Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.30 no.3 Bogotá jul./sep. 2015
The SD BIOLINE Rapid Test for Detection of Antibodies to HCV among High-Risk Patients
Julián David Martínez MD. (1,2), Martín A. Garzón MD (2), Juan M. Arteaga MD (1), Geovanny Hernández MD (2), Camilo Manrique MD. (2), Natán Hormaza MD (2), Jorge Lizarazo MD (2), Juan Marulanda MD (2), Juan C. Molano MD (2), Mario H. Rey MD (2), Carolina Salinas MD. (2)
(1) Faculty of Medicine at the National University of Colombia in Bogotá DC, Colombia.
(2) Gastroenterology Unit of the Hospital Universitario de La Samaritana in BogotáDC, Colombia.
Received: 09-02-15 Accepted: 21-07-15
Abstract
Introduction: Hepatitis C virus (HCV) infections are a public health problem throughout the world: the World Health Organization (WHO) estimates that over 180 million people are currently infected. The aim of this study is to determine the prevalence of anti-HCV in patients at high risk of infection through using a rapid test with capillary blood and confirmation of infection by PCR testing in real time.
Method: Patients were enrolled in the study from among those treated in the gastroenterology unit of the Hospital Universitario de La Samaritana. This is one of the principal referral centers in the department of Cundinamarca, but it cares for patients from across the country. Hepatitis C risk factors identified included previous history of hepatitis C, transfusions, hemodialysis, major surgery (SNC, thorax, abdomen, orthopedic), drug addiction, tattooing, piercing, acupuncture, time in prison, experience as a sex worker, HIV/AIDS, sexually transmitted diseases, health care work, chronic renal failure, and time spent living in the chronic renal failure the Orinoco and/or Amazon regions. Patients referred because of abnormal liver profiles, fatty liver disease, liver masses, cirrhosis (except when due to Hepatitis C virus), ascites, and esophageal-gastric varices were also included in the study. The SD BIOLINE HCV One Step Hepatitis C Virus Test (Standard Diagnostics, Inc. Korea), which is a commercially available kit, was used. This test contains a membrane which is pre-coated with recombinant HCV antigens (core, NS3, NS4, and NS5). A Protein A colloid is combined with the serum sample which then moves along the chromatographic membrane forming a visible line showing the antigen-antibody- Protein A reaction. This test has a high degree of specificity and sensitivity.
Results: Between January and October 2014, 391 patients were included in the study. Of this number, 161 were women (41%) and 230 were men (59%). Average patient age was 46.6 years, and the age range was 14 to 86 years. Four patients, three women and one man, tested positive for hepatitis C. All four diagnoses were confirmed by real time-PCR. The prevalence of HCV was 1.02%.
Conclusions: This study of a population selected especially for histories of risk factors associated with HCV showed this rapid test identified HCV in more than 1% of the population. This is what the true prevalence of HCV in this population would be expected to be based on the findings of 2012 and also based on the sensitivity of the rapid test.
Keywords
Hepatitis C, prevalence, rapid test.
INTRODUCTION
Hepatitis C infections (HCV) are a public health problem around the world: the World Health Organization (WHO) estimates that over 180 million people are currently infected (1, 2). Chronic infection develops in 80% of those who become infected and is responsible for a significant number of chronic liver diseases. It is estimated that 27% of cirrhosis and between 25% and 30% of hepatocellular carcinomas (HCC) are caused by this virus. HCC causes 360,000 deaths per year globally (3-6). The impact of chronic HCV infection is critical for the development of strategies for early diagnosis, management and eradication of HCV infections (6).
The diagnosis of HCV infection takes place through detection of antibodies to HCV (anti-HCV) in the serum of patients. Detection is based on recombinant antigens of the core particle and the NS3, NS4 and NS5 regions. The presence of these antibodies can indicate acute or chronic infection or past infections including those that have been cured. Multiple studies have shown sensitivities of over 99% and specificities between 80% and 90%. Nevertheless, false positives do occur, especially in immune-suppressed people with hematologic diseases and pregnant women (6-9). Reactivity for anti-HCV must be confirmed by molecular tests for the presence of viral RNA in all HIV-positive people (9-11).
