Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.31 no.1 Bogotá jan./mar. 2016
Clinical Characteristics and Decompensation in Patients with Liver Cirrhosis Treated at Two Hepatology Centers in Bogota DC from 2010 To 2014
Jhon E. Prieto O., MD. (1, 2), Santiago Sánchez P. MD. (1), Robin G. Prieto O. MD. (1), Ever L. Rojas D. MD. (1), Lupita González, MD. (2), Fredy Mendivelso MD. (3)
(1) Clínica Universitaria Colombia in Bogotá DC, Colombia
(2) Centro de Enfermedades Hepáticas y Digestivas in Bogotá DC, Colombia
(3) Clínica Reina Sofía in Bogotá DC, Colombia
Received: 13-04-05 Accepted: 26-01-16
Abstract
Introduction: Cirrhosis is the final medical condition of various progressive liver disease. Its prevalence varies from one country to another. Currently alcohol abuse, non-alcoholic fatty liver disease and chronic viral hepatitis are mentioned as the main causes. In our centers we have no clinical studies regarding this disease.
Materials and Methods: This is a retrospective, descriptive study of the clinical histories of patients who were diagnosed with liver cirrhosis between January 1, 2010 to March 31, 2014.
Results: The study included 419 patients, 50.1% of whom were women and 49.9% of whom were men. The average age at diagnosis of cirrhosis 63 years. 73% of patients had physical findings of chronic liver disease and 27% had normal physical examinations. The main etiologies in this series were nonalcoholic steatohepatitis (25.5%), alcoholic cirrhosis (14.8%), hepatitis C infection (14.6%), autoimmune cirrhosis (10%), nonalcoholic steatohepatitis plus alcohol (6.7%), and others (14.6%). The Child-Pugh classification could be calculated in 394 patients. Of these 59.1% were classified A, 32.4% were classified B, and 8.3% were classified C. The primary reasons for decompensation ascites (36.1%), bleeding varices (28.4%) and hepatocellular carcinoma (15.3%).
Conclusions: Local epidemiological behavior does not differ from those found elsewhere in the world. Attention needs to be paid to detection in early stages and NASH as the main etiological factor.
Keywords
Gastroenterology, liver cirrhosis, ascites, hepatitis non-alcoholic steatohepatitis (NASH).
INTRODUCTION
Cirrhosis is the end stage of chronic progressive liver diseases of various etiologies. (1) It is a common disease whose prevalence varies from country to country. Its peak incidence occurs between 40 and 50 years old, predominantly in men. In Western countries, 90% of all cases are caused by alcohol abuse, nonalcoholic fatty liver disease (NAFLD) and chronic viral hepatitis, especially hepatitis B and C (HBV and HCV). In almost 10% of cases, the etiology is unknown. (1-3)
Pathophysiologically, cirrhosis is the result of a process of continuous necrosis of hepatocytes with loss of hepatic parenchyma, inflammation, fibrogenesis, changes in cell regeneration and changes in macrocirculation and microcirculation. (4-6) Cirrhosis is a dynamic process which is reversible at some points. Research into cirrhosis aims at acquiring new tools and information to guide and focus proper management of this condition. (7-9) Special attention is being paid to the asymptomatic stage and to predicting decompensation and its results in ascites, variceal bleeding, hepatorenal syndrome, hepatopulmonary syndrome, encephalopathy, jaundice, bleeding disorders, hypoalbuminemia, spontaneous bacterial peritonitis and hepatocellular carcinoma (HCC). (10-12, 16) These forms of decompensation dramatically decrease the survival time of patients which is especially short for patients who develop hepatocellular carcinoma, (12-15) which occurs in about 7% of stable patients each year. (7, 12)
HCC is associated with advanced chronic liver disease, particularly with HCV and increasingly with non-alcoholic fatty liver disease NAFLD as well. (17-20)
The classic Child-Pugh scale for predicting the severity of liver disease is a good tool for clinical classification. Using it for early assessment is important for establishing changes in management and monitoring the disease. (21-23)
The purpose of this study is to describe and characterize a cohort of patients diagnosed with cirrhosis and to analyze the frequencies of decompensation in relation to disease etiologies.
