Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.30 no.3 Bogotá July/Sept. 2015
What is the Profile of Patients Waiting for New Hepatitis C Treatments?
João Alberto Martini Peruchi MD. (1), Emilia Tiemi Oshiro Bansho MD. (1), Débora Tonon MD. (1), Ariane Borgonovo, MD. (1), Dariana Maggi MD. (1), Esther Buzaglo Dantas-Corrêa MD. PhD. (1), Leonardo de Lucca Schiavon MD. PhD. (1), Janaína Luz Narciso-Schiavon MD. PhD. (1)
(1) Núcleo de Estudios en Gastroenterología y Hepatología, Universidad Federal de Santa Catarina (UFSC), Florianópolis, SC, Brazil.
Received: 04-05-15 Accepted: 21-07-15
Abstract
Introduction: Until recently, treatment with a combination of pegylated interferons (Peg-IFN) and ribavirin (RBV) was the gold standard treatment for hepatitis C. In anticipation of the arrival of new drugs, we evaluate current treatment outcomes and patients waiting for the new therapy.
Materials and Methods: This cross-sectional analytical study evaluated treatment outcomes among chronic hepatitis C patients, and then compared chronic non-responders and treatment naïve patients who were given interferon based-treatment.
Results: The study included 192 individuals among whom were 87 patients who received treatment. Among treated patients, we observed low rates of sustained viral response. A comparison of 105 treatment-naïve patients and 87 who had previously received IFN treatment showed that among patients waiting for new therapies, naïve individuals presented a higher proportion of genotype 1 (68% vs. 49%; p = 0.028) than did previously treated patients, lower ALT (91.1 ± 73.0 vs. 126.0 ± 73.40 U/L; p =017), lower AST (70.1 ± 51.3 vs. 89.7 ± 47.40 U/L; p = 050), lower GGT (85.3 ± 85.1 vs. 148.4 ± 123.9 U/L; p = 0.007) levels and a lower proportion of significant fibrosis (24.3% vs. 83.3%; p < 0.001).
Conclusions: SVR rates were low. Among potential candidates for HCV treatment, the majority are naïve, genotype-1 with mild histology.
Keywords
Hepatitis C, treatment outcome, interferon-alpha.
INTRODUCTION
Until recently, pegylated interferon-alfa (Peg-IFN) in combination with ribavirin was the gold-standard treatment for hepatitis C. In 2011, telaprevir and boceprevir were licensed for use in HCV genotype 1 infection. These two drugs are first-wave, first-generation direct-acting antivirals. Recently, new drugs have become available with fewer side effects and shorter treatment duration, especially in developed countries. These drugs have changed the standard of care to triple therapy (1). Second and third-generation protease inhibitors are not widely available in Brazil, and the dual scheme with standard interferon (IFN) is still recommended by the National Ministry of Health for non-cirrhotic patients with HCV genotypes 2 or 3. With the arrival of new drugs, we sought to assess the profile of experienced and naïve patients awaiting treatment. The objective of this study was to determine the clinical profile of treatment-experienced patients and naïve patients waiting for the new HCV therapy.
MATERIALS AND METHODS
This analytic cross-sectional study evaluated HCV carriers (adults) at the gastroenterology and hepatology outpatient clinic of a public university hospital between January and May 2014. Patients were excluded for the following reasons: insufficient registration of clinical data, refusal to participate in the study, acute hepatitis C, negative HCV-RNA, or undergoing interferon-based treatment. In a routine outpatient visit, individuals were invited to participate in the study and to sign the informed consent form. Clinical, laboratory, and histological data were collected from records on medical charts. HCV patients were defined as those with HCV ribonucleic acid (RNA) detectable by polymerase chain reaction (PCR). The clinical and laboratory variables analyzed were male gender, age, skin color, alcohol intake higher than 40 g per day, creatinine, platelets, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT), direct bilirubin, albumin, international normalized ratio, HCV genotype, and viral load. Sustained virological response (SVR) was defined as undetectable HCV RNA 24 weeks after treatment completion. Histological features were analyzed using the METAVIR group scoring system. Fibrosis was staged on a scale of F0 to F4 as follows: F0 = no fibrosis, F1 = portal fibrosis without septa, F2 = few septa, F3 = numerous septa without cirrhosis, and F4 = cirrhosis. Significant fibrosis was defined by the presence of F2, F3, or F4 METAVIR stages, and the occurrence of stages F3 or F4 characterized advanced fibrosis (2).
