Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.31 no.2 Bogotá Apr./June 2016
The experience of the Fundacion Valle Del Lili in Cali, Colombia with Combined Liver and Kidney Transplantation
Luis Armando Caicedo R. MD (1,3), Cristina Vernaza O. MD (2), María Lucía Valderrama M. MD (2), Eliana Manzi T. MD (2), Gabriel Jaime Echeverri J. MD (1,3), Diego Fernando Jimenez R. MD (1,3), Jairo Alberto García A. MD (1,3), Mauricio Sepúlveda C. MD (1,3), Liliana Mesa R. MD (1,3), Juan Guillermo Posada Ch. MD (1,3), Johana Schweineberg L. MD (1,3), Oscar Javier Serrano A. MD (1,3), Jorge Iván Villegas O. MD (1,3), Verónica Botero O. MD (1,3), Carlos Eduardo Durán R. MD (1,3)
(1) Transplant Unit of the Fundación Valle del Lili in Cali, Colombia.
(2) Clinical Research Center of the Fundación Valle del Lili in Cali, Colombia.
(3) CICAT (Centro para la investigación en cirugía avanzada y trasplantes - Center for research in advanced surgery and transplantation) at the Faculty of Health Sciences of University ICESI in Cali, Colombia.
Funding: There was no funding from sources other than the Fundación Valle del Lili.
This study was presented at the XXII Latin American and Caribbean Transplant Congress held in Buenos Aires, Argentina from December 1 to December 4, 2013.
Received: 21-07-15 Accepted: 18-04-16
Abstract
Introduction: Combined liver and kidney transplantation (CLK) has been shown to be a good alternative for patients with concomitant diagnosis of chronic kidney disease (CKD) and end-stage liver disease. Some studies have also shown immunological benefits from combined transplantation with decreased rates of kidney graft rejection.
Objective: The objective of this study was to describe the indications and clinical outcomes of CLK transplant recipients in a highly complex hospital.
Materials and Methods: CLK transplant patients were selected from the institutional transplant registry (Trenal) from 2000 to 2011. Demographic and clinical characteristics were described and survival of patients and grafts were estimated with the Kaplan-Meier method.
Results: Over a period of 11 years, 16 CLK transplants were performed. This was 1.51% of kidney transplants and 3.48% of liver transplants done in the institution during this period. Most recipients were male (10/16). The median age was 56.5 years. The median MELD was 17 (IQR: 12.5 to 20.5, range: 8 to 24). The most frequent diagnosis was liver cirrhosis due to NASH (4/16). All patients had been diagnosed with CKD: four cases were secondary to diabetes mellitus. The most common indications for transplants were difficult to manage ascites, recurrent encephalopathy and malnutrition. The average liver cold ischemia time was 7.3 hours, and the average kidney cold ischemia time 9.6 hours. The five-year liver graft survival rate was 87.5%, and the five-year kidney graft survival rate was 67.3%. Four patients died: two as the result of sepsis and two as the result of malignancy.
Conclusion: CLK transplantation results at the Fundación Valle del Lili have been satisfactory and comparable to those reported by other transplant centers.
Keywords
Combined liver-kidney transplant, survival, graft rejection.
INTRODUCTION
The first combined liver and kidney transplantation (CLK - Combined Liver-Kidney) was reported by Margreiter in 1984. (1) CLK transplantation has shown itself to be a good therapeutic alternative for patients with terminal liver disease and chronic kidney disease. (2) According to the OPTN (Organ Procurement and Transplantation Network), 5,816 CLK transplants were conducted in the United States between 1988 and June 2014. The recommendations of the American consensus on transplant CLK show the survival benefits for patients with pre-transplant serum creatinine> 2.0 mg / dL and who were on dialysis who underwent CLK compared to similar patients who received only liver transplants. (3). Various studies have found that liver transplantation protects the renal graft and decreases the rate of acute renal graft rejection. (4, 5) CLK transplantation also improves patient quality of life by allowing people with chronic kidney disease (CKD) to live independently of dialysis. In addition, it has benefits in terms of the costs involved. (6) The Fundación Valle del Lili is a clinic that has had extensive experience in solid organ transplantation since 1995. In 2000, the foundation performed its first CLK transplant. Since then, CLKs have been performed on 16 patients. The purpose of this study was to describe our experience in the management of patients with combined liver and kidney transplant.
PATIENTS AND METHODS
Data were obtained from the kidney and liver transplantation registry of TRENAL (Translational kidney research - from physiology to clinical application). Since 2010, this registry has existed. It includes information from renal transplants performed since 1995 at the Fundación Valle del Lili. All CLK transplant patients between 2000 and 2011 have been included. Demographic and clinical information related to the reason the transplantation was performed, early surgical and medical complications (in the first three months post-transplant) and late complications (occurring three months or more after transplant) were systematically collected. Admission to the waiting list was determined by pretransplant clinical evaluation, and each case was discussed on the board of the institution. At the time of transplant, the recipient was selected on the basis of blood group compatibility. No cytotoxicity tests of the donor were done prior to transplantation.
