Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.30 no.4 Bogotá oct./dic. 2015
Chronic Kidney Disease in Liver Transplant Patients in the Hospital Pablo Tobón Uribe 2005-2013
John Fredy Nieto Ríos MD. (1), Lina María Serna Higuita MD. (1), Juan David Vélez Rivera MD. (2), Henry Armando Giraldo Salazar (3), Jhonathan Ferney Vélez Morales (3), Verónica Pérez Guerra (3), Laura Ramírez Cardona (3), Oscar Mauricio Yepes Grajales (3), Juan Ignacio Marín Zuluaga MD. (4), Octavio Muñoz Maya MD. (4), Oscar Santos Sánchez MD. (4), Carlos Enrique Yepes MD. (5), Juan Carlos Restrepo MD (4)
(1) Nephrology and Renal Transplantation at the Hospital Pablo Tobon Uribe and the University of Antioquia in Medellín, Colombia.
(2) Hepatology Fellow at the University of Antioquia in Medellín, Colombia.
(3) Medical Students at the University of Antioquia in Medellín, Colombia.
(4) Hepatology and Liver Transplantation at the Hospital Pablo Tobon Uribe and member of the Gastrohepatology Group at the University of Antioquia in Medellín, Colombia.
(5) Clinical Epidemiologist at the Hospital Pablo Tobon Uribe and the University of Antioquia in Medellín, Colombia.
Received: 20-03-15 Accepted: 10-10-15
Abstract
Advances in immunosuppressive therapy have revolutionized long-term results of liver transplantation, but this has increased the prevalence of chronic kidney disease (CKD). Some risk factors including diabetes and hypertension are associated with the use of calcineurin inhibitors. The objective of this study is to determine the incidence of CKD in liver transplant patients at the Hospital Pablo Tobon Uribe from 2005 to 2013 and then assess associated complications.
Methods: This is a retrospective cohort study.
Results: This study evaluated 215 patients with liver transplants. Average patient age at transplant was 50.37 years (SD +/- 12.6), and 42.8% of the patients were women. Kidney replacements were required by 3.3% of the patients within the first month after liver transplantation. The most frequently used immunosuppressive therapy was cyclosporine which was used for 90.7% of the patients. During follow-up, the filtration rate decreased over time with a median of 86.2ml/min/1.73 (SD +/- 25.9) at transplant. This reached 74.2ml/min/1.73 (SD +/- 24.5) at 3 years follow-up. The rate of deterioration of renal function determined by generalized estimated equations was 3.5ml/year (95% CI 2.44 to 4.74, p = 0.000). At the time of liver transplantation 16.3% of patients had glomerular filtration rates of less than 60ml/min. After three years of follow-up, 29.6% of patients had glomerular filtration rates of less than 60ml/min. By grouping complications such as the presence or absence of renal dysfunction at follow-up, we found that, except for death, the presence of cerebrovascular disease and coronary heart disease was similar in both groups.
Conclusion: CKD is a frequent complication in liver transplant patients. Our recommendation is frequent monitoring of kidney damage markers.
Keywords
Liver transplantation, chronic kidney disease, glomerular filtration rate, calcineurin inhibitors.
INTRODUCTION
Chronic kidney disease (CKD) is characterized by a progressive and irreversible loss of kidney function. The new Kidney Disease | Improving Global Outcomes (KDIGO) 2013 guideline classify the disease according to the glomerular filtration rate and the albuminuria level (1). CKD occurs with increasing frequency in patients who have received a liver transplant because of the longer life expectancy of these patients (2). Approximately 10% to 20% of liver transplant patients develop renal insufficiency in the first five years after transplantation (3, 4), but the proportion is as high as 45% in longer term follow-ups (5-11). This wide range is due to the different definitions that have been used in studies of CKD, the different methods used to measure creatinine, the various techniques for calculating creatinine clearance and particular conditions of patients such as muscle mass.
The principal causes of CKD following liver transplantation are pre-transplant kidney disease, diabetes, hypertension, hepatitis C, perioperative acute renal damage and the use of nephrotoxic drugs. Prolonged use of calcineurin inhibitors has improved the prognosis for liver transplantation over the last 30 years, but these drugs can cause nephrotoxicity secondary to decreased renal blood flow, and this can lead to tubular atrophy, interstitial fibrosis, arteriolar hyalinosis which together cause progressive CKD (2, 12, 13).
