Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.30 no.3 Bogotá July/Sept. 2015
Clinical Efficacy of Azathioprine (AZA) for Treatment of Mild to Moderate Ulcerative Colitis (UC) in Patients Who Have Responded Inadequately to Steroids
Fabián Juliao B, MD (1), Yineth Agudelo Z. MD. (2), Carlos Yepes D. MD. (3), Luis I. Bejarano MD. (4), Jose G. Thorrens MD. (5), Fabián Jaimes B. MD. (6)
(1) Section of Gastroenterology and Digestive Endoscopy, Consultation Coordinator for Inflammatory Bowel Disease, Hospital Pablo Tobón Uribe in Medellin, Colombia. Professor at the University of Antioquia and Universidad Pontificia Bolivariana in Medellin, Colombia. Mail: fabianjuliao@hotmail.com
(2) Third year internal medicine resident at the University of Antioquia. Physician and Surgeon in the Gastrohepatology Research Group at the University of Antioquia. Mail: yineaz@gmail.com
(3) Research Unit Epidemiologist at the Hospital Pablo Tobón Uribe and Professor at the University of Antioquia in Medellin, Colombia.
(4) Third year internal medicine resident at the University of Antioquia. Physician and Surgeon at the Universidad Pontificia Bolivariana. Mail: ignaciomd200963@gmail.com
(5) Third year internal medicine resident at the University of Antioquia. Physician and Surgeon at the University of Cartagena. Mail: docthorrens@yahoo.es
(6) PhD in epidemiology, Johns Hopkins University. Master of Clinical Epidemiology, Pontificia Universidad Javeriana, Internal Medicine, University of Antioquia. Physician and Surgeon, Industrial University of Santander. Associate Professor Department of Internal Medicine University of Antioquia. Mail: fjaimes@hptu.org.co
Received: 09-02-15 Accepted: 21-07-15
Abstract
Introduction: Because there are very few studies of the use of azathioprine (AZA) for treatment of ulcerative colitis, and most are more than a decade old, we need to establish the efficacy of AZA in our environment for patients who have responded inadequately to steroids. Objective: The objective of this study is to evaluate short and long term clinical responses to AZA by ulcerative colitis patients in our population who have had inadequate responses to treatment with steroids. Materials and Methods: This is a retrospective study based on a database review of the medical records of 215 ulcerative colitis patients treated at the Hospital Pablo Tobon Uribe between August 2001 and May 2014. Sixty-nine patients (32%) had received AZA, and 30 patients had received at least 3 months of treatment with AZA at the optimal dose of at least 2.0 mg/kg after having responded inadequately to treatment with steroids. This group was included in the study. Results: The median follow-up time was 20 months with a range from three months to 72 months. After three months of treatment, clinical remission was found in 17 patients (57%) out of 30 patients, and partial responses were found in 12 out of 30 subjects (40%). A year after the initial follow-up, 16 patients (53%) maintained clinical remission, three (10%) continued to have partial responses, and five (17%) had relapsed and had received biological therapy. Sixteen patients (53%) achieved one year with steroid treatment suspended and no patients required colectomies. Conclusions: This study demonstrates a clinical remission rate of 53% for patients with mild to moderate ulcerative colitis who were treated with AZA. One year follow-ups showed that continuous suspension of steroid treatment had been achieved in 53% of patients. AZA is an inexpensive and safe therapeutic option which can be considered prior to initiating biological therapy for these patients.
Keywords
Ulcerative colitis, azathioprine, clinical remission, steroid suspension.
INTRODUCTION
Ulcerative colitis (UC) is a chronic inflammatory disease whose etiology is multifactorial. It is characterized by diarrhea, rectal bleeding, and erosion and/or ulceration of the colon mucosa. A previous article of ours showed that out of 202 patients diagnosed with inflammatory bowel disease (IBD), a higher proportion of patients presented UC (80.7%) than Crohn's disease (15.8%) (4.9: 1 ratio) with similar distributions by genders. This is different from what is observed in industrialized countries where the proportion of the two entities is very similar (1). The pharmacological management of patients with UC is done in stages with 5-ASA medications, steroids, and thiopurine immunosuppressants such as azathioprine. In recent years anti-tumor necrosis factors such as infliximab and adalimumab have also been used (2-4). Major clinical problems include steroid dependence, the oneyear dependence rate is 22% (5), and a high probability of colectomy within five years of initial treatment. Nine percent of patients with proctitis who do not respond to conventional management undergo colectomies, 35% of patients with extensive damage undergo this procedure (6).
