Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.30 no.3 Bogotá July/Sept. 2015
A Case of Duodenal Leiomyosarcoma
Rafael Parra Medina MD. (1), Paula Moreno L. (2), Julián Jiménez M. (2), Marcela Sánchez R. MD. (3), Edgardo Yaspe C. MD. (4), Patricia López C. MD. (5)
(1) Medical Epidemiologist and Pathology Resident at the Fundación Universitaria de Ciencias de la Salud in Bogotá, Colombia.
(2) Medical Students in the Semilleros de Investigación de Patología Program at the Fundación Universitaria de Ciencias de la Salud in Bogotá, Colombia.
(3) Pathology Resident at the Fundación Universitaria de Ciencias de la Salud in Bogotá, Colombia.
(4) Oncologist and Pathologist at the Hospital Infantil de San José in Bogotá, Colombia.
(5) Pathologist at the Hospital Infantil de San José in Bogotá, Colombia.
Received: 16-01-15 Accepted: 21-07-15
Abstract
Duodenal leiomyosarcoma is a rare disease with poor prognoses for patients. The disease does not have a clear set of signs and symptoms that allows easy diagnosis. Diagnosis is made on the basis of histopathological identification of a mesenchymal lesion composed of malignant tumor cells and immunohistochemistry positive for smooth muscle actin, desmin, muscle specific actin, calponin and caldesmon. We report a case of a 56 year old woman with primary duodenal leiomyosarcoma which had metastasized to the pancreas and the celiac artery.
Keywords
Leiomyosarcoma, duodenum, small intestine, mesenchymal tumors.
INTRODUCTION
Gastrointestinal mesenchymal tumors are a group of neoplasms which have similar histologic features but have different patterns of immunohistochemistry (1, 2). Gastrointestinal stromal tumor (GIST) tumors are the most common mesenchymal neoplasms that occur in the gastrointestinal tract. They are followed by smooth muscle and neural tumors (1).
Gastrointestinal smooth muscle tumors are rare. Arising in the muscle of the mucosa, they are most commonly located in the esophagus and then in the colon and rectum (3). These tumors are divided into leiomyomas which are benign and leiomyosarcoma tumors which are malignant tumors and rarer (3). Gastrointestinal leiomyosarcomas occur most commonly in the stomach. This type of tumor in this location accounts for 0.1% to 3% of all gastric tumors (4). The next most common location is the small intestine (5). Within the small intestine the most common locations are the jejunum and ileum with less frequent occurrence in the duodenum (6).
We report a case of a 56 year old woman who was diagnosed with primary leiomyosarcoma of the duodenum, and include a literature review of cases reported with adequate histopathological diagnoses.
CASE REPORT
The patient was a 56 year old woman with a history of non-insulin dependent diabetes mellitus, hypertension and hypercholesterolemia. She came to the hospital after 20 days of epigastric pain associated with watery stools, emesis, fatigue and weight loss.
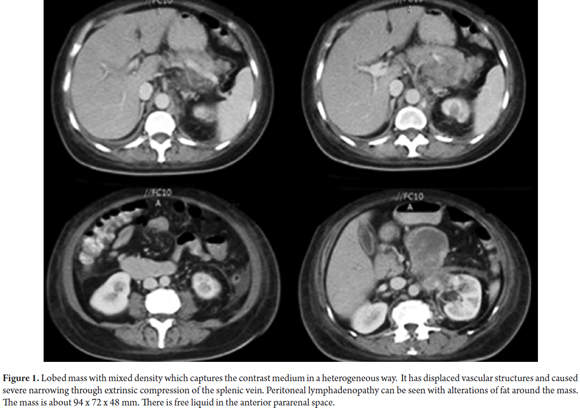
An abdominal CAT scan showed concentric thickening of the wall of the third portion of the duodenum wall together with a lesion with irregular margins measuring approximately 94mm x 72mm x 48mm that extended into the periduodenal tissue. In addition, multiple round and oval hypodense lesions were found in the topography of the celiac trunk (Figure 1). Complementary esophagogastroduodenoscopy (EGD) and endoscopic ultrasound (EUS) showed stenosis in the third portion of the duodenum.
