Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.31 no.1 Bogotá Jan./Mar. 2016
Idiopathic Hepatic Angiosarcoma and Heart Metastasis
Pedro Rosales Torres MD (1), Rafael Pila Pérez MD (1), Rafael Pila Peláez MD (1), Pedro León Acosta MD (1), Yoanis Socarras Vidal MD (1)
(1) Internal Medicine at Manuel Ascunce Domenech Provincial Teaching Hospital in Camagüey, Cuba.
Received: 22-06-15 Accepted: 26-01-16
Abstract
Hepatic angiosarcoma is a rare malignancy whose incidence is reported to be from 0.5% to 2% of primary liver tumors. Late diagnosis is defined as diagnosis after the liver has been compromised and surgical therapy is no longer possible. Most hepatic angiosarcomas (75%) are idiopathic, and clinical and radiological findings are nonspecific. Diagnosis is mainly by histopathology. We report the case of a 34-year-old male patient with no history of interest who had had a liver tumor for four months without specific symptoms. Cardiovascular disturbances led to death by cardiac failure. An autopsy and histopathology revealed liver angiosarcoma that had metastasized to the heart and pleural cavity.
Keywords
Liver angiosarcoma, liver tumors, cardiac metastases.
INTRODUCTION
Hepatic angiosarcoma (HA) is a mesenchymal neoplasm that accounts for 0.2- 2.0 % of all primary tumors of the liver. (1) HA is a rare type of sarcoma in which malignant cells express the morphological and functional properties of endothelial cells. It accounts for less than 1% of all sarcomas. (1) Because of its relative rarity, there are no large series in the literature, physicians have little experience with these tumors, and the criteria for handling them are approximate.
They occur between the second and eighth decades of life, are rare among children and teenagers, and have peak incidence between 60 and 70 years of age. Three times as many men as women develop these tumors. A series of cases in Britain and the United States reported that the annual incidence is 0.14 to 0.25 cases per million. (1, 2) Because it is a difficult disease to diagnose, most of the time it is reported in post mortem series reaching 100% of patients. (1, 3) The cause is idiopathic in 75% of cases, although they can be secondary to exposure to carcinogens, particularly vinyl chloride, thorotrast, arsenic, estrogenic compounds, anabolic steroids, cyclophosphamide and phenelzine. It can also be caused by Von Recklinghausens disease, hemochromatosis and cirrhosis. (1-4) Involvement of various mutations of genes has been postulated for these tumors. (5) Exposure to vinyl chloride causes mutations in the K-ras-2 and p 53 proteins which are commonly found in liver sarcomas. (5) Hypermethylation of the p16 tumor suppresser gene also plays a decisive role in the pathogenesis of HA. (5)
The aim of this paper is to present an extremely rare case of a young patient with idiopathic HA which had metastasized to the heart.
CLINICAL CASE
The patient was a 34 year old mestizo man who worked as a high school teacher. He had no personal or family history of pathological interest: he did not smoke, drink alcohol, or ingest or have contact with toxic substances. For three months prior to consultation he had had problems in the right upper quadrant. At first it was just a nuisance, but as time passed it became more intense, fixed, and persistent. It was accompanied by mild jaundice of the sclera and skin. He developed a fever which improved with antipyretics. He developed asthenia, was markedly anorexic and had lost about 8 kg from the onset of symptoms. He began to have very frequent palpitations, to feel fatigued and to experience difficult breathing to the point of dizziness. Upon consultation with a cardiologist he was referred to our department.
Physical examination showed serious constitutional syndrome with marked asthenia, anorexia, and a 390 C fever. He had jaundice of his skin and mucous membranes, pallid skin with petechiae and ecchymosis scattered across his chest and other parts of his body. He had peripheral lymphadenopathy and bilateral lower limb edema. His respiratory rate was 30 breathes per minute and he had bilateral vesicular murmurs. He had muted cardiovascular sounds, tachycardia and arrhythmia. His blood pressure was 110/70mmHg and his central heart rate was 112 beats per minute. He had a hard, 10 cm hepatomegaly which was painful and had a nodular consistency. He also had a splenomegaly that was grade II on the Boye scale. The rest of the physical examination showed no abnormalities.