METHODOLOGY
A descriptive crosscut study that includes clinical data and laboratory and pathological test results from a cohort of patients with confirmed liver cirrhosis who were treated at two hepatology centers in Bogotá DC during the period from January 1, 2010 to March 31, 2014.
All patients underwent extensive serological tests to study chronic liver disease. They included:
- Hepatitis B: [surface antigen (HBsAG), surface antibody (anti-HBs), total anti-hepatitis B core (anti-HBc, IgM and IgG) and/or anti-hepatitis B core (anti-HBc, IgM and IgG), viral load as appropriate].
- Hepatitis C [Second and third generation hepatitis C antibodies: EIA 2.0, EIA3, and HCV RNA] viral load as appropriate.
- Hemochromatosis (serum iron, transferrin saturation %, total iron binding capacity, ferritin and hemochromatosis gene (HFE) testing if indicated).
- Wilsons disease (ceruloplasmin levels).
- Autoimmune Hepatitis and Primary Biliary Cirrhosis (ANAS anti-nuclear antibodies, anti-smooth muscle antibodies, anti-mitochondrial antibodies).
- IgG, IgM levels and protein electrophoresis.
Diagnoses of NASH were based on the following criteria for nonalcoholic fatty liver disease: (24-26)
- Biopsy or imaging evidence of liver steatosis.
- Zero or minimal consumption of alcohol (less than 20 g of ethanol per week)
- Absence of serologic evidence of hepatitis B or hepatitis C infection.
- Absence of other causes of liver fat accumulation
- Absence of other factors for liver disease
- Presence of dyslipidemia diabetes or obesity.
Statistical analysis
Absolute frequency distributions and proportions were calculated for categorical variables, and measures of central tendency and dispersion were calculated for quantitative variables. Normal distributions of continuous variables were analyzed using the Shapiro-Wilk test and Kolmogorov-Smirnov test. Means of variables with normal distributions were compared using Students T distribution while those with nonparametric distributions were compared with the Mann Whitney test. The Pearsons asymptotic chi-squared test or Fishers exact test was used for bivariate analysis of categorical data. P values <0.05 were considered statistically significant. The data were processed and analyzed in Microsoft Excel spreadsheets and Stata 13.0®. In this study there were no interventions or intentional modifications of biological, physiological, psychological or social variables of individuals. This study was conducted in accordance with the principles stated in the Eighteenth World Medical Assembly (Helsinki, 1964).
RESULTS
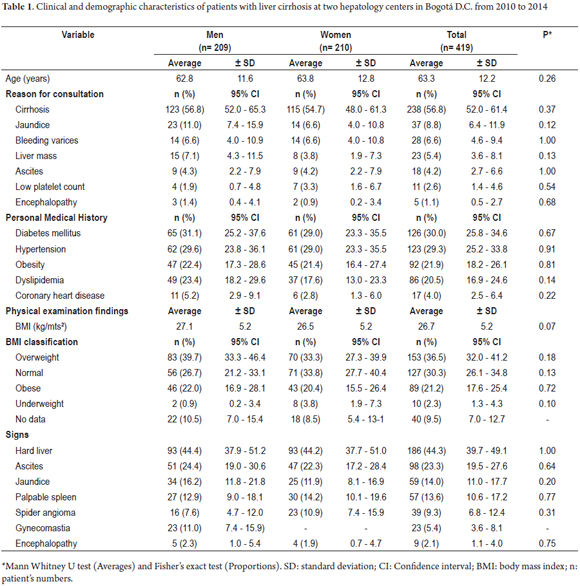
During the study period, 3,500 patients with liver diseases were treated. Diagnoses of cirrhosis were confirmed in 419 (11.9%, 95% CI: 10.9-13.0) by clinical criteria, imaging (ultrasound, portal venous system Dopler ultrasound, Computed Tomography or Magnetic Resonance Imaging), histological (liver biopsy) or a combination of these techniques. There were no statistically significant differences in the proportions of men and women diagnosed with cirrhosis (p> 0.05). However, a slightly higher percentage of women were affected (50.1%, 95% CI: 45.3-54.8). Average patient age at first consultation was 63.3 years (SD ± 12.2) with a minimum of 15 and a maximum of 91 years. Cirrhosis was the main reason for consultation among men (56.8%, 95% CI: 52.0-61.4) and women (54.7%, 95% CI: 48.0-61.3). Diabetes Mellitus (30.0%, 95% CI: 25.8-34.6) and arterial hypertension (29.3%, 95% CI: 25.2-33.8) were the primary antecedents. Being overweight (36.5%, 95% CI: 32.0-41.2) and hard liver (44.3%, 95% CI: 39.7-49.1) were the most frequent findings in men and women with cirrhosis (Table 1).