In the last decade rapid immunochromatographic tests (tests performed in hospitals) have been introduced for the diagnosis of HCV. The OraQuick Rapid HCV Antibody Test (OraSure Thechnologies) is a rapid test for detecting anti-HCV in venous or capillary blood samples (12). The SD Bioline HCV test is a qualitative immunoassay for the determination of specific antibodies against HCV in human serum or plasma. It has shown a sensitivity of 100% and diagnostic specificity of 99.4% (12). In the USA, Europe and several Asian countries, these tests are approved for use in doctors' offices and emergency rooms. All patients who test positive for HCV with rapid tests must have diagnoses confirmed with molecular testing for viral RNA (9, 13).
The aim of this study is to determine the prevalence of anti-HCV in patients with any risk factors for infection by using a rapid test with capillary blood and confirmation of infection by real time PCR testing.
MATERIALS AND METHODS
Patients were enrolled in the study from among those treated in the gastroenterology unit of the Hospital Universitario de La Samaritana in Bogotá, one of the principal referral centers for the department of Cundinamarca which also cares for patients from the entire country. The following factors were defined as risks for hepatitis C: transfusions, hemodialysis, chronic renal failure, sexually transmitted diseases, major surgery (central nervous system, thorax, abdomen, orthopedic), drug addiction and dependency, tattoos, piercings, acupuncture, imprisonment, sexual work, HIV/AIDS, work in the area of health care, and residency in the Orinoco or Amazon regions. Patients were enrolled who had been referred because of abnormal liver profiles, fatty liver disease, liver masses, ascites, and esophageal-gastric varices. Patients with cirrhosis were also enrolled except when cirrhosis was due to HCV.
Informed consent was obtained in writing and a form with the relevant data from the clinical history each patient was filled out. A commercially available kit, the SD HCV BIOLINE (Standard Diagnostics, INC. Korea) was used for initial diagnosis. This test contains a membrane which is pre-coated with recombinant HCV antigens (core, NS3, NS4, NS5). Protein A colloid is combined with the serum sample and moves along the chromatographic membrane forming a visible line of antigen-antibody- protein A reaction. It has a high degree of specificity and sensitivity.
After cleaning with rubbing alcohol, a sample of capillary blood is obtained from the base of one of the fingers of the nondominant hand. A drop of blood is placed in the cavity of the casing and 4 drops of reagent are added. The blood should fully disseminate through the slot of the kit. The result is interpreted according to the manufacturers instructions. The test is negative for HCV when a single band of color is displayed in the window, and the test is positive for HCV when two bands of color (T band and C-band) are displayed in the result window.
Patients who tested positive with the rapid test had their diagnoses confirmed with real time polymerase chain reaction (RT-PCR) using the Abbott m2000 RealTime System which has a linear range from 12 to 100 million Ul/ml.
RESULTS
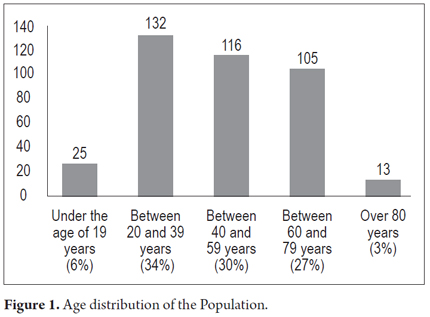
Between January and October 2014, 391 patients including 161 women (41%) and 230 (59%) men, were studied. The average age was 46.6 years and the age range was 14 to 86 years. Their age distribution was as follows: twenty-five (6%) were under 19, 132 (34%), were between 20 and 39, 116 (30%) were between 40 and 59, 105 (27%) were between 60 and 79 years, and thirteen (3%) were over 80 (Figure 1).
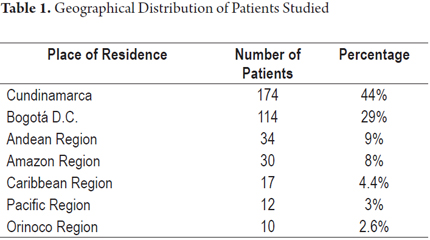
The geographic origin of the patients is shown in Table 1.
Risk factors for hepatitis C that patients were screened for are shown in Table 2.