ANALYTICAL STUDY
Hgb: 8.5 g/L; Hematocrit: 0.26 %; Leukocyte count: 12,000 × 10-9/L (normal differential count); Platelet count: 70,000 × 10-3/L; Erythrocyte sedimentation rate: 88 mm/hour; Glucose: normal; Creatinine: normal; Ions: normal; Kidney function: normal; Pancreatic amylase: normal; VDRL: normal; HIV: negative; Total Bilirubin: 23 mmol/L (normal range: 5-17 mmol∕L); Indirect Bilirubin: 8 mmol/L; Direct Bilirubin: 5 mmol/L (normal range: 0 - 4 mmol/L); AST: 300 IU/L (normal range: 5-30 U/L); ALT: 145 mmol/L (normal range: 5-30 mmol/L); Lactate dehydrogenase: 50 IU/L (normal range: 130-300 mmol/L); Gamma glutamyl transferase: 80 IU (normal range: 8-35 IU); Alkaline phosphatase: 300 IU/L (normal range: 40-100); Alpha-fetoprotein: 10 ng/ml (normal range 1-20); Surface antigen for hepatitis B: unreactive; Surface antigen for hepatitis C: unreactive; Prothrombin Time: C 14 P 26; Serum iron: 5 mmol/L (normal range 14-28); Reticulocyte count, clotting and bleeding: normal; Total serum protein: 50 g/L (normal range 60-80 g/L).
A chest x-ray showed moderate amounts of bilateral pleural effusion and increased volume of the cardiac area at the expense of both cavities. An ECG showed blockage of the left e branch of the bundle of His, and tachyarrhythmia due to atrial fibrillation. Abdominal ultrasound showed diffuse increase in the size of the liver which had an irregular appearance with 5-25 mm nodules scattered about. There was no bile duct dilatation, but the spleen was enlarged to 135 mm. An abdominal and chest CT scan showed pleural effusion, bilateral enlargement of atria and ventricles with pericardial effusion and the same abdominal characteristics found with ultrasound. It also showed a small amount of fluid in the abdominal cavity.
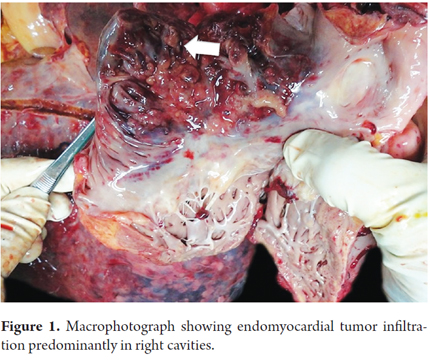
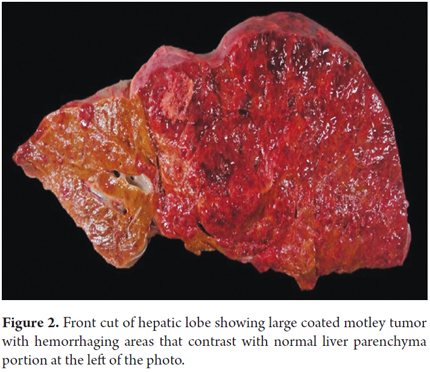
Seven days after admission, while the patient was being prepared for a CT guided liver biopsy, the patient developed cardiac tamponade which required pericardiocentesis. Approximately 1051 ml of serosanguineous liquid was extracted, but the patient died within two hours. An autopsy showed idiopathic hepatic angiosarcoma with metastases primarily in the heart and pleura. (Figure 1, 2, 3, 4, 5, 6 and 7).
DISCUSSION
The liver tumors most commonly described are hepatocellular carcinoma and cholangiocarcinoma. Primary HA are very difficult to differentiate with imaging techniques from other vascular tumors of the liver. (1, 2) These tumors are commonly multifocal and can also be found in the spleen and bone marrow. (3) In our case, it was multifocal, and both the liver and spleen were affected. Clinical presentation includes signs and symptoms related to liver disease such as hepatomegaly, ascites, abdominal pain and weight loss (62%) all of which were found in our patient. It may present as an acute abdomen hemoperitoneum due to rupture of the tumor (15%) or as splenomegaly (5%) which was found in our patient. It can also present as fever of unknown origin. (1-5)
Distant metastases occur mainly in the bones, lymph nodes, spleen and lungs (9%). Our patients case was striking because the tumor had metastasized to the heart and the pleura which had not been previously reported. (1-4,6) Liver failure resulting from replacement of hepatocytes by tumor cells leading to secondary necrosis is rare but occurred in our patient. (3, 5) Analytical parameters are not specific, but more than 50% of patients have thrombocytopenia, abnormal liver functioning, cholestasis more often than cytolysis and iron deficiency anemia syndrome. This is thought to be secondary to sequestration that originates in tumors and is associated with vascularity. Tumor markers are negative. (1-6) True thrombocytopenia occurs only in individuals with massive hemoperitoneum and platelet consumption. (2)
Imaging studies show various patterns due to necrosis and hemorrhaging. (1-3,7) CT scans show tumors with multiple hypodense masses. These findings are similar to those for hemangiomas. (7) MRIs show heterogeneous and hypervascular hemorrhagiing of all dominant masses. (7) Differential diagnosis must be established among visceral Kaposis sarcoma, hemorrhaging hepatocellular carcinoma, vascular leiomyosarcoma, malignant epithelioid hemangioendothelioma, diffuse metastatic processes and peliosis. (1-4, 6)