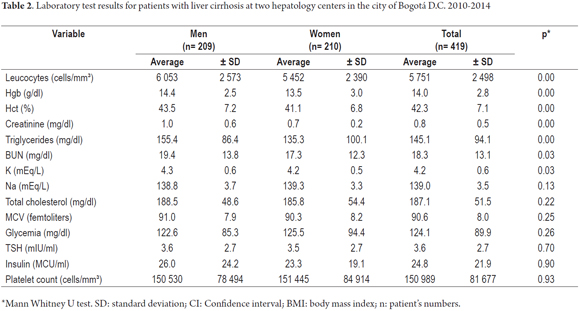
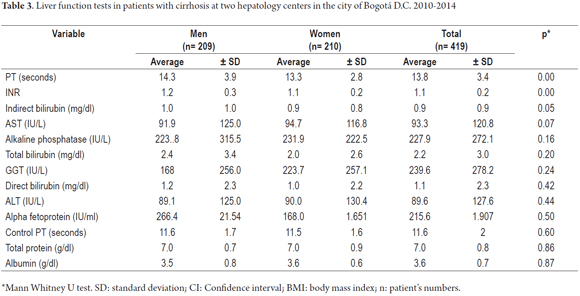
Statistically significant differences for paraclinical test results between men and women with cirrhosis were found leukocyte counts, hemoglobin, hematocrit, creatinine, triglycerides, urea nitrogen and potassium (p <0.05). Other results showed no such differences (Table 2). Specific liver function tests reported significant differences in the values of PT, INR and direct bilirubin (p <0.05) (Table 3).
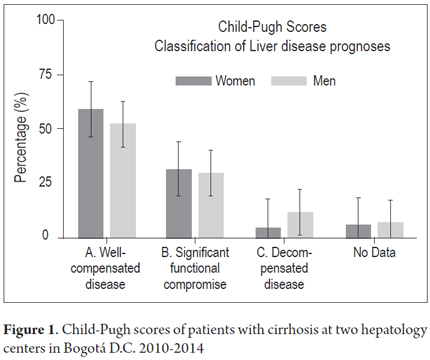
Child-Pugh scores were calculated for 394 patients. There were 233 Class A patients with well-compensated cirrhosis (59.1%, 95% CI: 54.2-63.8), 128 Class B patients with significant functional impairment (32.4% 95% CI: 28.0-37.2) and 33 Class C patients with decompensated disease (8.3%, 95% CI: 6.0 -11.5). There difference between (11.5%) and women (4.3%) in this group was significant (Figure 1).