Azathioprine (AZA) is a thiopurine which interferes with the metabolism of nucleic acids. It acts as a competitive antagonist that produces immunosuppression through a reduction of cellular proliferation and other mechanisms (7). Treatment of Crohn's Disease (CD) with AZA or 6-mercaptopurine (6-MP) is well supported by clinical evidence available in the literature, but this is not the case with UC. The data about the efficacy of this kind of treatment for UC are scarce, and most studies are more than a decade old and of low quality (8-10). There are no studies in Latin America that demonstrate the usefulness of AZA for managing UC although it is a relatively inexpensive drug in Colombia compared to biological therapy with infliximab or adalimumab. Considering the above and based on our experience at our center in the management of IBD, we designed a retrospective descriptive cohort in order to describe the short and long term effectiveness of UC treatment for AZA patients with inadequate responses to steroids.
MATERIALS AND METHODS
Type of study
A retrospective review of the medical records of patients treated for UC between August 2001 and July 2013 was done at the Hospital Pablo Tobón Uribe.
Area of study
The Hospital Pablo Tobón Uribe is in the city of Medellin, Colombia. It is a hospital that provides high complexity procedures for a large percentage of the population of the city, and is a referral center for treatment of difficult pathologies such as IBD. In 2001 we created an IBD clinic in the Hospital Pablo Tobón Uribe due to a special interest of the authors of this study. The objectives were to provide care exclusively for patients with UC and CD and to become the city's point of reference for the management of these entities.
Study population
The medical records of all patients with UC over 18 who received AZA for at least 3 months at therapeutic doses of at least 2 mg/kg were reviewed. In Colombia, AZA comes in 50 mg tablets and dosages are prescribed in a single daily dose. The diagnosis of UC was determined according to the criteria adopted by the European Organization for Crohn's Disease and Ulcerative Colitis (ECCO) (11), and patients were classified according to the Classification of Montreal (12). For patients with UC, the cues for prescribing AZA treatment was their dependence on steroids and a refractory pathology.
Definitive diagnostic criteria for UC are based on first excluding infectious, ischemic and neoplastic diseases and then finding three of four key sets of symptoms (11):
1. Diarrhea and/or bleeding, and/or mucus in the stools for more than six weeks or in repeated episodes
2. Colonoscopic findings of friable granular mucosa, with or without ulceration
3. Histological findings of acute or chronic inflammation compatible with IBD together with cryptitis and distortion of crypts associated with lymphoplasmacytic infiltrate, without granulomas
4. Absence of Crohn's disease which must be ruled out through radiological studies of the small intestine, ileocolonoscopy, or biopsies.
Endoscopic severity and extent of the UC were determined according to the last colonoscopy before the beginning of azathioprine treatment. Extension of UC was classified according to the Montreal classification.
Inclusion criteria
The patients included in this study were over 18 years of age, had been diagnosed with UC, and had received at least 3 months of treatment with AZA dosages of at least 2 mg/kg. Also, the indication for treatment with AZA was inadequate response to steroids, either because of steroid dependence or disease refractory to treatment with steroids.
- Dependency was defined as the inability to reduce steroid doses to below the equivalent of 10 mg of prednisone per day within 3 months (12 weeks) of the start of corticosteroids.
- Refractory disease was defined as disease that recurred within three months after the suspension of the steroid.
- Inadequate response to treatment with oral steroids (0.75 to 1 mg/kg/day of oral prednisone or equivalent) for more than four weeks (13).
Exclusion criteria
The following types of patients were excluded from the study:
- Patients whose medical record information was unavailable for various reasons.
- Patients with prior use of AZA or concomitant use of AZA and biological therapy with infliximab or adalimumab.
- Patients with colectomies prior to the start of AZA treatment.
- Patients with severe acute UC.
Measurement of outcomes and definitions
Primary outcome measured: The proportion of steroid dependent patients with sustained response to steroidfree AZA treatment, 12 months after the start of AZA treatment. Sustained response was defined as the maintenance of clinical remission (modified Mayo score <2) with full suspension of steroids and a minimum of six months without having returning to steroids.
Secondary outcomes measured:
- No clinical response: Patient's Mayo score either increases or decreases by less than 3 points or 30%.
- Partial response: Patient's Mayo score decreases by 3 points (30%) or more, but the patient does not reach a modified Mayo score below two.