Once liver transplant patients who have developed CKD begin renal replacement therapy the rates of morbidity and mortality increase and life expectancy becomes very short (13). In Latin America there are few reports on the behavior of renal functioning after liver transplantation (3). The aim of our study is to determine the frequency of chronic kidney disease in liver transplant patients at the Pablo Tobon Uribe (HPTU) Hospital between 2005 and 2013 and assess complications associated with the disorder.
METHODOLOGY
This is a retrospective study of a cohort of patients who received liver transplants from 2005 to 2013. Patients under 18 years of age, patients who had had combined kidney and liver transplants, and patients who had not had a minimum of three month follow-up were excluded. In addition, patients for whom sufficient data for statistical analysis was not available were also excluded.
The immunosuppressive regimen began with a 1000 bolus with 500 mg of methylprednisolone on the first day followed by a 1000 bolus with 250 mg of methylprednisolone on the second day. Beginning on the third day, a regimen of three daily doses one mg of prednisone per kg of patient weight began with progressively diminishing doses over the first month until a dose of 10 mg per day was reached. Cyclosporine was used for additional maintenance therapy starting with a dose of 10 mg/kg/day. This was diminished to C2 levels of 1000 to 1200 ng/ml during the first three months and then to 800 ng/ml after that. Alternate treatments included tacrolimus, azathioprine and sirolimus. Tacrolimus was used for additional maintenance therapy beginning at a dose of 0.1 mg/k/day which was diminished until C0 levels of 8-12 ng/ml were reached over the first three months and then further reduced to levels of 5-8 ng/ml. Azathioprine was administered at doses of 100 mg/day, and in some patients sirolimus was used at a dose of 3-5 mg/day. Neither monoclonal nor polyclonal antibodies were used.
All clinical data were extracted from the HPTU liver transplant group database. Clinical parameters evaluated included gender, age, etiology of liver disease, comorbidities, immunosuppressive therapy used, and complications associated with transplants. These included arterial hypertension (AH), dyslipidemia, diabetes, chronic occlusive arterial disease and death. The need for renal replacement therapy during the postoperative period and after transplantation was also evaluated. The main outcome evaluated was the glomerular filtration rate (GFR) which was assessed at transplant and during follow-up. GFR was calculated using the CKD-EPI equation (14).
The most important finding was the prevalence of loss of kidney function which was defined as GFR of less than 60 ml/min/1.73 m2. The KDIGO 2013 guideline definitions were used for classification of stages of CKD. Stage 1 of CKD is defined as GFR > 90 ml/min/1.73 m2 plus the presence of markers for kidney damage. Stage 2 of CKD is defined as GFR of 60-90 ml/min/1.73 m2 plus markers of kidney damage. Stage 3a of CKD is defined as GFR of 45-59 ml/min/1.73 m2. Stage 3b of CKD is defined as GFR of 30-44 ml/min/1.73 m2. Stage 4 of CKD is defined as GFR of 15-29 ml/min/1.73 m2, and stage 5 of CKD is defined as GFR of less than 15 ml/min/1.73 m2 (1).
The descriptive analysis of the data used calculations of frequencies and proportions for qualitative variables while quantitative variables were described as means or medians with their standard deviations or quartiles in accordance with data distributions identified by the Shapiro Wilk test. For evaluations between qualitative variables and acute kidney injury (AKI) the chi2 or Fisher's exact test was used with a significance level of 0.05. Comparisons of quantitative variables used the Student's t Test when they met assumed standard of normality and the Mann-Whitney test when variables did not meet this assumption. SPSS 18 and STATA 12 were used for these analyses.
The glomerular filtration rate was evaluated as a continuous variable during follow-up in order to determine decreases over time. GFR was assessed at transplant and at one, three, six, twelve, twenty-four and thirty-six months after transplant. These repeated measurements over time were analyzed using generalized estimating equations (GEE). An exchangeable correlation structure is assumed to obtain an estimated average change in GFR over time. In addition, patients were pooled according to stages of CKD and the percentage of patients was analyzed at each stage of CKD during follow-up.
In the second part of the analysis, a logistic regression model was used in STATA to analyze the relationship between renal dysfunction and several covariates chosen from the literature review and variables that showed statistical significance in the bivariate analysis. Maximum likelihood coefficients which express the maximum probability of the dependent variable were calculated using the Newton Raphson iterative method. Goodness of fit was assessed with the Hosmer-Lemeshow test, and the statistical significance of the regression coefficients produced by the model were evaluated with the Wald test.