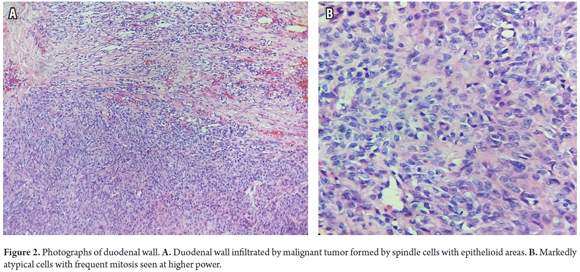
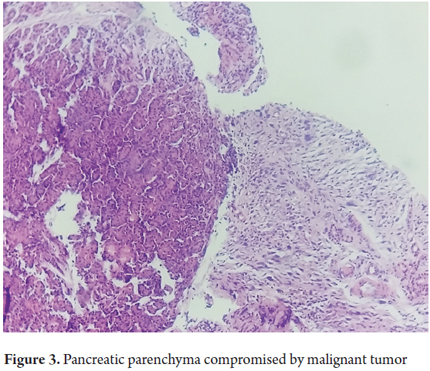
Histopathology of an excisional biopsy of the duodenal mass revealed a malignant mesenchymal tumor composed of epithelioid and fusiform areas (Figure 2). The tumor had markedly atypical with cytopathology with a mitotic count of 27 in 10 high power fields and necrosis compromising 20% of its area. An additional pancreatic biopsy showed that it was also compromised by a tumor (Figure 3).
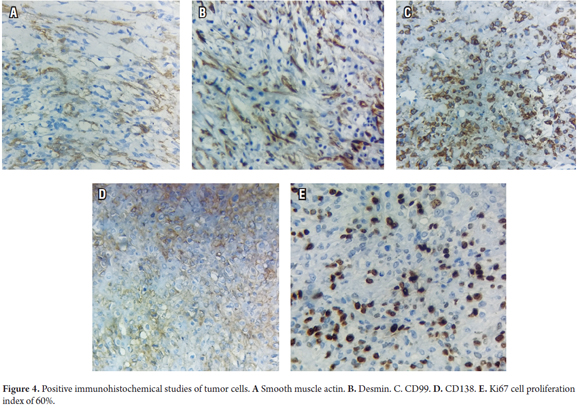
Immunohistochemistry of spindle cell areas was predominantly positive for smooth muscle actin, desmin, calponin and keratins. In addition, epithelial and spindle cell areas were also positive for CD 99 and CD138 (Figure 4), and positive for diffuse BCL2, with a proliferation index of 60% for Ki67. In addition, tests were negative for HMB45, S100, CD117, DOG1, CD34, EMA, INI1, and TL1.
The patient was treated for her comorbidities and remained hospitalized for twenty days at which time she passed away.
DISCUSSION
Gastrointestinal mesenchymal tumors are a group of neoplasms that arise from the stromal elements of the wall of the gastrointestinal tract. While they are histologically similar, the immunohistochemical profiles of the different types of gastrointestinal mesenchymal tumors vary (1). These tumors include GISTs, smooth muscle tumors, glomus tumors, schwannomas, inflammatory myofibroblastic tumors, plexiform fibromyxomas, synovial sarcomas, and Kaposi's sarcomas (7). These tumors are characterized by specific anatomical regions although they may not be exclusive to those regions (8).
The first reported case of primary leiomyosarcoma of the duodenum was published in 1920 by Von Salis (9). About 170 cases have been reported in the medical literature (6, 10-13). Cases of duodenal leiomyosarcomas that have metastasized to the corpus uteri have also been reported (14, 15).