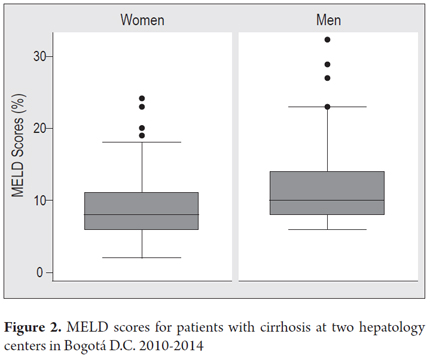
MELD (model for end-stage liver disease) scores were obtained for 341 patients (81%). Among these patients, 95% had scores less than or equal to 18 with an average score of 9 (SD ± 4.3). The highest score among the men was 32 points, while the highest score among the women was 24 points (Figure 2).
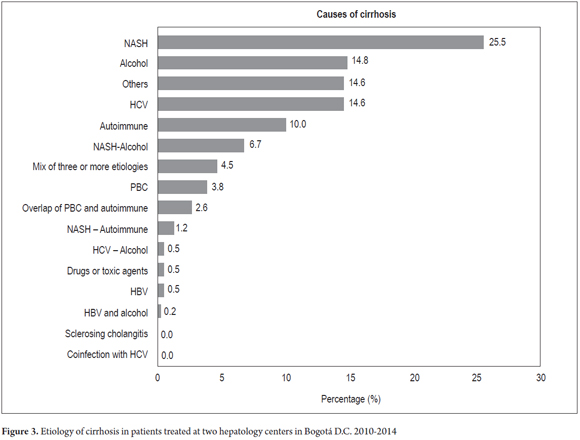
Nonalcoholic steatohepatitis (NASH in English) was the most frequent etiology for cirrhosis (25.5%, 95% CI: 21.6-29.9) followed by alcoholic cirrhosis (14.8%, 95%: 11.7-18.5), hepatitis C virus and other causes (heart, cryptogenic) (14.6%, 95% CI: 11.5-18.2) (Figure 3).
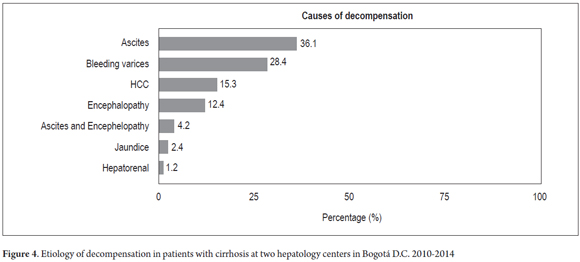
In 169 patients (40.3%), clinical decompensation was caused mainly by Ascites (36%, 95% CI 29.2- 43.5) followed by variceal bleeding (28.4%, 95% CI: 22.1-35.6) and HCC (15.3%, 95% CI: 10.7-21.5) (Figure 4). The average between diagnosis and first decompensation was 31 months (2.5 years).
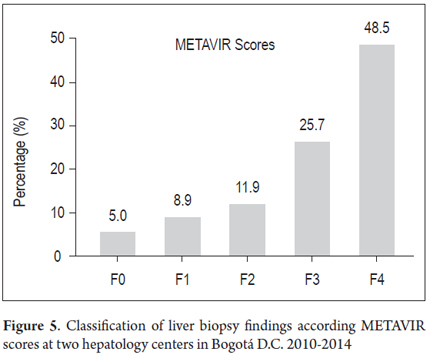
Liver biopsies of 108 patients (25.7%) were ranked with METAVIR fibrosis scores: 49 patients (48.5%) had F4 rankings, 26 patients (25.7%) had F3 rankings, 12 patients (11.9%) had F2, nine patients (8.9%) had F1, and five patients (5%) were ranked F0 (Figure 5).