Immunosuppression was administered according to the immunological and clinical conditions of each patient. Fifteen patients received a calcineurin inhibitor, thirteen received mycophenolate, two received azathioprine, and sixteen were given steroids. Induction of tolerance by monoclonal antibody therapy was used in three cases. Post-transplant follow-up was based on regular monthly check-ups of patients. Demographic and clinical characteristics of patients were described. Averages and interquartile ranges (IQR) or averages and ± standard deviation (SD) were used for quantitative variables. Proportions were used for qualitative variables. Patient survival time and renal and liver graft survival times were calculated with the Kaplan Meyer method using STATA 12.
RESULTS
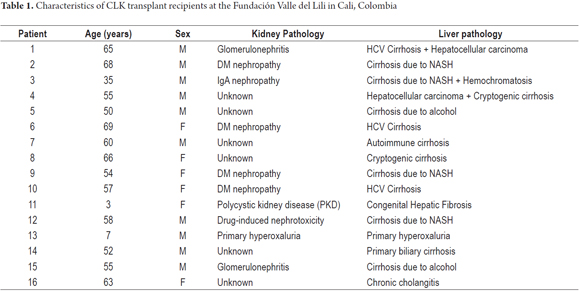
Between 2000 and 2011, 1059 kidney transplants and 459 liver transplants were done at this institution. In the same period, 16 CLK transplants CLK were performed (1.51% of all kidney transplants and 3.48% of all liver transplants). Ten patients were men, and six were women. The median age at transplantation was 56.5 years (IQR 50.7 to 64.5, range 3 to 69). This includes two pediatric patients aged three and seven years old. Patients' liver and kidney disease etiologies are described in Table 1. One patient had a pre-transplant liver biopsy which showed primary biliary cirrhosis. Three patients had pretransplant kidney biopsies two of which showed proliferative glomerulonephritis and one of which showed terminal kidney failure.
Patient's medical histories showed that six patients had Type 2 diabetes mellitus, four had dyslipidemia, three had arterial hypertension, and three had histories of malignancy. The median MELD (Model for End-stage Liver Disease) score was 17 (IQR 12.5 to 20.5, range 8-240,029) One of two pediatric patients had a PELD (Pediatric End-Stage Liver Disease) score of 1 and had been diagnosed with congenital hepatic fibrosis. The PELD score was not calculated for the other pediatric patient because the diagnosis indicated primary hyperoxaluria transplantation. The mean cold ischemia time for livers was 7.3 hours with SD ± 2.3 (range 4-12). For kidneys it was 9.6 hours with SD ± 2.2 (range 6-12). The average time on the waiting list was 157 days, with SD ± 112 (range 1-422). Two patients were transplanted at zero urgency. The median follow up time was 35 months (IQR 18-48, range from 0.07 to 156). Three patients received monoclonal antibody therapy to induce tolerance: one received daclizumab and two received basiliximab.
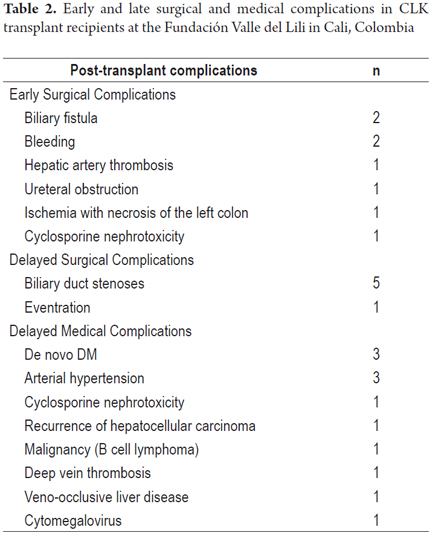
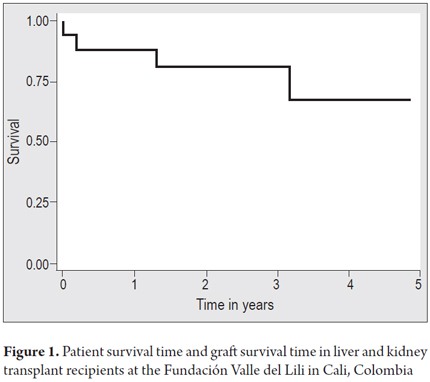
Table 2 shows surgical and medical complications. At least one complication was documented in each of 11 patients (68%). Three patients had early biliary complications, five patients had late biliary complications, two suffered from bleeding, and one developed a cytomegalovirus infection. Three patients were diagnosed with mild acute liver graft rejection. Two of these occurred between 3 and 9 months after transplant, and one occurred 10 years after transplantation. These diagnoses were made on the bases of clinical signs associated with abnormal liver profiles and confirmed by biopsies in two patients and in one by the patient's response to empirical treatment. The 7-year-old patient was diagnosed with acute renal graft rejection by biopsy three months after transplantation. All events of acute rejection were treated with steroids, and all patients responded to treatment. The one year survival rate for patients, liver grafts and kidney grafts was 87.5% while the three five year survival rates were all 67.3% (Figure 1). Two patients died as the result of sepsis: one in the immediate postoperative period and the other in the third month after transplantation. Two patients died of cancer after the first year after transplantation. One had B-cell lymphoma and the other suffered recurrence of hepatocellular carcinoma. Of the 12 patients whose grafts continue to function, none has required retransplantation.