This study was approved by the HPTU ethics committee and followed Resolution 008430 of 1993 the Ministry of Social Protection of the Republic of Colombia which regulates research involving human subjects. The researchers agreed to respect the confidentiality and privacy of the information contained in the medical records. This work involved no risks to participants because it involved no interventions in the study population such as direct physical examinations, laboratory testing and treatments.
RESULTS
Baseline Characteristics
From 2005 to 2013, 269 liver transplants were performed at the HPTU. Of these 215 patients had a minimum follow-up period of three months and a maximum of 121 months. Average age at transplant was 50.37 years (SD +/- 12.6); 123 of the patients 57.2% were men, 37.2% (80) were hypertensive, 22.8 (49) had diabetics, 17.7% (38) had dyslipidemia, and 3.3% (7) of the patients required renal replacement therapy during the first month after transplantation. The average GFR before transplantation was 86.3 ml/min/1.73 m2 (SD 25.89). At the moment of transplantation, 16.7% (36) of the patients had GFRs of less than 60 ml/min/1.73 m2.
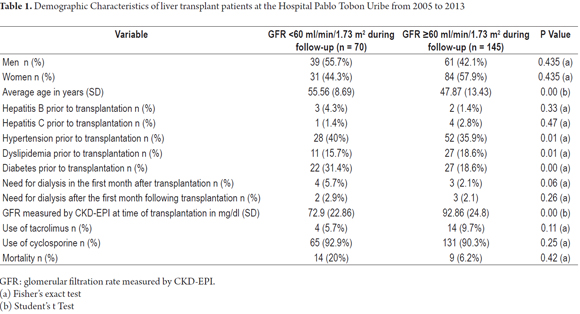
Table 1 shows patients' demographic characteristics grouped according to the presence or absence of renal injury at the end of follow-up. As can be seen, the only differences found are related to patient age with younger patients accounting for all those with GFRs over 60 ml/min/1.73 m2. Hypertension and diabetes were also more frequent in patients with renal insufficiency. At the moment of transplantation, GFRs were lower in patients who later had kidney injuries during follow-up.
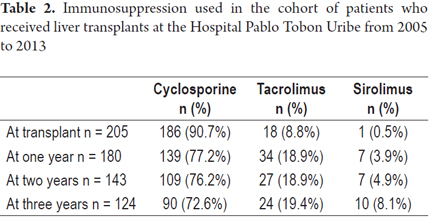
Cyclosporine was used to treat 90.7% of the patients and was by far the most commonly immunosuppressive therapy during the first year after transplantation. It was followed by tacrolimus which was administered to 8.8% of the patients and sirolimus which was administered to 0.5% of the patients. In the third year after transplantation use of tacrolimus increased to include 19.4% of the patients (Table 2).
Evolution of renal functioning during follow-up
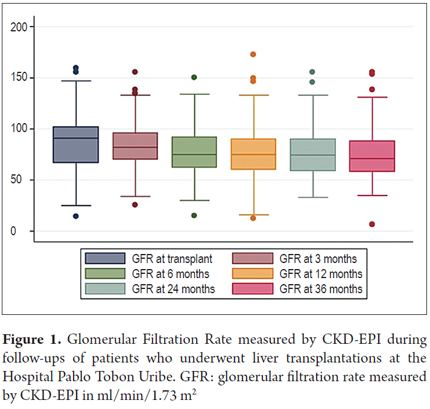
During follow-up the glomerular filtration rate decreased over time starting with an average of 86.2 ml/min/1.73 m2 (SD +/- 25.9) at transplant and decreasing to 74.2 ml/min/1.73 m2 3 (SD +/- 24.5) at 3 years of follow-up (Figure 1).
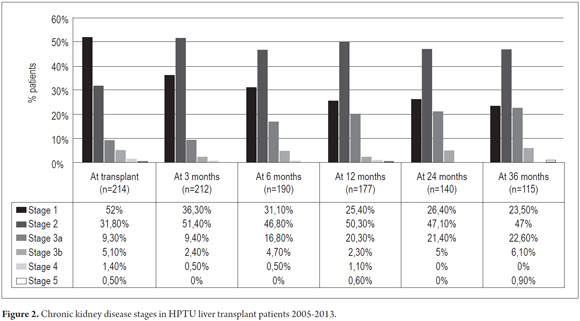
Renal functioning showed a decrease in glomerular filtration rate of 3.5 ml/year (95% CI 2.44 to 4.74, p = 0.000). At the time of liver transplantation, 39 patients (16.3%) had GFRs of less than 60 ml/min. This increased to 34 patients (29.6%) at three years of follow up. At three years of follow-up there were less patients in CKD stage 1 and more patients in CKD stages 2 and 3 (Figure 2).