Cirrhosis is the most important factor for radiological differential diagnosis, since more than 80% of hepatocellular carcinoma is associated with cirrhosis. (1-4, 6, 7) Macroscopically, the tumor is formed by gray areas which alternate with foci of hemorrhages with large cavities. (1-4) This was observed in our patient. In most cases the definitive diagnosis is based on histopathological findings showing large cavernous spaces with papillary projections into the light, tumor cells, pleomorphic cells, hyperchromatic cells, and occasionally multinucleated cells and with scant cytoplasm. Ideally, diagnosis should be confirmed with immunohistochemical markers such as CD-34 and CD-31. (8) In many cases samples obtained by fine needle aspiration (FNA) are inconclusive, so a liver biopsy is always recommended. To avoid bleeding from these highly vascularized tumors, the biopsy should be guided by imaging. (4)
The prognosis is poor for a patient who has developed HA: overall survival at 5 years is 35%. (1-6) Cases which have not metastasized have a 5-year survival rate of 60%, with median survival time of 7 months. (2, 3) This patients survival time from the onset of symptoms was three to four months with metastases mainly in the heart. Treatment for HA is primarily surgical. Nevertheless, due to the usually advanced stage at diagnosis, its role is limited to those cases for which it might improve the patients chances of survival. Laparoscopy should be the surgical technique of choice because it offers a direct view of the abdominal cavity and provides useful information about tumor stage and prognosis. (4, 9) Many authors believe that in case of advanced disease, ascites or peritoneal carcinomatosis, laparoscopic surgery should be the technique of choice. In general, the use of radiation therapy after resection of smaller localized sarcomas is recommended even though tumors may be resistant to radiation. (3) Whether or not liver transplantation is indicated is controversial, but the first choice of treatment for metastatic HA is cytotoxic chemotherapy even though the evidence is inconclusive. The main drugs used are anthracyclines, taxanes and iphosphamides. (3, 7 - 10)
CONCLUSIONS
Hepatic angiosarcoma is a type of liver cancer that appears in Kupffer cells and endothelial cells lining the blood vessels of the liver. It is an extremely rare malignancy that is considered to be idiopathic because possible etiologies are difficult to demonstrate. If not completely removed, the disease is fatal within six months. The prognosis is poor, since only 6-15% of these tumors can be removed with surgery.
Conflicts of interests
The authors have received no financial or other support for this study.
REFERENCES
1. Flores Rivera OI, Quintana Quintana M, Frias Aguirre YN, González Cervantes JG, Baena Ocampo L. Angiosarcoma hepático: reporte de un caso y revisión bibliográfica. Med Int Mex. 2012;28(5):520-530. [ Links ]
2. Granado López SL, Gómez Jiménez LM, Chávez Bravo NC, Sánchez Rodríguez C. Angiosarcoma hepático idiopático. Informe de un caso. Rev Med Inst Mex Seguro Soc. 2012;50(4):445-448. [ Links ]
3. Young RJ, Brown NJ, Reed MW, Hughes D, Xxoll PJ. Angiosarcomas. Lancet Oncol. 2010;11:983-991. [ Links ]
4. Poggi Macituca L, Ibarra Chirinos O, López del Aguila J, Villanueva Pffucker M, Camacho Zacarías F, Tagle Arroslpide M, et al. Angiosarcoma hepático: reporte de un caso y revisión de la literatura. Rev Gastroenterol Perú. 2012;32 (3):178-186. [ Links ]
5. Bhati CH S, Bhatt AN, Starkey G, Hubscher SG, Bramhatt SR. Acute liver failure due to primary angiosarcoma: A case report and review of literature. World J Surg Oncol. 2008;6:104-109. [ Links ]
6. Valenzuela J, Poveda MG. Angiosarcoma hepático. Presentación de dos casos. Rev Esp Enferm Dig. 2009;101(6):430-437. [ Links ]
7. Koyama T, Fletcher JG, Johnson CD, Kuo MS, Notohara K, Burgart LJ. Primary Hepatic Angiosarcoma: findings at CT, med MR imaging. Radiology. 2002;222(3):6667-673. [ Links ]
8. Ying CH. Liver Angiosarcoma a rare malignancy presented with intraabdominal bleeding due to ruptura: A case report. World J Surg Oncol. 2012;10:23-28. [ Links ]
9. Kim HR, RHA Sy, Cheon SH, Roh JK, Park YN, Yoo NC. Clinical features and treatment outcomes of advanced stage primary hepatic Angiosarcoma. An Oncol. 2009;20:780-787. [ Links ]
10. Yasunaka Y, Ikeda F, Kobashi H, Miyake Y, Takaki A, Iwasaki T, et al. Hepatic Angiosarcoma with characteristic laparoscopy findings. Digest Endosc: Official Jpn J Gastroenterol Endosc Soc. 2012;24(2):124. [ Links ]











 text in
text in