Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.30 no.4 Bogotá oct./dic. 2015
Alcoholic Hepatitis: A review with emphasis on liver transplantation and alternative medical treatment
David Ríos P. MD. (1), Dariana Pereira R. MD. (1), Juan Carlos Restrepo G. MD. (2)
(1) Surgeon, Gastro-hepatology Group at the University of Antioquia. Hospital Pablo Tobon Uribe in Medellín, Colombia.
(2) Internist and Hepatologist in the Hepatology and Liver Transplant Unit of the Hospital Pablo Tobon Uribe. Professor in the Faculty of Medicine and member of the Gastro-hepatology Group at the University of Antioquia in Medellín, Colombia. E-mail: jcrestrepo@hptu.org.co
Received: 24-02-14 Accepted: 20-10-15
Abstract
Alcoholic hepatitis is a clinical syndrome characterized by jaundice, ascites and acute liver failure secondary to alcohol consumption. The prevalence of the disease is increasing as a result of increased exposure to risk factors. Most patients are asymptomatic until significant compromise of liver function presents. This hinders early diagnosis and results in high morbidity and mortality rates. Liver transplantation is a valid treatment option for selected patients, with great prospects for the future, but it is still controversial. On the other hand, there are medical treatment options such as steroids, pentoxifylline and N-acetylcysteine, whose impact on morbidity and mortality is supported by evidence-based medicine. This review addresses current concepts of medical and surgical treatment of alcoholic hepatitis.
Keywords
Alcoholic hepatitis, liver transplant, treatment
INTRODUCTION
Alcoholic hepatitis (AH) is a clinical syndrome of acute onset that is characterized by jaundice, ascites, and acute liver failure. It occurs in people who consume alcohol chronically, usually for decades. In 50% of cases it coexists with cirrhosis and its comorbidities at diagnosis (1).
Alcoholic liver disease (ALD) is the second most common indication for liver transplantation in the western world (2). The actual prevalence of AH is probably underestimated since patients may be completely asymptomatic and often remain undiagnosed (3). For decades it has been known that the likelihood of developing cirrhosis in patients with AH is about 10% to 20% per year and that about 70% of patients with AH ultimately develop cirrhosis (4). In turn, it is estimated that 90% to 100% of people with alcohol dependence have steatosis, 10% to 35% have some degree of AH and 8% to 20% develop cirrhosis (5).
In 2007 in the United States about 8 million people were hospitalized due to AH, with mortality of 6.8%. Old age, sepsis, pneumonia, urinary tract infections, spontaneous bacterial peritonitis, acute renal failure, hepatic encephalopathy and coagulopathy were identified as predictors of mortality for these people (6).
The exact amount of alcohol that must be consumed to develop AH is unknown, but most patients report drinking more than 100 gr/day for an average of two decades (7). However, some reports show that consuming just 30-50 gr/day for more than five years is enough to produce a high risk for ALD. (8) The standard Unit of Alcohol (UBE) described in Table 1 is used to calculate the volume of alcohol consumed.
Besides alcohol there are other risk factors that influence the development of ALD. Among them is age at presentation which is usually between 40-50 years old and is rarely later than 60 (9). Women are at greater risk of developing the disease, as they have fewer gastric alcohol enzymes than men (10). However, the disease is more common in men because they are twice as likely to abuse alcohol as women (11).
ALD also has a known genetic basis. It has been linked to mutations in the alcohol dehydrogenase enzymes and aldehyde dehydrogenase (12). Recently, mutations in genes PNPLA3 and rs738409 which modulate steatosis, necroinflammation, and fibrosis in the liver have been identified. They may be directly related to persistent ALD (13, 14).
The incidence of cirrhosis from any cause and mortality from this disease are much more common among African and Hispanic people than among Caucasians (15). Another risk factor is obesity which increases the likelihood of liver disease and cirrhosis and enhances the severity of alcoholic liver disease at every stage (16, 17). Protein calorie malnutrition is present in most patients with AH and is directly proportional to mortality rates which reach 80% in patients with severe malnutrition (18, 19). Hepatitis C virus and alcohol abuse coexist in 14% of all people with chronic liver disease (20). These two agents act synergistically to accelerate the progression of inflammation and fibrosis so that mortality rates are higher than when only one of them is present (21).
Regarding pathophysiology, it is accepted that the way alcohol causes AH is by facilitating translocation of lipopolysaccharides from intestinal bacteria to the enterohepatic circulation which allows interaction with Kupffer cells and releases free radicals from NADPH oxidase. They induce the production of proinflammatory cytokines, mainly TNF alpha, IL4, IL13 and IL8 (22), that attract neutrophils, macrophages and stellate cells which cause damage in hepatocytes and cause inflammation and fibrosis (23, 24). Free oxygen radicals are released by CYP2E1 and mitochondria during the process of metabolizing alcohol. They cause peroxidation of lipids which combine with proteins and acetaldehyde to form neoantigens that can stimulate an autoimmune response. Glutathione transport into mitochondria is reduced and caspase 8 is activated causing mitochondrial damage, release of cytochrome C and activation of other caspases which contribute to hepatocyte apoptosis (23). Portal hypertension develops as a result of severe obstruction of terminal hepatic venules and dense sinusoidal fibrosis, but without showing the structural changes of cirrhosis (25).