DISCUSSION
In our group of patients, the average age of onset of cirrhosis was 63 years which is higher than that reported in the literature. This may have been due to etiological differences in our series. There were no differences between men and women. The main causes of cirrhosis in our patients were NASH, alcohol and HCV infections. The order of frequency in our study reversed the order of NASH and alcoholic etiology found in international studies. (2, 11, 19, 27, 28). At both of our institutions the main cause of consultation in the hepatology department is fatty liver disease (unpublished data), so the fact that NASH is the main cause of cirrhosis in this study is not strange. Instead, it alerts us to the possibility of similar data elsewhere in our country. In addition, globally about 30% of all cirrhosis patients have medical histories of metabolic syndrome and being overweight which may suggest a growing epidemic of fatty liver disease here just as elsewhere in the world. Fatty liver disease can progress to cirrhosis, and 11% of our patients presented it in the form of cirrhosis. (29, 30) In addition, between 2% and 12% of patients diagnosed with NASH have hepatocellular carcinoma. (15,20) This approaches the portion who have chronic hepatitis C infections (Two to six percent of cirrhotic patients develop HCC every year.) This reinforces the need for early detection and monitoring of patients with nonalcoholic fatty disease. (29)
Our cirrhotic patients were mostly in early stages of compensated disease: most were classified as Child-Pugh A and had MELD scores under 18 with low risks of mortality and infrequent needs for liver transplantation. (7, 11, 12, 29, 30) Women had less severe illness than men which could be due to more frequent and earlier consultations. Less than half of the patients were decompensated to one degree or another. On average they developed ascites, variceal bleeding or hepatocellular carcinoma two and a half years after diagnosis. All of these are easily diagnosed complications when there is proper monitoring as recommended by the guidelines. Patients should have ultrasound and laboratory tests every 6 months and endoscopy once a year. (12, 18, 26)
The diagnoses of most of our patients were based on clinical and radiological criteria, however 108 patients (less than a quarter) had liver biopsies taken. Of these, nearly half had fibrosis (F4 METAVIR score). The other half had other degrees of fibrosis including METAVIR scores of F0 (minimal proportion of zero). This is interpreted by the variability of the liver biopsy in relation to the reason it was done, the moment it was performed in relation to the evolution of the disease (months or years before diagnosis), sample quality and variability of interpretation among medical personnel reading the biopsy results, etc. (31-33).
CONCLUSIONS
NASH is the leading cause of cirrhosis in this study. This should alert us to a potential increase of the incidence of this disease in our midst. Ascites, variceal bleeding and hepatocellular carcinoma are the main reasons for decompensation in this series, however most of our patients had compensated cirrhosis. Multicentric studies are needed in order to compare and validate the data obtained in this series and to solidify statistics about cirrhotic patients so that we can generate own recommendations aimed at the prevention and management of cirrhosis in our country.
We recognize that the retrospective nature of this study has methodological weaknesses, nevertheless this study represents a starting point for research in this area in Colombia.
Acknowledgements
We thank our patients for allowing us to include them in this study.
Conflicts of Interests
The authors declare that they have no conflicts of interest.
Sources of Financing
The authors declare that they have no sources of funding for this study.