Patients' first treatments were evaluated retrospectively. They received standard interferon (IFN), peguilated-IFN (Peg-IFN), or fist-generation protease inhibitors telaprevir or boceprevir (PI) plus Peg-IFN. All treatment regimens included weight-based ribavirin for 24 or 48 weeks, depending on the HCV genotype or individual approach (cirrhosis, for example). Continuous variables with a normal distribution were expressed as a mean and standard deviation and compared using the Student's t-test. Categorical variables were represented by frequency (%) and analyzed via chi-squared test, linear-by-linear association chi-square, or Fisher's exact test, when necessary. P values of less than 0.05 were considered statistically significant.
Bivariate analysis was performed to identify the variables associated with naïve patients. Variables with p values less than 0.20 were included in the multivariate analysis. Binary logistic regression (enter) analysis was performed to identify variables independently associated with naïve patients. All tests were performed using the IBM Statistical Package for Social Sciences software, version 17.0 (SPSS Statistics, Chicago, Illinois, USA). The study protocol adheres to the ethical principles of the Declaration of Helsinki and was approved by the local ethics committee (number 832.915).
RESULTS
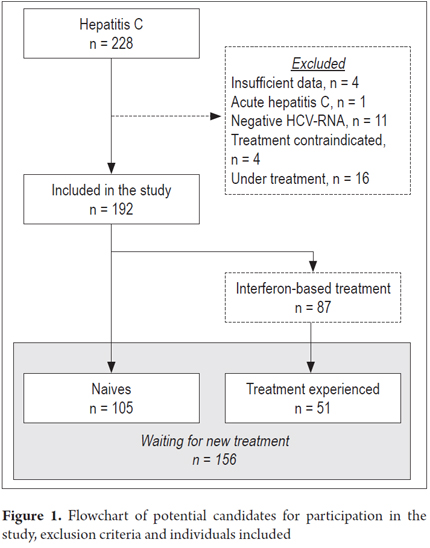
From January 2014 until May 2014, there were 228 patients treated for chronic HCV in our institution and evaluated for enrollment. Thirty-six patients were excluded for the following reasons: 11 repeatedly tested negative for HCV-RNA, 4 were under treatment, 4 had incomplete data on medical charts, and 4 had advanced liver disease (Figure 1). The study included 192 individuals with HCV with a mean age of 51.8 ± 11.8 (median 52.0) years; 47.4% were men, and 94.7% declared themselves to be Caucasian. Genotype was available for 165 patients and was distributed as follows: genotype 1 = 60%; genotype 2 = 3%; genotype 3 = 37%. Among subjects with genotype 1, 15.6% were genotype 1a, 1b genotype 6.7% and in 77.8% of the subjects the laboratory did not report the genotype subtype. Advanced fibrosis was observed in 45.9% of the sample and 33% presented cirrhosis. With respect to HCV treatment, 105 patients were naïve to treatment, and 87 had previously received IFN treatment.
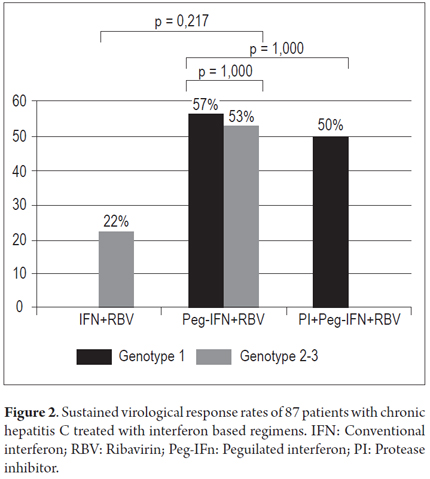
Among the 87 individuals submitted to interferon-based treatment, 84.5% had more than 40 years, 51.8% were genotype 1, 60.3% presented advanced fibrosis (45% were cirrhotic), and 54.5% presented viral load ≥ 800,000.00. Standard IFN was administered to 14.9%, Peg-IFN to 73.6%, and PI to 11.5% of the patients (p = 0.136). Among ten patients treated with PI, seven received telaprevir and three received boceprevir. Overall SVR was obtained for 50% of the treated patients: 56% of genotype 1 and 42.3% of non-1 genotype patients (p = 0.328). Among genotype non-1, 22.2% obtained SVR with conventional IFN + ribavirin therapy and 52.9% obtained SVR with Peg-IFN + ribavirin. Among genotype 1, 57.1% obtained SVR with Peg-IFN + ribavirin therapy and 50.0% obtained SVR with PI + Peg-IFN + ribavirin (Figure 2).