DISCUSSION
The combined liver and kidney transplant program at the Fundación Valle del Lili has a low incidence of acute rejection of renal allografts which is similar to those reported by other transplant groups (Table 2). (2,7,8) In the literature, the rate of occurrence of major complications in combined kidney and liver transplants is no higher than that of complications of liver transplants alone. (9-13) In our study population, five year patient, kidney graft and liver graft survival rates were all 67.3%. These data are similar to those reported in other series as well as in the database of United Network for Organ Sharing (UNOS) which reports a five year survival rate of 58%. (7,11,14-17) The five year survival rates for hepatic and renal grafts censored for death was 100%. Of the 16 combined liver and kidney transplants performed at our institution, only one pediatric patient (6.7%) had acute renal graft rejection in the third month after transplantation. Two patients (12.5%) had mild acute liver graft rejection in the first year. According to our statistics (unpublished data), in the same periods of time after transplantation incidences of acute rejection were higher for patients who received only a kidney transplant (20%) as well as for those who received only a liver transplant (21%).
Creput et al. reported that the incidence of acute rejection, for both kidney and liver grafts in patients with simultaneous liver and kidney transplant was lower than for patients with liver or kidney transplants alone. (17) Fong and colleagues have also reported a comparison of the incidence of acute kidney graft rejection in patients who had only kidney transplants with those who underwent CLK transplants showed a lower incidence in the CLK transplant patients. (14) A review of other related reports found that the incidence of acute renal graft rejection annually in patients receiving either liver or kidney transplantation is about 18%, while in patients with combined liver and kidney transplants the rate was 11.8%. (2) This is explained by publications describing the protective role of hepatic graft for kidney graft functioning not only in the initial phase after transplantation but also in long term maintenance. This occurs through reduction of levels of reaction to donor specific antibodies. (3) This may be a result of mediation by activation of the enzyme indoleamine 2,3-dioxygenase (IDO). (18)
CONCLUSIONS
The MELD score which incorporates renal function was created to improve efficiency, reduce mortality of patients on the waiting list and make entry onto the transplant list more objective. Its use has increased the number of combined liver and kidney transplants. Since this occurred, there has been a decrease of CKD among liver transplant recipients, shorter stays in the ICU and lower health care costs as a result of the immune protection of kidney graft by the liver in patients who undergo combined transplantation. (2) What is clear is that the use of calcineurin inhibitors, drug interactions and the risk of infection after liver transplantation cause kidney disease to progress. It also appears that the results of simultaneous combined liver and kidney transplantation are better than the results of a sequential transplantation of liver first followed by kidney. (2, 9)
Indications for simultaneous liver-kidney transplant are clear when the patient is on dialysis with irreversible kidney disease and for patients with glomerular filtration rates (GFR) less than 40 mL/min (Although some authors define the limit at 30 mL/min.) (16, 19) Independent of GFR, irreversible chronic renal failure is an indication for combined liver kidney transplantation given the nephrotoxic effects on native kidneys of calcineurin inhibitors which are used after liver transplantation. (9, 12, 15) Determination of the possibility of recovering functioning of native kidneys after liver transplantation has been of particular interest. In order to improve decision making about allocation of organs available for transplantation, studies have been conducted that include performance of radioisotope renography using either MAG3 or DTPA before and after transplantation and the use of biomarkers in serum creatinine. (20,21). Our study did not document changes in the functioning of native kidneys that may have occurred after transplantation, but this is an topic for future studies. Combined liver and kidney transplantation at our center compares well with those reported by medical centers in other countries which have more experience. The clinical outcomes have proven that this is a good therapeutic option for patients who have concurrent terminal liver disease and chronic kidney disease.