There is no specific clinical picture for AH. In fact, it has a broad spectrum, but the key sign is rapidly evolving jaundice which is sometimes associated with fatigue, lethargy, anorexia, right upper quadrant pain and fever. In addition AH often produces other signs of alcoholic liver disease such as painful hepatomegaly, signs of protein energy malnutrition, telangiectasia, facial flushing, gynecomastia, and ascites. Other symptoms and signs include the appearance of cholestatic hepatitis, hyperbilirubinemia, elevated transaminase (AST>2 ALT, the elevation is generally less than 300UI/ml) and increased alkaline phosphatase. Severe forms of presentation include liver failure, encephalopathy, coagulopathy, increased ascites, sepsis, and hepatorenal syndrome (26).
Differential diagnosis for AH must consider non-alcoholic steatohepatitis, chronic or acute viral hepatitis, drug-induced liver injury, fulminant Wilson's disease, autoimmune hepatitis, alpha 1-antitrypsin deficiency, liver abscesses, cholangitis, and decompensation associated with hepatocellular carcinoma.
Liver biopsy findings can confirm the diagnosis, but a biopsy is not necessary. The histology of AH is characterized by neutrophil infiltration, ballooning degeneration of hepatocytes, necrosis and irregular fibrosis in the perivenular space and the perisinusoidal space (or space of Disse) (chicken wire fibrosis), and Mallory hyaline inclusions (27).
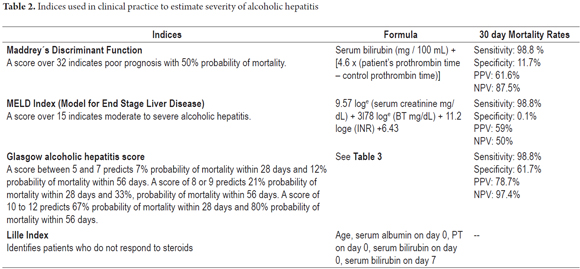
AH can be staged according to severity indexes which are vital for clinical decision making. A score that is greater than or equal to 32 in Maddrey's Discriminant Function confirms the diagnosis (28). For the MELD index (29) a score greater than or equal to 21 is required. For the Glasgow AH index a score higher than 9 is needed (Table 2 y 3) (30).
MEDICAL TREATMENT OPTIONS
Corticosteroids
The inflammatory process that characterizes alcoholic hepatitis is mainly limited to the centrilobular zone (26). This is the target of treatment with corticosteroids. They block the inflammatory process by blocking inflammatory pathways and preventing. Corticosteroids are currently considered the cornerstone of medical treatment of AH. No other drugs have similar results in terms of survival and reduced morbidity. They have been studied for over 40 years as a treatment option for severe AH (31, 32). In the ensuing years, many studies have shown different results, but the latest evidence supports them.
Since 1971, improvement in the survival of patients treated with corticosteroids has been demonstrated. The study included only 20 patients (33). In 1995, a meta-analysis did not recommend the routine use of corticosteroids to treat alcoholic hepatitis (34).
A 2008 Cochrane review (35) which included 15 randomized trials, concluded that corticosteroids did not have a significantly greater effect than placebos or no treatment on mortality in patients with AH (RR 0.83, 95% CI: 0.63 to 1.11). However, an important reduction of mortality has been demonstrated in patients whose Maddrey's Discriminant Function is over 32 and among those who have hepatic encephalopathy (RR 0.37 95% CI 0.16 to 0.86).
In 2011, a meta-analysis of five randomized double blind trials concluded that corticosteroids significantly improve survival in patients with Maddrey's Discriminant Functions over 32. Their survival at 28 days was 84.6% vs. 65.1% in patients on placebos. The difference was not significant for patients with Maddrey's Discriminant Functions under 32 and encephalopathy (91.7% in patients on placebos and 89.5% in patients with corticosteroids) (36). In the same study, the Lille Model for Alcoholic Hepatitis was used to divide patients into complete responders, partial responders, and non-responders. The study showed large 28 day survival benefits for patients who fully responded (91.1±2.7%), lesser 28 day survival benefits for those who partially responded (79.4±3.8%), and poorest 28 day survival for those who did not respond (53.3±5.1%) (p<0.0001).
A randomized study comparing the use of corticosteroids with pentoxifylline concluded that survival after one month was greater with the use of corticosteroids (74.5 with pentoxifylline vs. 87.0 with prednisolone). After three months the difference was not greater (37). Another study concluded that combination therapy does not provide any additional survival advantage over the use of only corticosteroids (38). The combined therapy group after one month had a result of 72.2 percent compared to the group with prednisolone alone whose result was 73.5 percent. At six months there was no significant difference.
Pentoxifylline
In ALD, and especially in AH, serum levels of tumor necrosis factor-α (TNF-α) are usually high. Pentoxifylline is a drug that inhibits phosphodiesterase and consequently decreases the levels of anti-TNF-α. In 1991 it was suggested that pentoxifylline decreased the incidence of hepatorenal syndrome in acute AH and would not negatively impact mortality unlike other drugs that inhibit TNF-α such as infliximab and etanercept (39).
A meta-analysis of five randomized trials with 336 patients suggested that a positive effect on mortality and morbidity of patients with severe acute hepatitis was possible since there was a statistically significant reduction in these parameters with the use of the drug. However, the authors did not state their conclusions since the results were not reproducible through sequential analysis of trials. They emphasized the need for more research. More recent studies have concluded that pentoxifylline is superior to placebos and not inferior to corticosteroids (40) for reducing mortality and the risk of hepatorenal syndrome. Some authors even dare to suggest that there is a likelihood that it is superior (41). This justifies the use of pentoxifylline when steroids are contraindicated in cases of severe acute AH is as stated by the American Association for the Study of Liver Diseases (AASLD). The European Association for the Study of the Liver (EASL) recommends pentoxifylline if there is sepsis (42).