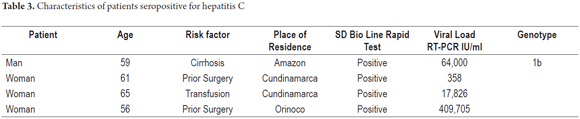
Four patients (3 women and 1 man) tested positive and all were confirmed by RT-PCR. This indicates a prevalence of the infection in this patient population of 1.02%. Table 3 shows the characteristics of these four patients.
DISCUSSION
Globally, studies show that those at greatest risk for HCV infection are individuals who received transfusions before the year 1994, intravenous drug addicts, patients on hemodialysis, sex workers, inmates, those with piercing or tattoos. Also at risk, but in a much smaller proportion, are health care workers (2, 3, 14). It is estimated that 6,800,000 to 8,900,000 adults are positive for anti-HCV in Latin America. This region has been very proactive in screening samples from blood banks thus minimizing the risk of infections from transfusions.
Intravenous drug use is less of a problem in Latin America than it is in the United States and Europe. This suggests that other risk factors play a major role in new infections. Suspected factors include nosocomial infections, inappropriate needle deposits, dental procedures, tattoos and other procedures that compromise contact with infected blood such as cosmetic pedicure procedures (6, 15).
In 1993 Decree 1571 made HCV analysis of blood components used in humans compulsory in Colombia. In 1995, Beltran et al. studied 368,000 units of blood from 172 blood banks in the country and found that 1% of these were reactive for antibodies to HCV (anti-HCV) (16). In 1997, a study of blood banks in the department of Valle del Cauca by Cortes et al. found that 0.98% of donors of Cali were seropositive for anti-HCV. Donors from other municipalities showed seropositivity of 0.47% (17). In 2002, Farfan et al. studied 6,009 blood donors in our hospital and found a prevalence of 0.6% for anti-HCV and a prevalence of 0.06% using real time PCR (18). These results were similar to those of Bedoya et al. who studied 65,535 blood donors in the department of Antioquia and found an HCV antibody prevalence of 0.6% (19). In the 2012 national health indicators report, 0.49% of 746,000 units of blood from Colombian blood banks were seropositive for anti-HCV (20). In 2014, Arroyave et al. reported on a group of 166 people who were transfused before 1994. Anti-HCV prevalence was 11/166 (6.6%). Seven of these eleven were positive in realt time PCR testing. Viral genotype 1 was identified in four samples. No risk factors other than transfusions were found for the individuals with positive markers for HCV (14).
The small proportion of patients with HCV who are diagnosed early when they are still asymptomatic delays treatment and has a negative impact on health services. Cirrhosis and its complications cause huge economic costs. Complications include hepatocellular carcinoma and liver transplantation. The costs related to these complications greatly exceed the cost of treatment to eradicate the virus. As noted previously, knowledge of the impact of chronic infection is critical for the development of strategies for early diagnosis, management and eradication of HCV infections (6). This includes identification of individuals with chronic infections who may be candidates for antiviral therapy. One problem throughout the world is that a significant number of infected people, more than 75%, are unaware that they have HCV and are often only diagnosed when they are in advanced stages of the disease. This has become the most common cause of liver transplantation all over the world (5, 6). This is caused in part by lack of access to health services for timely diagnostic testing and lack of knowledge of the disease in general both in the medical community and in the general population (15). In Latin America the percentage of people diagnosed with HCV ranges from 0.1% to 0.89% of the population (15). Some models have shown that early diagnosis and treatment of a small proportion of the total infected patients worldwide can contribute significantly to controlling the impact of the disease (21). The greatest reduction in morbidity and mortality related to HCV virus occurs when early diagnosis is combined with treatment with highly effective therapies. Some analyses and projections have shown that treatment of only 10% of the infected patients could achieve the elimination of HCV (a reduction of more than 90% of infections by 2030). It has also demonstrated reduced that such a reduction in morbidity mortality associated with the virus can be achieved by switching to treatments with greater sustained viral responses such as the new direct-acting antiviral drugs and interferon-free therapies which effective in more than 90% of patients with fewer adverse effects with shorter treatment times. This impact has already been magnified in countries that have reached treatment rates of 2.8% - 4.5% such as the Netherlands, Luxembourg and Norway (21).