Duodenal leiomyosarcomas most frequently occur in the sixth decade of life and are slightly more common among men than among women. The most common location is in the second portion of the duodenum (16). Like other sarcomas, these tumors can disseminate through the blood, especially to the lungs and liver. Lymphatic dissemination can also occur and has been described primarily to the pancreas, mesocolon and transverse colon (17). Metastases to the skin and skeletal muscles have also been reported (6, 18). Patients with this disease have poor prognoses: the five year survival rate is 5% while the ten year survival rate is 0.0% (19, 20). One case of reoccurrence six years after treatment and two years free of tumors has been reported (10).
The clinical diagnosis is complex because the symptoms are not specific. Symptoms include abdominal pain, gastrointestinal tract bleeding, intestinal obstruction, weight loss, jaundice, emesis, fever, diarrhea, anemia, palpable masses, and fistulas (10, 16). Diagnoses using the various endoscopic techniques are not always conclusive (5), and even though CT scans and MRIs may show masses in the duodenum, a definitive diagnosis must be made through histopathological examination, specifically by immunohistochemistry (2).
Macroscopically, leiomyosarcomas are characterized by their irregular appearances that are lobed with sectors of necrosis (21). Microscopically these tumors are clearly distinguished from benign analogs by high cellularity and their cytological features (3). The morphologic spectrum ranges from fusiform to epithelioid variants (1). Tumor cells are characterized by eosinophilic cytoplasm and hyperchromatic elongated nuclei and nuclear pleomorphism (19). Mitotic activity is often greater than 50 mitoses per 10 high power fields. Mitotic counts over 10 in 10 high power fields are associated with tumor size. Compromised tissue at deeper levels and metastases indicate poor prognoses (19, 20).
The immunohistochemistry of leiomyosarcomas is positive for smooth muscle actin, desmin, muscle specific actin, caldesmon, calponin and CD34. In both epithelioid and spindle cell areas, fifty to sixty percent are positive for CD 138, twenty-five percent are positive for calretinin (25%), and thirty percent are positive for CD 99. They rarely test positive for positive for CD117 or HMB45, but this can be seen in epithelioid areas (22, 23). Some cases also test positive for cytokeratins (23).
Differential diagnosis is primarily concerned with GISTs since they are the most common mesenchymal tumors. This requires immunohistochemistry in which over 95% of these tumors positively express CD 117 and DOG1 (24).
In 1935 Whipple described a pancreatoduodenectomy for treatment of duodenal neoplasms (6). Nevertheless, there is no current consensus on appropriate surgical treatment for these tumors. Treatment options include tumor excision, segmental resection of the duodenum and pancreatoduodenectomy. Management with radiation therapy has demonstrated therapeutic utility locally, but has not been proven to have an impact on long-term survival (17, 19).
REFERENCES
1. Miettinen M, Lasota J. Gastrointestinal stromal tumors - definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438(1):1-12. [ Links ]
2. Yamamoto H, Handa M, Tobo T, Setsu N, Fujita K, Oshiro Y, et al. Clinicopathological features of primary leiomyosarcoma of the gastrointestinal tract following recognition of gastrointestinal stromal tumours. Histopathology. 2013;63(2):194-207. [ Links ]
3. Miettinen M, Sarlomo-Rikala M, Sobin LH, Lasota J. Esophageal stromal tumors: A clinicopathologic, immunohistochemical, and molecular genetic study of 17 cases and comparison with esophageal leiomyomas and leiomyosarcomas. Am J Surg Pathol. 2000;24(2):211-22. [ Links ]