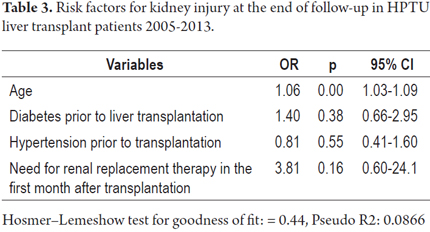
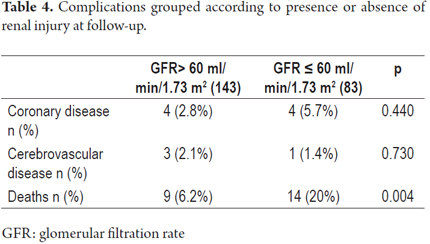
Of the total number of patients, seven (3.25%) required renal replacement therapy during the first month after transplantation. Of these, four (57.1%) had GFRs of less than 60 ml/min/1.73 m2 at the end of follow-up. Of the patients who did not require renal replacement therapy during the first month after transplantation, sixty-six (31.7%) had GFRs of less than 60 ml/min/1.73 m2 at the end of follow-up (Table 3). However, this difference was not statistically significant (p = 0.218). Logistic regression analysis of renal injury at the end of follow-up showed that the only independent predictor of chronic kidney disease was age.
Complications found
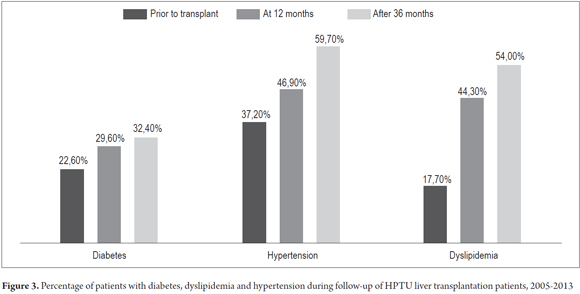
The percentages of patients with diabetes, hypertension and dyslipidemia increased during the first three years after transplantation (Figure 3). Coronary disease occurred in eight patients (3.7%), cerebrovascular disease in four patients (1.9%), chronic occlusive arterial disease in one patient (0.4%) and twenty-three patients died (10.7%). The causes of death were infections in six patients, malignancies in three patients, renal injuries in two patients, chronic rejection in two patients, cardiac complications in two patients, pulmonary complications in two patients, recurrence of cholangiocarcinoma in one patient, portal vein thrombosis in one patient and four patients with unlisted causes of death.
Complications can also be grouped according to presence or absence of renal dysfunction at follow-up. We found that except for death, the presence of cerebrovascular disease and coronary heart disease was similar in both groups (Table 4).
Survival curves
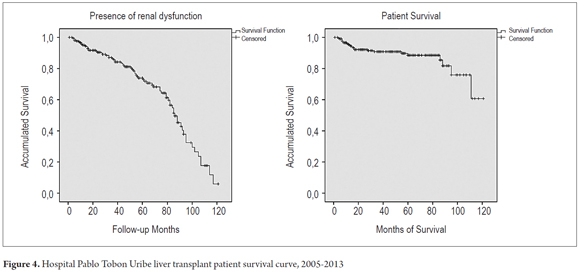
The presence of renal impairment defined as GFR less than 60 ml/min/1.73 m2 was evaluated using a Kaplan Meier curve: 98.1% of Patients had GFRs greater than 60 ml/min/1.73 m2 at 6 months, 95% at one year, 86.4% at three years and 74% at five years, and 50% of patients had GFR≤ 60 ml/min/1.73 m2 at 84 months of follow up. The patient survival rate at six months after liver transplantation was 99%, at one year it was 95.5%, at three years it was 91%, and at five years it was 88.5%. Mortality occurred in 23 patients (10.7%) (Figure 4).
DISCUSSION
The survival times of liver transplant patients has increased in recent years. This is mostly due to improvement of technical factors related to the surgical procedure, improvement of management in intensive care units, and safer immunosuppressive regimens. However, one factor that negatively impacts survival in these patients is chronic kidney disease which may be present before transplantation or which can develop later as the result of several different causes (10, 15). CKD increases the risk of complications in the short and long term. This study evaluates the incidence of CKD in liver transplant patients at our institution and classifies CKD stages according to GFR measured by the formula recommended by KDIGO.