A recent systematic review that included 10 publications about 884 participants in whom the effects of pentoxifylline were compared to the placebo effect (4 publications) and corticosteroids (6 publications) found that pentoxifylline is superior to placebos for preventing fatal hepatorenal syndrome (RR 0.47, 95% CI: 0.26 to 0 86, P = 0.01). Nevertheless, the articles were heterogeneous in terms of the control groups and the definitions of primary results. The review found that there was no benefit in reducing short-term mortality (RR 0.58, 95% CI 0.31-1.07, P = 0.06), and it was not possible to establish superiority or inferiority of pentoxifylline or corticosteroids (43).
Elsewhere, efforts have been made to establish whether therapy combining steroids and pentoxifylline improves short and medium term mortality rates and serious complications caused by severe acute alcoholic hepatitis more than the use of corticosteroids only (44). Some years ago it was believed that there was some benefit to this treatment, especially with regard to short-term mortality, however current randomized studies dismiss any positive impact on survival and are inconclusive regarding the net effect on complications like hepatorenal syndrome (45-48).
N-acetyl cysteine
Because of the important role of oxidative stress in the pathophysiology of alcohol-mediated hepatotoxicity (49, 50), antioxidant therapy has been explored, although increasingly less often. It has been shown that ethanol consumption generates endogenous antioxidant deficiency, especially in patients with severe AH (51). However, chronic consumption of ethanol is required for this to produce selective deficiency in the availability of glutathione in mitochondria mediated by alteration in the functioning of mitochondrial carriers that translocates glutathione from cytosol to the mitochondrial matrix to exert its antioxidant effect (52, 53).
Several years ago it was demonstrated that N-acetylcysteine (NAC) was inferior to corticosteroids (prednisolone 30 mg/day) for treating severe AH. A randomized non-inferiority trial showed that mortality at 30 days was significantly higher in patients receiving NAC (30% vs. 46%). A m ore recent randomized double-blinded study has evaluated the impact of NAC and other antioxidants on mortality at six months. It found a statistically insignificant difference in favor of those who did not receive them (52.8% vs. 55.8%, p = 0.699). The mortality rate was not affected by steroids (54, 55).
Subsequently, a randomized multicenter study conducted in Belgium compared the performance of a proper nutrition plan combined with intravenous N-acetylcysteine (NAC) against placebos for 14 days. Survival rates at 1 month and for the placebo and NAC groups were 70.2% and 83.8% (p = 0.26) respectively, and 62.4% vs. 67.1% (p = 0.60) respectively at 6 months. The incidence of hepatorenal syndrome was not significantly different between the two groups (14.8% NAC vs. 30% placebo, p= 0.21). Similarly, the incidences of documented infections within the first month of treatment (40.7% placebo vs. 31.6% NAC) (p = 0.53) were also not significantly different (56).
In recent years its effectiveness in combination with steroids has been evaluated, but the results have been inconclusive. One randomized study has compared the performance of intravenous NAC combined with prednisolone against prednisolone alone for one month. Overall mortality was 38% in the monotherapy group (34 of 89), and 27% in the prednisolone plus NAC group (23 of 85). In other words, there was a 62% reduction of the mortality rate with combination therapy, although the confidence interval was wide enough to exclude statistical significance (HR, 0.62; 95% CI: 0.37 to 1.06). The frequency of hepatorenal syndrome and mortality from this entity was significantly higher in the monotherapy group (mortality 9% vs. 22%, OR: 2.79; 95% CI: 1.08 to 7.42) (25% vs. 12%, OR: 0.41; 95% CI: 0.17 to 0.98). On the other hand, the authors noted that a secondary outcome was that one-month mortality was higher in patients receiving monotherapy (21 of 89) than in patients receiving combination therapy (HR, 0.58; 95% CI: 0.14 to 0.76).
Today it is accepted that the NAC is useless both as monotherapy and in combination with other substances that are not steroids. Because the evidence is not yet enough, administration of NAC plus steroids is still not recommended (57).
SURGICAL TREATMENT OPTION
Liver transplant
Liver transplantation is the only intervention that has demonstrated an increase in the survival of patients diagnosed with alcoholic cirrhosis who have Child-Pugh scores of C and/or a MELD score of 15 or more. Consequently, liver transplantation is becoming the alternative therapy of choice in these cases (58). In Europe, the number of patients undergoing liver transplantation for ALD increased by 8.3% in the period between 1988 and 1995 over the number recorded between 1996 and 2005. The survival rates have been high: 84% at 1 year, 73% at 5 years and 58% at 10 years after transplantation (59).
In recent years, the frequency of liver transplantation for ALD has also increased in the United States. It is estimated that 23% (3,563) of liver transplants performed in that country in 2011 were due to this cause, mostly for alcoholic cirrhosis (60).
Although it is clear that terminal ALD is a good indication for liver transplantation according to multiple studies (61-63), there is much controversy about whether to transplant patients with alcoholic hepatitis. The idea that liver transplantation should not be used in these cases is based on the low rates of abstention from alcohol following transplants which allegedly leads to worse prognoses thus contraindicating the procedure (64). However, recent studies and transplant groups around the world have modified this view on the relevance of transplantation with favorable results.