Some studies have demonstrated the cost effectiveness of HCV treatment programs of people based on age rather than in high-risk populations. In the United States, screening of baby boomers born between 1945 and 1965 has recently been recommended because studies have shown that they have a high prevalence of HCV infections (22, 23) Similarly it has been shown that the prevalence of patients who are positive for anti-HCV increases when patients with risk factors (up to 7%) are screened. About half (47%) of patients who are diagnosed are candidates for treatment (24).
We must insist that the medical community has an urgent need to perform diagnostic tests for HCV on people with known risk factors. Most especially, testing needs to be done for people who had transfusions before 1994 and people from the Amazon and Orinoco regions. The latter is based on findings from Brazilian who have reported a high prevalence of hepatitis C in the Amazon basin. Oliveira et al. have reported serological survey of 161 indigenous people which anti-HCV antibodies in 8.8% of those tested. Of these, 62.5% were found to have viral RNA in blood samples (25). Reports from the Ministry of Health of Brazil show a higher prevalence of anti-HCV antibodies in blood donors from the Amazon region (0.62%) than in donors from central (0.28%) and southern regions (0.43%) of that country (26). In Colombia, the study of Alvarado-Mora et al. of 697 people in four departments (Amazonas, Chocó, Magdalena and San Andres Islands) showed the highest prevalence of anti-HCV in Amazonas (5.68%) and the lowest in San Andres Island (0.66%) (27).
This study found HCV prevalence of about 1% in the selected population of patients who are at risk for the disease. This prevalence is very similar to that reported by Beltran et al. in 1995 and by Cortés et al. It is higher than previously reported by our group's blood bank study (18). This study of the use of a rapid HCV test to diagnose a population selected for medical histories and HCV risk factors found that more than 1% of the population had HCV. In all of these patients, the rapid test diagnosis was confirmed by PCR. One of these patients was confirmed to have Hepatitis genotype 1b. This is consistent with previous reports in which genotype 1b is the most prevalent (66%) in patients with HCV in Colombia (28). Samples from the other patients were not genotyped because of technical and economic constraints that prevent us from learning the genotypes of most patients even though this is information of great importance for therapeutic purposes and patient prognosis.
In conclusion, early diagnosis of patients infected with the Hepatitis C virus together with access to more effective new interferon-free therapies and direct acting antivirals with fewer adverse effects can reduce the impact of this disease and can reduce the morbidity and mortality associated with its progression and the need for liver transplantation. Health care systems should provide screening programs to identify people infected with HCV who do not have clinical manifestations of the disease. This type of rapid test can provide a quick and inexpensive way to achieve this goal.
Acknowledgements
The authors would like to thank Merck Sharp & Dome for providing the diagnostic kits used in this study.
REFERENCES
1. Poynar T, Ratzui V, Charlotte F, Gooodmanz M, Mc Hutchinson J, Albrecht J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis C. J Hepatol. 2001;34:730-9. [ Links ]
2. World Health Organization: Hepatitis C. 2011. Disponible en: http://ecdc.europa.eu/en/publications/Publications/TER_100914_Hep_B_C%20_EU_neighbourhood.pdf. [ Links ]
3. States M. Global policy report on the prevention and control of viral hepatitisin. WHO Member States. 2013. [ Links ]
4. Perz JF, Armstrong GL, Farrington L, Hutin YJF, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529-38. [ Links ]
5. Gonzalez S, Davis G. Demographics of hepatitis C virus today. Clin Liver Disease. 2012;1(1):2-5. [ Links ]
6. Corona-Lau C, Muñoz L, Wolpert E, et al. Hepatitis C screening in the general population. Rev Inves Clin. 2015;67:104-8. [ Links ]