The incidence of CKD varies greatly and depends on the definition and the methods used for determining GFR. The incidence of CKD varies from 18% to 45% in 10-year follow-ups (3, 11). The principal finding of our study was a high prevalence of chronic kidney disease in liver transplant patients at follow-up. On average, there was a decline in glomerular filtration rates of 3.5 ml per year which can be compared to the general population in which GFRs decrease by 0.5 ml to 1 ml/min/year from age 40.
Before liver transplantation average GFR was 91 ml/min/1.73 m2, and 16.9% of patients were classified as having chronic renal insufficiency. The cumulative rates of chronic kidney disease in this cohort was 1.9% at 6 months, 5% at 1 year, 13.6% at 3 years, and 26% at 5 years. This is comparable to the findings of studies such as Allen et al. in which the prevalence at 5 years was 27% (2), but lower than rates found by studies such as Giusto et al. in which a rate of 45% at 5 years was reported. Akinlolu et al. have described CKD incidence in liver transplant patients of 13.9% at 3 years and 18.1% at 5 years (11). However, it is noteworthy that GFR was measured in different ways in these studies. In some studies, it was measured by a formula, but others such as the study by Allen et al. performed measured GFR with ethanolamine (2). That study showed that when comparing standard GFR measures and measurement using ethanolamine, the incidence of CKD was far lower in those measured with the standard formula (2).
Factors associated with low GFRs found in the bivariate analysis were age, hypertension, diabetes, dyslipidemia and GFR at the time of transplantation. Average initial GFR was 92.8 ml/min/1.73 m2 in patients with GFR greater 60 ml/min/1.73 m2 at the end of monitoring and 72.9 ml/min/1.73 m2 in those with GFR less than 60 ml/min/1.73 m2 at the end of follow-up. However, in the multivariate analysis only age was correlated with the risk of CKD with GFR less than 60 ml/min/1.73 m2 at follow-up. Giusto et al. found that factors associated with the onset of CKD included prior renal dysfunction, hypertension, diabetes and severe infections (11). Allen et al. reported that the only factor associated with the development of CKD was of perioperative AKI (2).
The hepatitis C virus has been described as a risk factor associated with CKD after liver transplantation (4). In this study no significant relationship between hepatitis C viral infections and CKD was demonstrated. However, this can be explained by the low proportion in our series of patients transplanted because of hepatitis C in the time period evaluated.
Post-transplant CKD is not only important because of the potential need for renal replacement therapy, but because it increases cardiovascular risks, and increases the frequency of hypertension, dyslipidemia and diabetes. When added to the effects of calcineurin inhibitors and steroids, the implication is that it is important to optimize monitoring of renal functioning during and after liver transplantation (4). Previous studies suggest that elderly patients are at increased risk of CKD, so it is important to use lower doses of calcineurin inhibitors (3). Our study found that cardiovascular risk factors like hypertension, diabetes and dyslipidemia increase during the first and third years following transplant. Increased incidence of hypertension, diabetes and dyslipidemia may be associated with higher doses of calcineurin inhibitors used in this post-transplant stage. In addition to the association with steroids, there is also increased risk of insulin resistance and hypertension. In our study, the presence of cardiovascular disease was not significantly different between the two groups, but mortality was significantly higher in the group with less than 60 ml/min/1.73 m2 GFR.
Among the limitations of this study it is that markers of kidney damage such as albuminuria were not identified through urinalysis, ultrasound, or biopsies even though they are important for determining the early stages of CKD. Another important limitation is the calculation of GFR by formulas which is not well validated in the transplant population. In addition, calculation of GFR before liver transplantation is inaccurate because of the clinical condition of cirrhotic patients who have low muscle masses and suffer from malnutrition with consequent low levels of creatinine which cause overestimation of GFRs. An additional difficulty of this study was the loss of patients during long-term monitoring which did not allow for a proper assessment of the incidence of CKD over time.