- Adverse effects of AZA: onset within 4 weeks after initiation of treatment with AZA of any of the following: platelet count <100,000, leukocytes <3000, elevated transaminase or bilirubin (more than twice the reference value), acute pancreatitis, opportunistic infections or lymphoma arising at any time during monitoring.
The Mayo Scoring System for Assessment of Ulcerative Colitis Activity Clinic score was used (14). This score includes four dimensions: number of stools, presence of bleeding, the global assessment by the physician, and endoscopic severity. Remission is defined as a score ≤ 2, a partial response that leads to a 30% or 3 point decrease of the score, or an endoscopic mucosal healing score less than or equal to 1. For the oneyear clinical response in the follow-up, the Mayo Clinic score was modified by excluding the endoscopic results. AZA treatment failure is defined as discontinuation due to intolerance, loss of clinical response, or switching to biological therapy. Sustained clinical response is defined as the absence of AZA treatment failure during the followup.
We built a database in Excel which included the following data for each patient:
1. Age at UC diagnosis
2. Current age
3. Gender
4. Location and severity of ulcerative colitis (Montreal classification)
5. Mayo Clinic score at the beginning of treatment with azathioprine
6. Steroid dosage at the start of AZA
7. Starting dosage of AZA
8. Adverse effects associated with azathioprine (thrombocytopenia, leukopenia, abnormal liver function, acute pancreatitis, lymphoma, opportunistic infections)
9. Use of biological therapy.
For the patient population:
1. Percentage of patients who stopped using steroids
2. Percentage of patients who underwent colectomies
3. Percentage of patients who relapsed during follow-up.
Statistical Analysis
A descriptive statistical analysis was performed. Qualitative variables were characterized by frequencies and proportions, while quantitative variables were described by their means, their standard deviations (SD), their medians (Me), and their interquartile ranges (IQR). Also, their distributions were studied with the ShapiroWilk test. For bivariate analysis, qualitative variables were analyzed with Pearson's chisquared test or Fisher's exact test.
Ethical issues
This study's protocol was submitted to the Research and Research Ethics Committee at the Hospital Pablo Tobón Uribe where it was approved. The confidentiality of information is guaranteed, and informed consent was not necessary since this study only involves collecting information retrospectively from clinical records and does not involve additional intervention in patients. This makes this study a "No Risk" research project according to Article 11 of Resolution 8430 of 1993 of the Ministry of Health of Colombia.
RESULTS
A total of 208 patients with UC were recorded in our database. Of these 69 (33%) had received AZA. Thirty of the 69 patients met the inclusion criteria previously mentioned and had full information available in their medical histories. Thirty patients were receiving steroids at the start of treatment with AZA and all used AZA simultaneously with mesalazine. As for sex, 47% were male and 53% female. The average age of onset of azathioprine was 43 years, and 80% of subjects were between 20 and 60 years old. According to the classification of Montreal, 57% of patients had extensive colitis, 33% leftsided colitis, and only 10% had damage in the rectum. A summary of the demographic characteristics of the patients is presented in Table 1.
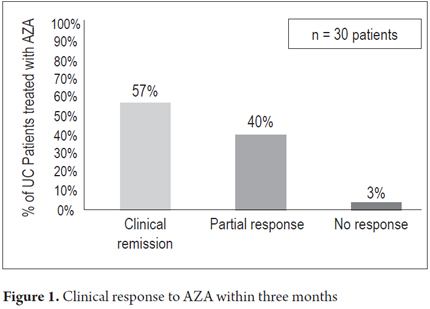
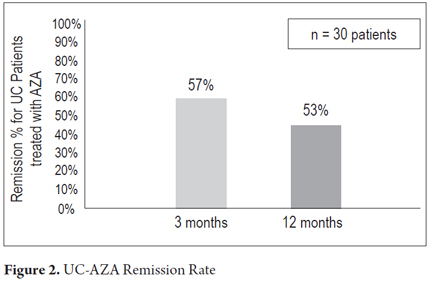
The average patient follow-up was 20 months (3 months to 72 months). Clinical remission was found in 17 patients (57%) and partial responses in 12 patients (40%) three months into treatment. One patient (3%) did not exhibit any primary response and required biological therapy (Figure 1). One year after the initial response, 16 patients (53%) maintained clinical remission, 3 patients (10%) continued to have partial responses. Five patients (17%) had relapsed and received biological therapy (Figure 2). Steroid treatment had been suspended in sixteen patients (53%) by one year after the beginning of treatment. Only 10 patients were followed for at least 2 years. Of these, nine (90%) had sustained responses, but one patient had stopped responding to AZA and required biological therapy. No significant association between clinical remission and extent of disease was found. No patients required colectomies during the followup.