While in most cases immediate and lasting abstinence is the key to the recovery from AH (26), the evolution of patients with severe AH when they do abstain remains unsatisfactory, and death is very common in the short-term (65). When these patients are treated with corticosteroids, the rate of treatment failure is 40% (26), and they most likely will not respond to other medical treatment alternatives such as Pentoxifylline (66). In addition, when severe AH is unresponsive to steroid treatment, the mortality rate at 6 months is 70% (67, 68).
On the other hand, survival in transplanted patients with AH appears to be similar to that of patients without AH. A case-control study that compared five-year survival of a group of 55 transplant patients who had been diagnosed with AH with that of a group 165 transplanted patients diagnosed with alcoholic cirrhosis reported survival rates of 82% and 80%, respectively,. Added to this was a loss of implants in 25% of the case group, and 26% in the control group. The Cox regression model ruled out influence of the reason for transplantation on survival of patients and implants. Also, the causes of death were not related to alcohol (69).
In another case-control study conducted in seven hospitals in France, 26 patients with AH at high risk of death underwent liver transplant immediately after not responding to drug treatment. The selected patients had good social support, belonged to a program to stop drinking, and had no prior episodes of alcoholic hepatitis or evidence of severe psychiatric illness. These patients were compared with 26 controls with severe alcoholic hepatitis who did not undergo transplantation. The survival rate at six months was significantly higher (77%) among transplanted patients than among those who did not receive transplants (23%). The relation between the two year survivial rates was similar: 71% in the transplant group and 23% in the control group (70).
In addition, only three of the patients who underwent transplantation consumed alcohol again. At 720, 740 and 1140 days respectively, two consumed from 30 to 50 gr/day of alcohol and one consumed 10 gr/week. This is very similar to documented recurrence rates among patients who receive transplants because of alcoholic cirrhosis (71, 72).
The studies suggest that liver transplantation in patients with AH can save lives and provides long-term results that are as good as those for liver transplantation to treat alcoholic cirrhosis, and even better in strictly selected patients. There are other aspects to be analyzed that could support the idea of the relevance of the transplant. Regarding the relevance of abstinence from alcohol, since 1997, when the American Society of Transplantation and the American Association for the Study of Liver Diseases determined the minimum criteria to include adult patients on liver transplant waiting list, a golden rule was established: "Anyone with alcoholic liver disease must comply with a minimum period of abstinence of 6 months" (73).
Patients with severe acute AH were implicitly ruled out. Nevertheless understanding of the pathophysiology of the disease and the prognoses of these patients were much lower at that time than they are today.
The 6-month rule is arbitrary because it is not based on evidence and does not guarantee that patients who meet it will not consume liquor again. A cohort study of 167 patients who were eligible for liver transplants made a broad psychosocial assessment that found that patients went on the waiting list they were recorded as having been sober for at least 180 days, but one year after transplantation 22% had consumed alcohol at least once, and five years after 42% had. In addition, 26% had consumed alcohol to intoxication and 22% had consumed it for 4 consecutive days (74). Something similar was found in a nested study of 100 patients in a prospective database conducted in Australia. It found that the duration of pre-transplant abstinence was not significant for predicting relapses of alcohol consumption (75).
In fact, the new 2006 consensus regarding indications for liver transplantation clearly states that the six months rule should not be the determining factor for considering a patient with ALD. It also states that one episode of alcohol poisoning does not necessarily mean a relapse into dependence, although this is definitely not compatible with the required immunosuppressive treatment and can increase the risk of graft rejection. This consensus also highlights the commonly known reality that alcoholic patients should be considered to be suffering from both liver and alcoholic diseases and that the latter does not depend exclusively on will. Therefore, a comprehensive strategy for intervention that reduces the risk of relapse, not just consumption, should be established (76).
The limitations of medical treatment also lend support to the relevance of transplantation. There is currently no therapeutic alternative to steroids that achieves similar margins of effectiveness, but not all patients respond favorably to steroids, and in some cases they are contraindicated. An important tool for determining which patients will not respond to the first cycle of steroids is the index of Lille Model for Alcoholic Hepatitis developed by Mathurin et al. This index gives special relevance to levels of total bilirubin in the first 7 days of treatment which is a parameter that is closely related to the effectiveness of steroids (67). This tool can select patients who will actually benefit from transplantation.
One of the most difficult issues to address in relation to liver transplantation is its degree of social acceptance. If we assume that an organ from a deceased donor is a valuable public good which any citizen theoretically has the right to, it is essential that a person who really needs it should receive it. However, it is clear that a good proportion of these patients will continue to consume alcohol even though abstinence of up to two years has been documented (72). This fact could translate into criticism from the community, moralistic judgments and an eventual decrease in organ donation (5). In fact, the perception of the community about the liver transplantation has been assessed and there is a dismissal of the relevance of transplant patients who consume alcohol when compared to transplant patients with other diagnoses (77).
A valid counterargument would be that it is still unclear whether the abstinence period prior to transplantation is associated with longer periods of post-transplant abstinence. While some studies support the idea that prior abstinence is an important predictor of relapse into consumption after transplantation, other research shows that it is a poor predictor. Also, since there are no documented differences in survival rates between drinkers and non-drinkers, allegations of mismanagement of public property have no support (72, 78, 79).
A second argument is that alcoholic liver disease has a known genetic basis which predisposes some people to suffer from it, so its development is not necessarily the result of continuous and abundant consumption of liquor (16).