Twenty patients underwent a second treatment regimen, all of which were more than 40-years old and presented advanced fibrosis; 40% were men, and 42.1% were genotype 1. One (5%) received standard IFN, 13 (65%) received Peg-IFN, and 6 (30%) were treated with IP. SVR rates of the second treatment were available for 16 patients, indicating 43.8% SVR: 27.3% for Peg-IFN and 80% for PI (p = 0.106). Two patients underwent a third treatment regimen with Peg-IFN for 72 weeks, and both achieved SVR.
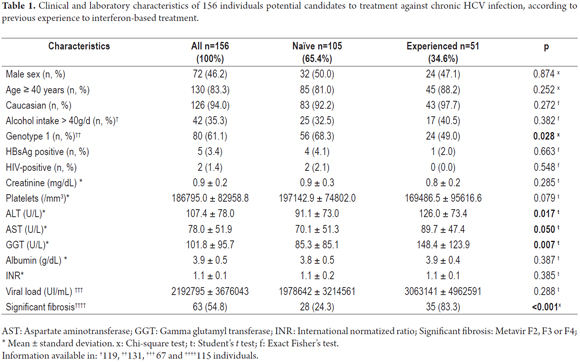
Among 156 potential candidate patients for HCV treatment, 105 were naïve and 51 were experienced (Table 1). Almost 2/3 of the naïve patients were genotype 1 and presented high mean viral loads. Nonetheless, only 1/4 of them presented advanced fibrosis. Half of experienced patients presented HCV genotype 1, and 3/4 had advanced fibrosis. Experienced patients presented higher mean levels of ALT, AST, and GGT in comparison to naïve patients (Table 1). In the multivariate analysis, the following variables were included: non-1 genotype, ALT, AST, GGT, and non-significant fibrosis. Binary logistic regression (enter) analysis revealed that non-1 genotype (OR = 1.006; 95% CI 1.0011.011; p = 0.031) and non-significant fibrosis were independently associated with treatment-naïve patients (OR = 0.071; 95% CI 0.0190.271; p < 0.001).
DISCUSSION
In regard to the variables age, gender, ethnicity, and genotype, the present study sample does not differ from what has been observed in Brazil and other countries. It is noteworthy that almost 100% of the patients auto declared themselves Caucasians, especially in a country of miscegenation like Brazil. In southern Brazil, colonization is predominantly of European origin (3), and the majority of the population is Caucasian, as reported by other southern Brazilian studies evaluating individuals with HCV (9299%) (4, 5).
Since 2000, it has been well known that in patients with chronic hepatitis C, a regimen of Peg-IFN + RBV is more effective than a regimen of IFN + RBV given three times weekly (39% vs. 19%, p=0.001) (6). Among patients with HCV genotype 1 infection, the corresponding SVR rates were 42-46% (7, 8), while the rate for patients with genotypes 2 and 3 infections is about 80%, independently of the IFN regimen (7). This was one of the justifications used in Brazil to keep the scheme with IFN + RBV as first-line treatment of non-cirrhotic patients with genotypes 2 and 3. Among non-1 genotype individuals, similar SVR rates were observed between those who received IFN + RBV or Peg-IFN + RBV (Figure 1), probably due to the small number of patients evaluated with non-1 genotype (n = 24). We have to pinpoint the abovementioned fact that in Brazil Genotype non-1 patients can only receive Peg-IFN if they present advanced fibrosis. The others may only be treated with biosimilar standard IFN, which cheaper in terms of public health but has proven to be worse than Peg-IFN in efficacy. Vigani et al. have demonstrated that Peg-IFN + RBV are associated to higher SVR rates when compared to biosimilar standard IFN regardless of fibrosis stage (79.3% vs. 49.1%, p = 0.0001) (9).