As for the safety of azathioprine during follow-up, six of 30 patients (20%) developed leucopenia which was resolved by decreasing the dose without discontinuing the medication. There was one case of acute pancreatitis which required the suspension of AZA, and only one individual had AZA therapy discontinued because of gastrointestinal symptoms. No hepatic toxicity, opportunistic infections, or lymphoma were observed in any of the 30 patients during their followups.
DISCUSSION
Purine analogs such as AZA and 6-mercaptopurine have been used for over 30 years for the maintenance of remission in patients with UC that are refractory to management with 5-ASA and steroids. They have also been used as a "steroid saving" treatment with patients that are dependent on steroids, with the clearly established risk/benefit profile of maintaining remission in the longterm in 20% of patients with UC, according to various studies (15). Our study shows that AZA is effective in 65% of patients who have refractory UC or who are steroid-dependent one year into followup with an adequate safety profile. This is the first Latin American study on this particular topic.
Studies of AZA for treatment of UC are scarce and most are more than a decade old. The first study, by Jewell and Truelove, dates back to 1974. That study failed to demonstrate that AZA is useful for inducing or maintaining remission in patients with ulcerative colitis. It was even less effective than sulfasalazine (16). The first double-blind placebo-controlled study was published by Hawthorne et al. in the United Kingdom (17). This study was done with patients in remission from UC as the result of treatment with AZA for at least six months. Thirty three patients who had been treated with AZA at average daily doses of 100 mg were followed for one year. The authors found a relapse rate of 36% compared to 59% in 34 subjects who suspended medication and were given placebos. Other retrospective or open studies in tertiary care centers have shown response rates of 84% and 63% and remission rates of 65% and 69% (18).
The largest retrospective study was done in Oxford. The study, in which 346 patients were followed for over 30 years, found a remission rate of 58% (19). A more recent Spanish study of 34 patients found a rate of maintenance of remission of 63.6% in patients with 6mercaptopurine treatment. It showed that methotrexate was less effective than 6mercaptopurine for ulcerative colitis (20). An Italian study by Ardizzone et al. of 72 steroiddependent patients who were randomly assigned to receive 2 mg/kg/day of azathioprine or 3.2 g/day of 5-ASA, found 6 month remission rates of 53% and 21% respectively (OR: 4.78) (21). All of these results are similar to those presented in our study, regarding both overall response and clinical remission. A systematic review and meta-analysis of all immunosuppressants used for UC was recently published. Two of the studies evaluated AZA for maintaining remission (130 patients) and pointed towards the existence of benefits with AZA therapy (RR = 0.5, 95% CI = 0.7-1.0) (22). A more recent systematic review by Cochrane of 6 studies with a total of 286 UC patients showed that AZA is significantly superior to placebos for maintaining remission in 51 of 115 patients in the AZA group compared with 76 of 117 patients in the placebo group (23).
Additionally, there is a risk of relapse of UC in individuals who suspended AZA therapy. A study with UC patients in clinical remission who suspended AZA use showed that five years into the followup, 60% had had relapses (24). A more recent study found a relapse rate of 26% at 24 months in 108 patients with UC who stopped AZA treatment deliberately due to clinical remission (25).
A weakness of our study is the sample size. The size was due to the fact that many of the patients who initially come to our IBD clinic continue their medical services at other sites afterwards, and we lose track of them. Because of this, we do not have complete information. In addition, some patients that come to us receive doses of AZA lower than 2mg/kg, and therefore did not meet the inclusion criteria and were excluded from the study. More randomized, placebo-controlled, clinical trials (with more patients) are needed to confirm the results presented in the various studies cited here.
The results of this study are very important because they show that AZA is an effective drug for management of individuals with UC who have inadequate responses or intolerance to steroids. AZA is inexpensive and delivers long-lasting clinical remission in patients who respond and tolerate the drug. Consequently, it is a valid alternative for treating UC prior to the use of biological therapy with anti-TNFs.