CONCLUSION
Chronic kidney disease is a frequently occurring serious complication in liver transplant patients. It is an independent prognostic factor for morbidity and mortality. The development of this complication is related to comorbidities such as hypertension and diabetes, to the use of nephrotoxic drugs, and to perioperative acute kidney injury. The authors recommend a full renal assessment before liver transplantation, consideration of combined kidney-liver transplantation in cases of advanced CKD, limitation of the use of nephrotoxic drugs, protection of the kidney from iodinated contrast agents, calculation of GFR at each follow-up appointment, frequent monitoring of markers of kidney damage and early evaluation by a nephrology group for patients in whom abnormalities have been detected.
Acknowledgements
The authors wish to thank the Sustainability Project of the Office of the Vice-Rector for Research of the University of Antioquia.
REFERENCES
1. Founding KDIGO Co-Chairs. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013;3(1):1-136. [ Links ]
2. Allen AM, Kim WR, Therneau TM, Larson JJ, Heimbach JK, Rule AD. Chronic kidney disease and associated mortality after liver transplantation--a time-dependent analysis using measured glomerular filtration rate. J Hepatol. agosto de 2014;61(2):286-92. [ Links ]
3. Smoter P, Nyckowski P, Grat M, Patkowski W, Zieniewicz K, Wronka K, et al. Risk Factors of Acute Renal Failure After Orthotopic Liver Transplantation: Single-center Experience. Transplant Proc. Elsevier Inc. 2014;46(8):2786-9. [ Links ]
4. Bahirwani R, Shaked O, Kurd S, Bloom R, Reddy KR. Chronic Kidney Disease After Orthotopic Liver Transplantation : Impact of Hepatitis C Infection. Clin Transl Res. 2011;91(11):1245-9. [ Links ]
5. Gonwa T, Mai M, Melton L, Hays S, Goldstein R, Levy M. Endstage renal disease (ESRD) after orthotopic liver transplantation (OLTX) using calcineurin-based immunotherapy: risk of development and treatment. Transplantation. 2001;72:1934-9. [ Links ]
6. Burra P, Senzolo M, Masier A, Prestele H, Jones R, Samuel D. Factors influencing renal function after liver transplantation. Results from the MOST, an international observational study. Dig Liver Dis. 2009;41:350-6. [ Links ]
7. Sharma P, Welch K, Eikstadt R, Marrero J, Fontana R, Lok A. Renal outcomes after liver transplantation in the model for end-stage liver disease era. Liver Transpl. 2009;15:1142-8. [ Links ]
8. LaMattina J, Mezrich J, Fernandez L, D'Alessandro A, Djamali A, Musat A, et al. Native kidney function following liver transplantation using calcineurin inhibitors: single-center analysis with 20 years of follow-up. Clin Transpl. 2013;27:193-202. [ Links ]
9. Moreno J, Cuervas-Mons V, Rubio E, Pons F, Herreros T, Turrion V, et al. Chronic renal dysfunction after liver transplantation in adult patients: prevalence, risk factors, and impact on mortality. Transpl Proc. 2003;35:1907-8. [ Links ]
10. Fisher N, Nightingale P, Gunson B, Lipkin G, Neuberger J. Chronic renal failure following liver transplantation: a retrospective analysis. Transplantation. 1998;66:59-66. [ Links ]
11. Giusto M, Berenguer M, Merkel C, Aguilera V, Rubin A, Ginanni Corradini S, et al. Chronic kidney disease after liver transplantation: pretransplantation risk factors and predictors during follow-up. Transplantation. 2013;95(9):1148-53. [ Links ]
12. Nacif LS, David AI, Pinheiro RS, Diniz MA, Andraus W, Cruz Junior RJ, et al. An analysis of tacrolimus-related complications in the first 30 days after liver transplantation. Clinics (Sao Paulo). 2014;69(11):745-9. [ Links ]
13. Ojo A, Held P, Port F, Wolfe R, Leichman A, Young E, et al. Chronic Renal Failure after Transplantation of a Nonrenal Organ. N Engl J Med. 2003;349(10):931-40. [ Links ]
14. Shaffi K, Uhlig K, Perrone RD, Ruthazer R, Rule A, Lieske JC, et al. Performance of creatinine-based GFR estimating equations in solid-organ transplant recipients. Am J Kidney Dis. Elsevier Inc. 2014;63(6):1007-18. [ Links ]
15. Sato K, Kawagishi N, Fujimori K, Ohuchi N, Satomi S. Renal function status in liver transplant patients in the first month post-transplant is associated with progressive chronic kidney disease. Hepatol Res. 2014;4:1-8. [ Links ]











 texto en
texto en