SVR for genotype 1 individuals who received Peg-IFN + RBV was 57.1%, which is higher than that the values reported in other Brazilian studies of 43-52% (10, 11) and lower than that in international randomized clinical trials. In a retrospective study on Southern Brazilian patients with chronic hepatitis and HCV genotype 1 infection, SVR was achieved in only 35.3% of patients (114/323), although this may have occurred because a large proportion of the patients presented advanced fibrosis (F3/F4 = 74%) (12). In the present study, 52% of genotype 1 patients presented advanced fibrosis, a higher proportion than that reported in registry studies, where SVR rates for Peg-IFN + ribavirin varied from 42% to 52% and 24% to 55% presented advanced fibrosis (7, 8).
It is known that among the variables that most interfere with the SVR are age, race, sex, body mass index, viral load, degree of fibrosis (1, 7, 13). When we evaluate the variables associated with better rates of SVR, there was a tendency to lower viral loads only (SVR 1,172,172±1,342,722 vs. no-SVR 4,079,651±5,275,628; p = 0.054). This difference probably was due to the small number of patients included in subgroups of different treatment regimens. Although there was a preponderance of Caucasian individuals in our sample, many of the variables associated known as associated with SVR were unfavorable in the majority of the patients who received treatment: age, genotype (97% of the sample presented genotype 1 or 3), advanced fibrosis and viral load. And these findings, although they have not been shown statistically significance (except from viral load) cannot be overlooked as a possible influence for the low SVR rates described in this sample.
Only ten patients in the present study were treated with PI, and therefore, we cannot know if the SVR rates represent the actual results of our clinic. The SVR rate of 50% found in our cohort is well below the rates in the first trials (14). In phase III clinical trials, SVR rates in treatment-naive patients were reported as 66% and 75 % for patients treated with boceprevir and telaprevir-based regimens, respectively (15, 16). In registry studies, 20 to 50% of the studied patients presented advanced fibrosis (15, 16). In the Spanish clinical setting treated with PI boceprevir, a total of 80% had fibrosis F3/F4, and RNA negativation was 48.8% at week 8 (17). In the USA, a real-life cohort with 200 patients demonstrated 53% SVR for telaprevir and 40 % for boceprevir (18).
The high efficacy and safety of new drugs brings hope for universal treatment of those infected with HCV. However, the high cost of mass treatment may hinder use. There are currently four drugs approved by ANVISA, the company that regulates medicines in Brazil: Sofosbuvir, Daclatasvir, Simeprevir and "3D" (ABT-450, ritonavir, ombitasvir, and dasabuvir) but none of them is available for treatment in Public Health System (SUS) yet. The probable therapeutic approach is under public consultation and physicians are unaware of what will be available in the near future and who will be treated according to Government guidelines. Prices are estimated to be U$ 2,000.00 - 2,500.00 per capita per month, which may restrict patient selection to non-responders and those with advanced fibrosis(19). In the future, the arrival of new drugs to the market may lower prices and facilitate an unrestricted access to medication to patients who is in need of treatment.
We acknowledge some limitations to our analysis. The primary and most obvious shortcoming of single-center studies is their potentially limited external validity, although they allow larger, multicenter studies to be planned appropriately and supported. This study was conducted in a referral specialized service for hepatitis C treatment, and the studied sample is comparable to other populations in the world. Secondly, the relatively small number of patients included could limit the interpretation of the results. However, the collected data represent the reality of clinical practice.
In conclusion, double treatment with Peg-IFN and RBV is the most commonly IFN-based regimen administrated for HCV patients, and it exhibits similar SVR rates to those of other schemes. SVR demonstrated in this study are lower than those reported in the literature. Among potential candidates for HCV treatment, the majority were naïve with genotype 1 and mild histological findings. Half of the experienced patients were genotype 1, and the majority presented advanced fibrosis.