Finally, a third argument is that the transplantation is commonly performed in situations in which a toxic substance has been consumed knowingly whether it is acetaminophen or a hepatotropic virus acquired by unprotected sex. Even when the cause of liver disease is unknown, which to date has not represented an obstacle to transplantation, transplantation has saved thousands of lives.
In addition to everything already mentioned, other conditions must be present for a favorable transplant scenario. A very important issue is the need to change the legal framework, especially Colombian Law 73 of 1988 and Law 919 of 2004 which are insufficient given the growing demand for urgent transplants among patients who today die while waiting for their names to reach the top of the transplant list. Today, the organ shortage is largely due to the absence of a legal framework that helps reduce the loss of potential donors for reasons such as negative answers from families and absence of information about the willingness to donate.
It is important for the reader to remember that an interdisciplinary approach which aims to work through all factors of vulnerability to relapse is vital for defining who will receive a transplant and for guaranteeing the success of transplantation. Therefore, the contributions of professionals in toxicology, psychiatry and psychology are fundamental pillars for decreasing the likelihood of treatment failure. The fundamental objectives should always be maintaining therapeutic adherence, preventing relapse, improving quality of life and reducing potential psychiatric comorbidity. The American Association of Psychiatry has published strategies that could be the most useful for reducing and eliminating alcohol dependency even though they lack solid evidence. These strategies include community reinforcement, marital behavioral therapy for abuse and dependence, training in social skills, and exposure to stimuli. Other strategies commonly used are the facilitation therapy approach based on the 12 step program, group therapy, social behavior therapy, and work networks (80).
The use of various medications to support behavioral strategies can also prevent relapses. Topiramate has demonstrated that it can reduce excessive alcohol consumption and increase withdrawal time in two clinical trials (81, 82). This effect is believed to be related to facilitation of GABA function and antagonism to glutamate. It is a generally well tolerated drug whose most common adverse effects are paresthesia, taste perversion, anorexia, and difficulty concentrating.
Topiramate treatment should be initiated at a 25 mg/day dose titrated over a period of 8 weeks to 300 mg/day. It is suggested that most patients need to be treated at least 6 months to increase the chance of remission (83). Another option is baclofen, a derivative of GABA with an initial dosage of 5 mg three times a day during the first 3 days, and then a maximum dose of 10 mg three times a day. Special precaution should be taken to gradually reduce the dosage to avoid withdrawal. Another drug that has been used successfully is disulfiram, a competitive inhibitor of aldehyde dehydrogenase that should be prescribed with care since consumption of small amounts of alcohol can trigger poisoning. Still another is naltrexone which antagonizes the effects of endorphins that are released during consumption of alcohol thereby reducing the desire to consume (84).
Finally, proper nutrition is essential for patients with chronic liver disease whether or not they are transplant candidates. A recently published meta-analysis shows that nutritional support sufficient to meet the metabolic demands of patients with alcoholic hepatitis and liver cirrhosis can reduce mortality rates by up to 8 times over the rates of patients who receive no nutritional intervention (85). The consensus of the European Society for Parenteral and Enteral Nutrition of patients with liver disease recommends from 1.2 to 1.5 g protein/kg/day for proper nitrogen balance. Also, it is necessary to modify the diet to prevent malnutrition by providing frequent meals during the day and by supplementing meals with preparations enriched with branched-chain amino acids, that are without aromatic amino acids, and which are combined with glucose and insulin if necessary to decrease protein degradation (86). Of course these recommendations neither replace proper monitoring nor the professional judgments of nutritionists and dietitians.
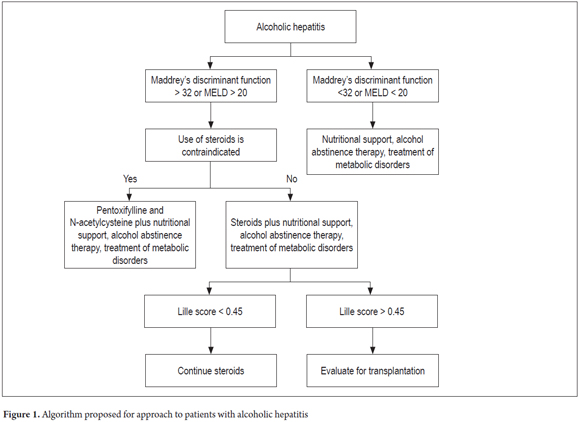
We propose the algorithm of management outlined in Figure 1 to readers. It is worth mentioning that this is an approximation because each patient has a unique clinical picture.
In conclusion, early liver transplantation is becoming a relevant and effective alternative for treatment of alcoholic hepatitis in selected patients. Nevertheless, more studies with better quality are needed to establish a clear judgment. Until then, medical treatment, efficient in many respects but limited for patients with the poorest prognoses, will continue to be the cornerstone of treatment.
Acknowledgments
The authors wish to thank the Sustainability Project of the Office of the Vice-Rector for Research, of the University of Antioquia.