4. Weledji EP, Enoworock G, Ngowe MN. Gastric leiomyosarcoma as a rare cause of gastric outlet obstruction and perforation: A case report. BMC Res Notes. 2014;7:479. [ Links ]
5. Jabr FI, Skeik N. A leiomyosarcoma of the small bowels causing obscure gastrointestinal bleeding diagnosed by capsule endoscopy. J Med Liban. 2010;58(4):238-40. [ Links ]
6. Cappellani A, Di Vita M, Lo Menzo E, Zanghì A, Lanzafame S, Veroux P, et al. Muscular metastasis from mesocolic and duodenal leiomyosarcoma. A case report and a review of the literature. Ann Ital Chir. 2011;82(5):383-7. [ Links ]
7. Bosman FT, World Health O, International Agency for Research on C. WHO classification of tumours of the digestive system. Lyon: International Agency for Research on Cancer; 2010. [ Links ]
8. Voltaggio L, Montgomery EA. Gastrointestinal tract spindle cell lesions-just like real estate, its all about location. Mod Pathol. 2015;28(Suppl 1):S47-66. [ Links ]
9. von Salis HW. Uber das Sarkom des Duodenum insbesondere das Myosarkom. Deut Z Chirurgie. 1920;160(3-4):180-204. [ Links ]
10. Colović R, Micev M, Zogović S. Leiomyosarcoma of the duodenum. A successful reoperation after recurrency. Acta Chir Iugosl. 2001;48(2):45-8. [ Links ]
11. Petralia GA, Hansen PD, Bowyer RC, Williamson RC. Duodenal leiomyosarcoma. Dig Surg. 1999;16(1):22-5. [ Links ]
12. Miettinen M, Kopczynski J, Makhlouf HR, Sarlomo-Rikala M, Gyorffy H, Burke A, et al. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum: A clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. Am J Surg Pathol. 2003;27(5):625-41. [ Links ]
13. Kefalas CH, Altrabulsi B, Milvenan JS, Goldschmiedt M. Endoscopic electrosurgical snare resection of leiomyosarcoma of main duodenal papilla. Gastrointest Endosc. 2004;59(6):743-5. [ Links ]
14. Patel JK, Cervellione KL, Sulh M, Patel AA, Gintautas J. A rare case of uterine leiomyosarcoma metastasis to the duodenum. Proc West Pharmacol Soc. 2009;52:8-10. [ Links ]
15. Msakni I, Bouraoui S, Ben slama S, Lahmar-boufaroua A, Goutallier ben fadhel C, Ben sassi L, et al. Duodenal metastatic leiomyosarcoma of the uterus. A case report. Ann Chir. 2005;130(9):584-6. [ Links ]
16. Olurin EO, Solanke TF. Case of leiomyosarcoma of the duodenum and a review of the literature. Gut. 1968;9(6):672-7. [ Links ]
17. Jerraya H, Guirat A, Frikha F, Beyrouti I. Leiomyosarcoma of the duodeno-jejunal angle: Two case reports and literature review. SS Surgical Science. 2013;4(7):313-6. [ Links ]
18. Corcoran S, Hogan AM, Nemeth T, Bennani F, Sullivan FJ, Khan W, et al. Isolated cutaneous metastasis of uterine leiomyosarcoma: Case report and review of literature. Diagn Pathol. 2012;7:85. [ Links ]
19. Serrano C, George S. Leiomyosarcoma. Hematol Oncol Clin North Am. 2013;27(5):957-74. [ Links ]
20. Aggarwal G, Sharma S, Zheng M, Reid MD, Crosby JH, Chamberlain SM, et al. Primary leiomyosarcomas of the gastrointestinal tract in the post-gastrointestinal stromal tumor era. Ann Diagn Pathol. 2012;16(6):532-40. [ Links ]
21. Shenoy S. Primary small-bowel malignancy: update in tumor biology, markers, and management strategies. J Gastrointest Cancer. 2014;45(4):421-30. [ Links ]
22. Fenoglio-Preiser CM. Gastrointestinal pathology: An atlas and text. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2008. [ Links ]
23. Dabbs DJ. Diagnostic Immunohistochemistry. 4th edition. London: Elsevier Health Sciences; 2013. [ Links ]
24. Corless CL. Gastrointestinal stromal tumors: What do we know now? Mod Pathol. 2014;27(Suppl 1):S1-16. [ Links ]











 text in
text in