REFERENCES
1. Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383(9930):1749-61. [ Links ]
2. Lefton HB, Rosa A, Cohen M. Diagnosis and epidemiology of cirrhosis. Med Clin North Am. 2009;93:787-99 [ Links ]
3. Hatzakis A, Wait S, Bruix J, Buti M, Carballo M, Cavaleri M, et al. The state of hepatitis B and C in Europe: Report from the hepatitis B and C summit conference. J Viral Hepat. 2011:18Suppl1:1-16. [ Links ]
4. Anthony PP, Ishak KG, Nayak NC, Poulsen HE, Scheuer PJ, Sobin LH. The morphology of cirrhosis. Recommendations on definition, nomenclature, and classification by a working group sponsored by the World Health Organization. J Clin Pathol. 1978;31: 395-414. [ Links ]
5. Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25:195-206. [ Links ]
6. Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-69. [ Links ]
7. García-Tsao G, Friedman S, Iredale J, Pinzani M. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445-9. [ Links ]
8. Desmet VS, Roskams T. Cirrhosis reversal: A duel between dogma and myth. J Hepatol. 2004;40:860-7. [ Links ]
9. Friedman SL, Bansal MB. Reversal of hepatic fibrosis-fact or fantasy? Hepatology. 2006;43(2)Suppl1:S82-8. [ Links ]
10. Benvegnù L, Gios M, Boccato S, Alberti A. Natural history of compensated viral cirrhosis: A prospective study on the incidence and hierarchy of major complications. Gut. 2004;53:744-9. [ Links ]
11. Asrani SK, Kamath PS. Natural history of cirrhosis. Curr Gastroenterol Rep. 2013 Feb;15(2):308. [ Links ]
12. D'Amico G, García-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. J Hepatol. 2006;44:217-31. [ Links ]
13. Schuppan D, Afdhal NH: Liver cirrhosis. Lancet. 2008; 371:838–51. [ Links ]
14. D'Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: An evidence-based approach. Semin Liver Dis. 1999;19:475-505. [ Links ]
15. Nusrat S, Khan MS, Fazili J, Madhoun MF. Cirrhosis and its complications: Evidence based treatment. World J Gastroenterol. 2014 May 14;20(18):5442-60. [ Links ]
16. Starr SP, Raines D. Cirrhosis: Diagnosis, management, and prevention. Am Fam Physician. 2011; Dec 15;84(12):1353-9. [ Links ]
17. Parkin DM, Bray F, Ferlay J, and Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:2:153–6. [ Links ]
18. Bruix J and Sherman M. Management of hepatocellular carcinoma. Hepatology, 2005; 42, 5,1208–36. [ Links ]
19. White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10:1342. [ Links ]
20. Bruix J. Hepatocellular carcinoma: Paving the road for further developments. Bruix J. Semin Liver Dis. 2014;Nov;34(4):361. [ Links ]
21. Child, CG, Turcotte, JG. Surgery and portal hypertension. In: The Liver and Portal Hypertension, Child, CG (Ed), Saunders, Philadelphia 1964. p.50. [ Links ]
22. Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973; 60:646. [ Links ]
23. Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464. [ Links ]
24. Brunt, EM. Nonalcoholic steatohepatitis: Definition and pathology. Semin Liver Dis. 2001;21:3. [ Links ]
25. Brunt, EM. Pathology of fatty liver disease. Mod Pathol. 2007; 20 Suppl 1:S40. [ Links ]
26. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142(7):1592. [ Links ]
27. Louie K, St Laurent S, Forssen U, Mundy L Piementa J. The high comorbidity burden of the hepatitis C virus infected population in the United States. BMC Infectious Disease. 2012;1286 [ Links ]
28. Aman W, Mousa S, Shiha G, Mousa S. Current status and future directions in the management of chronic hepatitis C. Virology J. 2012, Marzo 2, 9:57 [ Links ]
29. Prieto JE, Sánchez S, Rojas L, Huertas S. Hígado graso: aspectos clínicos en un centro de tercer nivel en Bogotá: periodo 2009-2013. Rev Col Gastroenterol. 2014;29(3),247-253. [ Links ]
30. Prieto JE, Sánchez S, Rojas L, Huertas S. Hepatitis C crónica: aspectos clínicos, serológicos y de tratamiento en dos centros de atención en Bogotá, Colombia. Rev Col Gastroenterol, 2014,29 (4),424-432. [ Links ]
31. Al Knawy B, Shiffman M. Percutaneous liver biopsy in clinical practice. Liver Int. 2007 Nov;27(9):1166-73. [ Links ]
32. Brunt EM. Nonalcoholic fatty liver disease: What the pathologist can tell the clinician. Dig Dis. 2012;30 Suppl 1:61-8. [ Links ]
33. Law K Brunt EM. Nonalcoholic fatty liver disease. Clin Liver Dis. 2010 Nov;14(4):591-604. [ Links ]











 texto em
texto em