REFERENCES
1. Juliao F, Florez JF, Ruiz MH, et al. Fenotipo e historia natural de la enfermedad inflamatoria intestinal en un centro de referencia en Medellín-Colombia. Rev Col Gastroenterol. 2010;25(3):240-51. [ Links ]
2. Juliao F. Tratamiento médico enfermedad inflamatoria intestinal. Rev Col Gastroenterol. 2007;22(4):313-30. [ Links ]
3. Girardin MM, Manser C, Biederman L, Wanner Roger, Frei Pascal, Safroneeva E, et al. First line therapies in inflammatory bowel disease. Digestion. 2012;86(1):6-10. [ Links ]
4. Dignass A, Lindsay JO, Sturm A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis Part 2: Current management. J Crohns and Colitis. 2012;6:991-1030. [ Links ]
5. Faubion WA Jr, Loftus EV Jr, Harmsen WS, et al. The natural history of corticosteroid therapy for inflammatory bowel disease: A population based study. Gastroenterology. 2001;121:255-60. [ Links ]
6. Langholz E, Munkholm P, Davidsen M, Binder V. Colorectal cancer risk and mortality in patients with ulcerative colitis. Gastroenterology. 1992;103:1444-51. [ Links ]
7. Chouchana NC, Beaune P, Loriot MA, Roblin X. The benefits of pharmacogenetics for improving thiopurine therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35(1):15-30. [ Links ]
8. Ghosh S, Chaudhary R, Carpani M, et al. Is thiopurine therapy in ulcerative colitis as effective as in Crohns disease? Gut. 2006;55:6-8. [ Links ]
9. Ginsburg PM, Dassopoulos T. Steroid dependent ulcerative colitis: Azathioprine use is finally "Evidence-Based". Inflamm Bowel Dis. 2006;12(6):921-2. [ Links ]
10. Sands BE. Immunosuppressive drugs in ulcerative colitis: Twisting facts to suit theories? Gut. 2006;55:437-41. [ Links ]
11. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut. 2006;55:749-53. [ Links ]
12. Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis Part 1: Definitions and diagnosis. J Crohns and Colitis. 2012;6:965-90. [ Links ]
13. Panaccione R, Rutgeerts P, Sandborn WJ, et al. Review article: Treatment algorithms to maximize remission and minimize corticosteroid dependence in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2008;28:674-88. [ Links ]
14. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis: A randomized study. N Engl J Med. 1987;317:1625-9. [ Links ]
15. Louis E, Irving P, Beaugerie L. Use of azathioprine in IBD: Modern aspects of an old drug. Gut. 2014;63(11):1695-9. [ Links ]
16. Jewell DP, Truelove SC. Azathioprine in ulcerative colitis: Final report on a controlled therapeutic trial. BMJ. 1974;4:627-30. [ Links ]
17. Hawthorne AB, Logan RF, Hawkey CJ, et al. Controlled trial of azathioprine withdrawal in ulcerative colitis. BMJ. 1992;305:20-2. [ Links ]
18. Paoluzi OA, Pica R, Marcheggiano A, et al. Azathioprine or methotrexate in the treatment of patients with steroid-dependent or steroid resistant ulcerative colitis: Results of an open-label study on efficacy and tolerability in inducing and maintaining remission. Aliment Pharmacol Ther. 2002;16:1751-9. [ Links ]
19. Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: A 30 year review. Gut. 2002;50;485-9. [ Links ]
20. Mate-Jimenez J, Hermida C, Cantero-Perona J, et al. 6-Mercaptopurine or methotrexate added to prednisone induces and maintains remission in steroid-dependent inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2000;12:1227-33. [ Links ]
21. Ardizzone S, Maconi G, Russo A, et al. Randomised controlled trial of azathioprine and 5-aminosalicylic acid for treatment of steroid dependent ulcerative colitis. Gut. 2006;55:47-53. [ Links ]
22. Khurram J, Khan M. Efficacy of immunosuppressive therapy for inflammatory bowel disease: A systematic review and meta-analysis. Am J Gastroenterol. 2011;106(4):630-42. [ Links ]
23. Timmer AMJ, Tsoulis DJ, Macdonald JK. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;9:CD000478. [ Links ]
24. Cassinotti A, Actis GC, Duca P, et al. Maintenance treatment with azathioprine in ulcerative colitis: Outcome and predictive factors after drug withdrawal. Am J Gastroenterol. 2009;104:2760-7. [ Links ]
25. Kennedy NA, Kalla R, Warner B, et al. Thiopurine withdrawal during sustained clinical remission in inflammatory bowel disease: Relapse and recapture rates, with predictive factors in 237 patients. Aliment Pharmacol Ther. 2014;40:1313-23. [ Links ]











 text in
text in