitt PG, Jamieson GG. Expandable metallic stents should not be used in the treatment of benign esophageal strictures. J Gastroen Hepatol. 2001;16:484-7. [ Links ]
31. Brown RE, Abbas AE, Ellis S, et al. A prospective phase II evaluation of esophageal stenting for neoadjuvant therapy for esophageal cancer: Optimal performance and surgical safety. J Am Coll Surg. 2011;212:582-8; discussion 8-9. [ Links ]
32. Lopes TL, Eloubeidi MA. A pilot study of fully covered self-expandable metal stents prior to neoadjuvant therapy for locally advanced esophageal cancer. Dis Esophagus: Official journal of the International Society for Diseases of the Esophagus / ISDE 2010;23:309-15. [ Links ]
33. Hirdes MM, Siersema PD, Vleggaar FP. A new fully covered metal stent for the treatment of benign and malignant dysphagia: A prospective follow-up study. Gastrointest Endosc. 2012;75:712-8. [ Links ]
34. Castaño R, Ruiz M, Juliao F, Sanín E. Eficacia de un nuevo stent de nitinol fabricado localmente, en el tratamiento de la obstrucción maligna esofágica. Rev Col de Gastroenterol. 2003;18:211-21. [ Links ]
35. Castaño R, Puerta JD, Restrepo JI, et al. Manejo actual de la obstrucción maligna colorrectal: grandes incisiones, pequeñas incisiones o sin incisiones. Rev Col Gastroenterol. 2007;23:57-66. [ Links ]
36. Puli SR, Spofford IS, Thompson CC. Use of self-expandable stents in the treatment of bariatric surgery leaks: A systematic review and meta-analysis. Gastrointest Endosc. 2012;75:287-93. [ Links ]
37. Schweigert M, Dubecz A, Solymosi N, Ofner D, Stein HJ. Times and trends in the treatment of spontaneous perforation of the esophagus: From herman boerhaave to the present age. Am Surgeon. 2013;79:902-8. [ Links ]
38. Takimoto Y, Nakamura T, Yamamoto Y, Kiyotani T, Teramachi M, Shimizu Y. The experimental replacement of a cervical esophageal segment with an artificial prosthesis with the use of collagen matrix and a silicone stent. J Thorac Cardiov Surg. 1998;116:98-106. [ Links ]
39. van Boeckel PG, Dua KS, Weusten BL, et al. Fully covered self-expandable metal stents (SEMS), partially covered SEMS and self-expandable plastic stents for the treatment of benign esophageal ruptures and anastomotic leaks. BMC gastroenterology. 2012;12:19. [ Links ]
40. Hunerbein M, Stroszczynski C, Moesta KT, Schlag PM. Treatment of thoracic anastomotic leaks after esophagectomy with self-expanding plastic stents. Ann Surgery. 2004;240:801-7. [ Links ]
41. Dai YY, Gretschel S, Dudeck O, Rau B, Schlag PM, Hunerbein M. Treatment of esophageal anastomotic leaks by temporary stenting with self-expanding plastic stents. Brit J Surg. 2009;96:887-91. [ Links ]
42. Eisendrath P, Cremer M, Himpens J, Cadiere GB, Le Moine O, Deviere J. Endotherapy including temporary stenting of fistulas of the upper gastrointestinal tract after laparoscopic bariatric surgery. Endoscopy. 2007;39:625-30. [ Links ]
43. Gelbmann CM, Ratiu NL, Rath HC, et al. Use of self-expandable plastic stents for the treatment of esophageal perforations and symptomatic anastomotic leaks. Endoscopy. 2004;36:695-9. [ Links ]
44. Schubert D, Scheidbach H, Kuhn R, et al. Endoscopic treatment of thoracic esophageal anastomotic leaks by using silicone-covered, self-expanding polyester stents. Gastrointest Endosc. 2005;61:891-6. [ Links ]
45. Freeman RK, Van Woerkom JM, Vyverberg A, Ascioti AJ. Esophageal stent placement for the treatment of spontaneous esophageal perforations. Ann Thorac Surg. 2009;88:194-8. [ Links ]
46. Langer FB, Wenzl E, Prager G, et al. Management of postoperative esophageal leaks with the Polyflex self-expanding covered plastic stent. Ann Thorac Surg. 2005;79:398-403; discussion 4. [ Links ]
47. Salminen P, Gullichsen R, Laine S. Use of self-expandable metal stents for the treatment of esophageal perforations and anastomotic leaks. Surg Endosc. 2009;23:1526-30. [ Links ]
48. Tuebergen D, Rijcken E, Mennigen R, Hopkins AM, Senninger N, Bruewer M. Treatment of thoracic esophageal anastomotic leaks and esophageal perforations with endoluminal stents: Efficacy and current limitations. J Gastrointest Surg: Official journal of the Society for Surgery of the Alimentary Tract. 2008;12:1168-76. [ Links ]
49. Zhou JH, Gong TQ, Jiang YG, et al. Management of delayed intrathoracic esophageal perforation with modified intraluminal esophageal stent. Dis Esophagus: Official journal of the International Society for Diseases of the Esophagus / ISDE 2009;22:434-8. [ Links ]
50. Shim CS, Cho YD, Moon JH, et al. Fixation of a modified covered esophageal stent: Its clinical usefulness for preventing stent migration. Endoscopy. 2001;33:843-8. [ Links ]
51. Mayoral W, Fleischer D, Salcedo J, Roy P, Al-Kawas F, Benjamin S. Nonmalignant obstruction is a common problem with metal stents in the treatment of esophageal cancer. Gastrointest Endosc. 2000;51:556-9. [ Links ]
52. Hirdes MM, Siersema PD, Houben MH, Weusten BL, Vleggaar FP. Stent-in-stent technique for removal of embedded esophageal self-expanding metal stents. Am J Gastroenter. 2011;106:286-93. [ Links ]
53. Babor R, Talbot M, Tyndal A. Treatment of upper gastrointestinal leaks with a removable, covered, self-expanding metallic stent. Sur Laparo Endo Per. 2009;19:e1-4. [ Links ]
54. Blackmon SH, Santora R, Schwarz P, Barroso A, Dunkin BJ. Utility of removable esophageal covered self-expanding metal stents for leak and fistula management. Ann Tthorac Surg. 2010;89:931-6 [ Links ]
55. Doniec JM, Schniewind B, Kahlke V, Kremer B, Grimm H. Therapy of anastomotic leaks by means of covered self-expanding metallic stents after esophagogastrectomy. Endoscopy. 2003;35:652-8. [ Links ]
56. Han XW, Li YD, Wu G, Li MH, Ma XX. New covered mushroom-shaped metallic stent for managing anastomotic leak after esophagogastrostomy with a wide gastric tube. Ann Thorac Surg. 2006;82:702-6. [ Links ]











 texto en
texto en