Financial support
No financial support.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
Paper presented as a requirement for obtaining the Medical Doctor (MD) degree from the Federal University of Santa Catarina (UFSC).
REFERENCES
1. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2014. J Hepatol. 2014;61:373-95. [ Links ]
2. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-93. [ Links ]
3. Pena SD, Di Pietro G, Fuchshuber-Moraes M, Genro JP, Hutz MH, Kehdy Fde S, et al. The genomic ancestry of individuals from different geographical regions of Brazil is more uniform than expected. PLoS One. 2011;6:e17063. [ Links ]
4. Martins T, Machado DF, Schuelter-Trevisol F, Trevisol DJ, Vieira e Silva RA, Narciso-Schiavon JL, et al. Prevalence and factors associated with HCV infection among elderly individuals in a southern Brazilian city. Rev Soc Bras Med Trop. 2013;46:281-7. [ Links ]
5. Silveira L, Schiavon L de L, Silva KP, Lopes TB, Zaccaron Mda R, Narciso-Schiavon JL. Clinical and epidemiological profile of blood donors with positive serology for viral hepatitis in southern Brazil. Rev Soc Bras Med Trop. 2011;44:269-73. [ Links ]
6. Zeuzem S, Feinman SV, Rasenack J, Heathcote EJ, Lai MY, Gane E, et al. Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med. 2000;343:1666-72. [ Links ]
7. Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-65. [ Links ]
8. Hadziyannis SJ, Sette H Jr., Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346-55. [ Links ]
9. Vigani AG, Goncales ES, Pavan MH, Genari F, Tozzo R, Lazarini MS, et al. Therapeutic effectiveness of biosimilar standard interferon versus pegylated interferon for chronic hepatitis C genotypes 2 or 3. Braz J Infect Dis. 2012;16:232-6. [ Links ]
10. Narciso-Schiavon JL, Schiavon L de L, Carvalho-Filho RJ, Sampaio JP, Batah PN, Barbosa DV, et al. Gender influence on treatment of chronic hepatitis C genotype 1. Rev Soc Bras Med Trop. 2010;43:217-23. [ Links ]
11. Silva GF, Polonio RJ, Pardini MI, Corvino SM, Henriques RM, Peres MN, et al. Using pegylated interferon alfa-2b and ribavirin to treat chronic hepatitis patients infected with hepatitis c virus genotype 1: are non-responders and relapsers different populations? Braz J Infect Dis. 2007;11:554-60. [ Links ]
12. de Almeida PR, de Mattos AA, Amaral KM, Feltrin AA, Zamin P, Tovo CV, et al. Treatment of hepatitis C with peginterferon and ribavirin in a public health program. Hepatogastroenterology. 2009;56:223-6. [ Links ]
13. Di Marco V, Covolo L, Calvaruso V, Levrero M, Puoti M, et al. Who is more likely to respond to dual treatment with pegylated-interferon and ribavirin for chronic hepatitis C? A gender-oriented analysis. J Viral Hepat. 2013;20:790-800. [ Links ]
14. Kwo PY, Lawitz EJ, McCone J, Schiff ER, Vierling JM, Pound D, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376:705-16. [ Links ]
15. Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-16. [ Links ]
16. Poordad F, McCone J Jr., Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-206. [ Links ]
17. Crespo J, Berenguer M, Perez F, Fernandez I, Gonzalez O, Barcena R, et al. Lead-in period and week 8 as predictive tools for response to boceprevir therapy: a retrospective study of Spanish real clinical practice. Gastroenterol Hepatol. 2015. Pii:S0210-5705(15)00100-4. [ Links ]
18. Vo KP, Vutien P, Akiyama MJ, Vu VD, Ha NB, Piotrowski JI, et al. Poor sustained virological response in a multicenter real-life cohort of chronic hepatitis C patients treated with pegylated interferon and ribavirin plus telaprevir or boceprevir. Dig Dis Sci. 2015;60:1045-51. [ Links ]
19. Ministério da Saúde/CONITEC. Simeprevir, sofosbuvir e daclatasvir no tratamento da hepatite crônica tipo C e coinfecções. Brasil: Ministério da Saúde/CONITEC; 2015. [ Links ]











 text in
text in