REFERENCES
1. O'Shea R, Dasarathy S, McCullough A. Alcoholic liver disease. Hepatology. 2010;51:307-28. [ Links ]
2. Burra P, Lucey M. Liver transplantation in alcoholic patients. TransplInt. 2005;18:491-98. [ Links ]
3. Ceccanti M, Attili A, Balducci G, Attilia F, Giacomelli S, Rotondo C, et al. Acute alcoholic hepatitis. J Clin Gastroenterol. 2006;40:833-84. [ Links ]
4. Bird G, Williams R. Factors determining cirrhosis in alcoholic liver disease. Mol Aspects Med. 1988;10:97-105. [ Links ]
5. Sorrell M, Mukherjee S. Non-Alcoholic Steatohepatitis (NASH). Curr Treat Options Gastroenterol. 1999;2:447-50. [ Links ]
6. Suthat L. Clinical Characteristics and Mortality of Hospitalized Alcoholic Hepatitis Patients in the United States. J Clin Gastroenterol. 2011:45. [ Links ]
7. Naveau S, Giraud V, BorottoE, Aubert A, Capron F, Chaput J. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997;25:108-11. [ Links ]
8. Becker U, Deis A, Sørensen T, Grønbaek M, Borch-Johnsen K, Müller CF, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23:1025-9. [ Links ]
9. Lischner M, Alexander J, Galambos J. Natural history ofalcoholic hepatitis. I. The acute disease. Am J Dig Dis. 1971;16:481-94. [ Links ]
10. Seitz H, Egerer G, Simanowski A, Waldherr R, Eckey R, Agarwal P, et al. Human gastric alcohol dehydrogenase activity: effect of age, sex, and alcoholism. Gut. 1993;34:1433-37. [ Links ]
11. Mandayam S, Jamal M, Morgan T. Epidemiology of alcoholic liver disease. Semin Liver Dis. 2004;24:217-32. [ Links ]
12. Day P, Bassendine, F. Genetic predisposition to alcoholic liver disease. Gut. 1992;33:1444-47. [ Links ]
13. Stickel F, Hampe J. Genetic determinants of alcoholic liver disease. Gut. 2011;67:150-59. [ Links ]
14. Stickel F, Buch S, Lau K, zu Schwabedissen M, Berg T, Ridinger M, et al.Genetic variation in the PNPLA3 gene is associated with alcoholic liver injury in caucasians. Hepatology 2011;53(1):86-95. [ Links ]
15. Stinson F, Grant B, Dufour M. The critical dimension of ethnicity in liver cirrhosis mortality statistics. Alcohol Clin Exp Res. 2001;25:1181-87. [ Links ]
16. Raynard B, Balian A, Fallik D, Capron F, Bedossa P, Chaput JC, et al. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology. 2002;35:635-38. [ Links ]
17. Mathurin P, Beuzin F, Louvet A, Carrie-Ganne N, Balian A, Trinchet JC, et al. Fibrosis progressionoccurs in a subgroup of heavy drinkers with typical histological features. Aliment Pharmacol Ther. 2007;25:1047-54. [ Links ]
18. Mendez-Sanchez N, Meda-Valdes P, Uribe M. Alcoholic liver disease. An update. Ann Hepatol. 2005;4:32-42. [ Links ]
19. Crabb DW. Pathogenesis of alcoholic liver disease: newer mechanisms of injury. Keio J Med. 1999;48:184-8. [ Links ]
20. Singal A, Anand B. Mechanisms of synergy between alcohol and hepatitis C virus. J Clin Gastroenterol. 2007;41:761-72. [ Links ]
21. Singal A, Sagi S, Weinman S, Snyder N. Impact of hepatitis C on the outcome and severity of acute alcoholic hepatitis. Eur J Gastroenterol and Hepatol. 2011;23:204-9. [ Links ]
22. Crews F, BecharaR, Brown L, Guidot D, Mandrecker P, Oak S, et al. Cytokines and alcohol. Alcoholism: Clinical & Experimental Research. 2006;30:720-30. [ Links ]
23. Lucey M, Mathurin P, & Morgan T. Alcoholic hepatitis. New England Journal of Medicine 2009;360(26):2758-2769. [ Links ]
24. Rao R, Seth A, ShethP. Recent advances in alcoholic liver disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. American Journal of Physiology e Gastrointestinal and Liver Physiology. 2004;286:881-84. [ Links ]
25. Changqing M, Elizabeth M. Histopathologic Evaluation of Liver Biopsy for Cirrhosis. Adv Anat Pathol. 2012;19:220-30. [ Links ]
26. Gregory D, Levi D. The clinical-pathologic spectrum of alcoholic hepatitis. Am J Dig Dis. 1972;17:479-89. [ Links ]
27. Purohit V, Russo D. Cellular and molecular mechanisms of alcoholic hepatitis: introduction and summary of the symposium. Alcohol. 2002;27:3-6. [ Links ]
28. Kulkarni K, Tran T, Medrano M, Yoffe B, Goodgame R. The role of the discriminant factor in the assessment and treatment of alcoholic hepatitis. Journal of clinical gastroenterology. 2004;38:453-59. [ Links ]
29. Dunn W, Jamil L, Brown L, Wiesner R, Kim W, Menon K, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41:353-58. [ Links ]
30. Forrest E, Evans C, Stewart S, Phillips M, Oo Y, McAvoy N, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut. 2005;54:1174-9. [ Links ]
31. Conn H. Steroid treatment of alcoholic hepatitis. The yeas and the nays. Gastroenterology. 1978;74:319-22. [ Links ]
32. Helman R, Temko M, Nye S, Fallon H. Alcoholic hepatitis. Natural history and evaluation of prednisolone therapy. Ann Intern Med. 1971;74:311-21. [ Links ]
33. Porter H, Simon F, Pope C,Volwiler W, Fenster F.Corticosteroid Therapy in Severe Alcoholic Hepatitis - A Double-Blind Drug Trial. N Engl J Med. 1971;284:1350-5. [ Links ]