7. Liang TJ. Current progress in development of hepatitis C virus vaccines. Nat Med. 2013;19:869-878. [ Links ]
8. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatitis C virus infection. J Hepatol. 2011;55:245-64. [ Links ]
9. Center for Disease Control and Prevention (CDC). Testing for HCV infection: An update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep. 2013;62:362-5. [ Links ]
10. Ansaldi F, Orsi A, Sticchi F, Bruzzone B, Icardi G. Hepatitis C virus in the new era: Perspectives in epidemiology, prevention, diagnostics and predictors of response to therapy. World J Gastroenterol. 2014;20(29):9633-52. [ Links ]
11. Cobb B, Heilek G, Vilchez RA. Molecular diagnostics in the management of chronic hepatitis C: Key considerations in the era of new antiviral therapies. BMC Infectious Diseases. 2014;14(Suppl 5):S5-S8. [ Links ]
12. Grakoui A, Wychowski C, Lin C, Feinstone SM, Rice CM. Expression and identification of hepatitis C virus polyprotein cleavage products. J Virol. 1993;67(3):1385-95. [ Links ]
13. Mutimer D, Aghemo A, Diepolder H, Negro F, Robaeys G, Ryder S, et al. EASL Clinical Practice Guidelines: Management of hepatitis C virus infection. J Hepatol. 2014;60:392-420. [ Links ]
14. Arroyave J, Álvarez C, Correa G, Balcázar N, Arbeláez MP, Navas MC. Infección por hepatitis C en individuos transfundidos antes de 1994 en Antioquia, Colombia. Rev Col Gastroenterol. 2014;29(4):383-9. [ Links ]
15. Kershenobich D, Razavi H, Sanchez-Avila JF, et al. Trends and projections of hepatitis C virus epidemiology in Latin America. Liver Inter. 2011;31(2):18-29. [ Links ]
16. Beltrán M, Raad J, Ayala M, Ching R. Tamizaje de enfermedades infecciosas en bancos de sangre, Colombia, 1995. Biomédica. 1997;17:137-42. [ Links ]
17. Cortés AB, Beltrán MDM, Olaya B, Sc M, Hernández M. Riesgo de enfermedades infecciosas transmitidas por transfusión en el Valle del Cauca, Colombia. Colomb Med. 1999;30:13-8. [ Links ]
18. Farfán Y, Garzón M, Rey MH, Molano J, Lizarazo J, Marulanda J. Prevalencia de hepatitis C por reacción en cadena de polimerasa (PCR) en donantes del banco de sangre. Rev Col Gastroenterol. 2007;22(4):308-12. [ Links ]
19. Bedoya JA, Cortés MM, Cortés JA. Seroprevalence of markers of transfusion transmissibble infection in blood bank in Colombia. Rev Saude Publica. 2012;46(6):950-9. [ Links ]
20. Informe Nacional de Indicadores. Red Nacional de Bancos de Sangre y Servicios Transfusionales. Instituto Nacional de Salud; 2012. [ Links ]
21. Gane D, Kershenobich D, Seguin-Devaux C, et al. Strategies to manage hepatitis C virus (HCV) infection disease burden - volume 2. J Viral Hepatitis. 2015;22(Suppl 1):46-73. [ Links ]
22. Smith BD, Beckett GA, Falck-Ytter Y, Holtzman D, Teo CG, Jewett A, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61(RR-4):1-32. [ Links ]
23. McGarry LJ, Pawar VS, Panchmatia HR, et al. Economic model of a birth cohort screening program for hepatitis C virus. Hepatology. 2012;55:1344-55. [ Links ]
24. Mallette C, Flynn M, Promrat K. Outcome of screening for hepatitis C virus infection based on risk factors. Am J Gastroenterol. 2008;103:131-7. [ Links ]
25. Oliveira C, Silva C, Kemper F, et al. Hepatitis B and C infection among Brazilian Amazon riperians. Rev Soc Bras Med Trop. 2011;44(5):546-50. [ Links ]
26. Brazil Ministry of Health for capacity on epidemiological surveillance on viral hepatitis. Ministry of Health, Brazilia, Brazil, 2008. Disponible en: http://www.aids.gov.br/sites/default/files/cbve hepatites.pdf. [ Links ]
27. Alvarado-Mora MV, Gutiérrez MF, Gomes-Gouvea M, Azevedo R, Carrilho F, Rebello J. Hepatitis B (HBV), Hepatitis C (HCV) and Hepatitis delta (HDV). Viruses in the Colombian Population - How is the epidemiological situation? PLOS One. 2011;6(4):e18888. [ Links ]
28. di Filippo D, Cortés F, Beltrán M, Arbeláez MP, Jaramillo S, Restrepo JC, et al. Molecular charaterization of hepatitis C virus in multi-transfused Colombian patients. J Virol. 2012;9:242. [ Links ]











 texto en
texto en