34. Christensen E, Gluud C. Glucocorticoids are ineffective in alcoholic hepatitis: a meta-analysis adjusting for confounding variables. Gut. 1995;37:113-8. [ Links ]
35. Rambaldi A, Saconato H, Christensen E, Thorlund K, Wetterslev J, Gluud C. Systematic review: glucocorticosteroids for alcoholic hepatitis–a CochraneHepato-Biliary Group systematic review with meta-analyses and trial sequential analyses of randomized clinical trials. Aliment Pharmacol Ther. 2008;27:1167-78. [ Links ]
36. Mathurin P, O'Grady J, Carithers RL, Phillips M, Louvet A, Mendenhall CL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut. 2011;60:255-60. [ Links ]
37. Kim D, Suk K, Park S, Tak W, Yim H, Um S, et al. Short-term survival in patients with severe alcoholic hepatitis treated with pentoxifylline vs. corticosteroid: a non-inferiority trial. Journal of Hepatology. 2013;58,219-20. [ Links ]
38. Singh S, Goyal O, Singla P, Gupta D, Sood A, Singh R, et al. Corticosteroid Plus Pentoxifylline Is Not Better than Corticosteroid Alone for Improving Survival in Severe Alcoholic Hepatitis (COPE Trial). Dig Dis Sci. 2012;57:1664-71. [ Links ]
39. McHutchison J, Runyon B, Draguesku J, et al. Pentoxifylline may prevent renal impairment (hepatorenal syndrome) in severe acute alcoholic hepatitis. Hepatology. 1991;14:96. [ Links ]
40. Whitfield K, et al. Pentoxifylline for alcoholic hepatitis. Cochrane Database Syst Rev 4 2009. [ Links ]
41. Garcõa J, Hernandez G, Lopez A, et al. Pentoxifilina versus esteroide en la sobrevivencia a corto plazo en hepatitis aguda alcohólica severa. Medicina Interna de México. 2012;28:228. [ Links ]
42. O'Shea R, Dasarathy S, McCullough A. Alcoholic liver disease. Hepatology. 2010;51:307-28. [ Links ]
43. Parker R, et al. Systematic review: pentoxifylline for the treatment of severe alcoholic hepatitis. Alimentary pharmacology & therapeutics. 2013. [ Links ]
44. De B, Gangopadhyay S, Dutta D, et al. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial. World J Gastroenterol. 2009;15:1613. [ Links ]
45. Mathurin P, Louvet A, Duhamel A, et al. Prednisolone With vs Without Pentoxifylline and Survival of Patients With Severe Alcoholic Hepatitis: A Randomized Clinical Trial. JAMA. 2013;310(10):1033-41. [ Links ]
46. Sidhu S, Goyal O, Singla P, et al. Corticosteroid plus pentoxifylline is not better than corticosteroid alone for improving survival in severe alcoholic hepatitis (COPE trial). Dig Dis Sci. 2012;57:1664-71. [ Links ]
47. Mathurin P. Addition of pentoxifylline to prednisolone for severe alcoholic hepatitis does not improve 6-month survival: results of the Corpentox trial. Hepatology. 2011;54(1):81. [ Links ]
48. Louvet A, Dao T, Nahon P, et al. Pentoxifylline does not improve shortterm survival in severe alcoholic hepatitis in combination with corticosteroids: results of a randomized controlled trial. J Hepatol. 2012;56:533. [ Links ]
49. Anderson H, Baumburg B. Alcohol in Europe. A public health perspective. London: Institute of Alcohol Studies; 2006. [ Links ]
50. Zatonski W, Sulkowska U, Manczuk M, Rehm J, Boffetta P, Lowenfels A, et al. Liver cirrhosis mortality in Europe, with special attention to Central and Eastern Europe.Eur Addict Res. 2010;16:193-201. [ Links ]
51. Paredes S, Kozicki P, Kukuda H, Rossetti M, Batlle A. S-Adenosyl-Lmethionine: its effect on aminolevulinatedehydratase and glutathione in acute ethanol intoxication. Alcohol. 1987;4:81-85. [ Links ]
52. Fernandez-Checa J, Ookhtens M, Kaplowitz N. Effect of chronic ethanol feeding on rat hepatocytic glutathione. Compartmentation, efflux, and response to incubation with ethanol. J Clin Invest. 1987;80:57-62. [ Links ]
53. Fernandez-Checa J, Ookhtens M, Kaplowitz N. Effect of chronic ethanol feeding on rat hepatocytic glutathione. Relationship of cytosolic glutathione to efflux and mitochondrial sequestration. J Clin Invest. 1989;83:1247-52. [ Links ]
54. Phillips M, et al. Antioxidants versus corticosteroids in the treatment of severe alcoholic hepatitis -a randomised clinical trial. Journal of hepatology. 2006;44:784-90. [ Links ]
55. Stewart S, et al. A randomized trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. Journal of hepatology. 2007;47:277-83. [ Links ]
56. Moreno C, et al. Enteral nutrition with or without N-acetylcysteine in the treatment of severe acute alcoholic hepatitis: A randomized multicenter controlled trial. Journal of hepatology. 2010;53:1117-22. [ Links ]
57. Nguyen-Khac E, et al. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. New England Journal of Medicine. 2011;365:1781-9. [ Links ]
58. European Association for the Study of Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol. 2012;57(2):399-420. [ Links ]
59. Burra P, Senzolo M, Adam R, Delvart V, Karam V, Germani G, et al. Liver transplantation for alcoholic liver disease in Europe: a study from the ELTR (European Liver Transplant Registry). Am J Transplant. 2010;10(1):138-48. [ Links ]
60. Annual Report - OPTN (Internet). Citado 11 de noviembre de 2015. Recuperado a partir de: http://optn.transplant.hrsa.gov/converge/data/annualReport.asp [ Links ]
61. Biselli M, Gramenzi A, Del Gaudio M, et al. Long term follow-up and outcome of liver transplantation for alcoholic liver disease: a single center case–control study. J Clin Gastroenterol. 2010;44:52-57. [ Links ]
62. Pfitzmann R, Schwenzer J, Rayes N, et al. Long-term survival and predictors of relapse after orthotopic liver transplantation for alcoholic liver disease. Liver Transpl. 2007;13:197-205. [ Links ]
63. Bjornsson E, Olsson J, Rydell A, et al. Long-term follow-up of patients withalcoholic liver disease after liver transplantation in Sweden: impact of structured management on recidivism. Scand J Gastroenterol. 2005;40:206-16. [ Links ]
64. Dureja P, Lucey M. The place of liver transplantation in the treatment of severe alcoholic hepatitis. J Hepatol. 2010;52:759-64. [ Links ]
65. Ceccanti M, Attili A, Balducci G, et al. Acute alcoholic hepatitis. J ClinGastroenterol. 2006;40:833-41. [ Links ]
66. Louvet A, Diaz E, Dharancy S, et al. Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in non-responders to corticosteroids. J Hepatol. 2008;48:465-70. [ Links ]
67. Louvet A, Naveau S, Abelnour M, et al. The Lille model: a new tool fortherapeutic strategy in patients with severe alcoholic hepatitis treated withsteroids. Hepatology. 2007;45:1348-54. [ Links ]
68. Mathurin P, O'Grady J, Carithers RL, et al. Corticosteroids improve short termsurvival in patients with severe alcoholic hepatitis: meta-analysis ofindividual patient data. Gut. 2011;60:255-60. [ Links ]
69. Singal A, Bashar H, Anand B, et al. Outcomes after liver transplantation for alcoholic hepatitis are similar to alcoholic cirrhosis: exploratory analysis from the UNOS database. Hepatology. 2012; 55:1398. [ Links ]
70. Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011;365:1790. [ Links ]
71. Beresford T, Everson G. Liver transplantation for alcoholic liver disease: bias, beliefs, 6 month rule and relapse - but where are the data. Liver Transpl. 2002;6:777-8. [ Links ]
72. Tandon P, Goodman K, Ma M, et al. A shorter duration of pre-transplant abstinence predicts problem drinking after liver transplantation. Am J Gastroenterol. 2009;104:1700-6. [ Links ]
73. Lucey M, Brown K, Everson G, Fung J, Gish R, Keeffe E, et al. Minimal criteria for placement of adults on the liver transplant waiting list: a report of a national conference organized by the American Society of Transplant Physicians and the American Association for the Study of Liver Diseases. Liver TransplSurg. 1997;3:628-37. [ Links ]
74. DiMartini A, Day N, Dew M, et al. Alcohol consumption patterns and predictors of use following liver transplantation for alcoholic liver disease. Liver Transpl. 2006;12:813-20. [ Links ]
75. Kelly M, Chick J, Gribble R, et al. Predictors of relapse to harmful alcohol after orthotopic liver transplantation. Alcohol. 2006;41:278-83. [ Links ]
76. Consensus conference: indications for liver transplantation, January 19 and 20, 2005 Lyon-Palais de Congres: text of recommendations. Liver Transplant. 2006;12:998-1011. [ Links ]
77. Neuberger J, Adams D, MacMaster P, Maidment A, Speed M. Assessing priorities for allocation of donor liver grafts: survey of public and clinicians. BMJ. 1998;317:172-5. [ Links ]
78. McCallum S, Masterton G. Liver transplantation for alcoholic liver disease: a systematic review of psychosocial selection criteria. Alcohol. 2006;41:358-63. [ Links ]
79. Wells J, Adnan S, Rashmi A, Tome S, Hughes S, Dureja P et al. The impact of acute alcoholic hepatitis in the explanted recipient liver on outcome after liver transplantation. Liver Transplantation. 2007;13:1728-35. [ Links ]
80. Chambless DL, Baker MJ, Baucom DH, et al: Update on empirically alidated therapies, II. TheClinical Psychologist. 1998;51:3-16. [ Links ]
81. Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, Javors MA, Ma JZ. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361:1677-85. [ Links ]
82. Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O'Malley SS, Swift RM, Topiramate for Alcoholism Advisory Board, Topiramate for Alcoholism Study Group. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298:1641-51. [ Links ]
83. Medication treatment of different types of alcoholism. Am J Psychiatry. 2010;167(6):630-9. [ Links ]
84. Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, Abenavoli L, D'Angelo C, Caputo F, Zambon A, Haber PS, Gasbarrini G. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370:1915-22. [ Links ]
85. Fialla AD, Israelsen M, Hamberg O, Krag A, Gluud LL. Nutritional therapy in cirrhosis or alcoholic hepatitis: a systematic review and meta-analysis. Liver Int. 2015;35(9):2072-8. [ Links ]
86. Marchesini G, Bianchi G, Rosi B, Brizi M, Melchionda N. Nutritional treatment with branched chain aminoacids in dvanced liver cirrhosis. J Gastroenterol. 2000;35:7-12. [ Links ]











 texto en
texto en