REFERENCES
1. Margreiter R, Kramar R, Huber C, Steiner E, Niederwieser D, Judmaier G, et al. Combined liver and kidney transplantation. Lancet. 1984;1(8385):1077-8. [ Links ]
2. Simpson N, Cho YW, Cicciarelli JC, Selby RR, Fong T-L. Comparison of renal allograft outcomes in combined liver-kidney transplantation versus subsequent kidney transplantation in liver transplant recipients: Analysis of UNOS Database. Transplantation. 2006;82(10):1298-303. [ Links ]
3. Eason JD, Gonwa TA, Davis CL, Sung RS, Gerber D, Bloom RD. Proceedings of Consensus Conference on Simultaneous Liver Kidney Transplantation (SLK). Am J Transplant. 2008;8(11):2243-51. [ Links ]
4. Olausson M, Mjörnstedt L, Nordén G, Rydberg L, Mölne J, Bäckman L, et al. Successful combined partial auxiliary liver and kidney transplantation in highly sensitized cross-match positive recipients. Am J Transplant. 2007;7(1):130-6. [ Links ]
5. Alqurashi S, Alsayyari AA, Abdullah K, Alwan A, Hajeer AH. Combined liver and kidney transplantation in a highly sensitized and positively cross-matched patient. Saudi J Kidney Dis Transpl. 2011;22(4):757-60. [ Links ]
6. Rosselli D. CS3 Un modelo de costo-utilidad del trasplante renal en Colombia. Value in Health. 2009;12(7):A484. [ Links ]
7. JL GB. Evolución del injerto renal en los pacientes con trasplante hepático asociado. En: Portillo Martín JA BDR, Zubillaga Guerrero S, Ramos Barselo E, Campos Sañudo JA, ed., Actas Urol Esp. 2008:220-4. [ Links ]
8. Fung J, Makowka L, Tzakis A, Klintmalm G, Duquesnoy R, Gordon R, et al. Combined liver-kidney transplantation: analysis of patients with preformed lymphocytotoxic antibodies. Transplant Proc. 1988;20(1 Suppl 1):88-91. [ Links ]
9. Martin EF, Huang J, Xiang Q, Klein JP, Bajaj J, Saeian K. Recipient survival and graft survival are not diminished by simultaneous liver-kidney transplantation: an analysis of the united network for organ sharing database. Liver Transpl. 2012;18(8):914-29. [ Links ]
10. Ma Y, Wang G, He X, Li Q, Li J, Zhu X, et al. Simultaneous liver and kidney transplantation: analysis of a single-center experience. Chin Med J. 2010;123(10):1259-63. [ Links ]
11. Ruiz R, Kunitake H, Wilkinson AH, Danovitch GM, Farmer DG, Ghobrial RM, et al. Long-term analysis of combined liver and kidney transplantation at a single center. Arch Surg. agosto de 2006;141(8):735-741-742. [ Links ]
12. Veras FJ de O, Coelho GR, Feitosa-Neto BA, Cerqueira JBG, Garcia RCFG, Garcia JHP. Combined liver-kidney transplantation: experience at a Brazilian university hospital. Arq Bras Cir Dig. 2014;27(1):53-5. [ Links ]
13. González MR, Ramírez P, Cascales P, Domingo J, López MD, Rios A, et al. Thirteen cases of liver-kidney transplantation. Transplant Proc. 2010;42(8):3162-3. [ Links ]
14. Fong T-L, Bunnapradist S, Jordan SC, Selby RR, Cho YW. Analysis of the United Network for Organ Sharing database comparing renal allografts and patient survival in combined liver-kidney transplantation with the contralateral allografts in kidney alone or kidney-pancreas transplantation. Transplantation. 2003;76(2):348-53. [ Links ]
15. Xing T, Zhong L, Chen D, Peng Z. Experience of combined liver-kidney transplantation for acute-on-chronic liver failure patients with renal dysfunction. Transplant Proc. 2013;45(6):2307-13. [ Links ]
16. Haad CR, Rodriguez-Benot A, Martinez-Vaquera S, Navarro-Cabello MD, Aguera-Morales ML, Ruiz de Mier MVP, et al. Combined liver-kidney transplantation: survey of a single center in Spain. Transplant Proc. 2013;45(10):3640-3. [ Links ]
17. Creput C, Durrbach A, Samuel D, Eschwege P, Amor M, Kriaa F, et al. Incidence of renal and liver rejection and patient survival rate following combined liver and kidney transplantation. Am J Transplant. 2003;3(3):348-56. [ Links ]
18. Ingelsten M, Gustafsson K, Oltean M, Karlsson-Parra A, Olausson M, Haraldsson B, et al. Is indoleamine 2,3-dioxygenase important for graft acceptance in highly sensitized patients after combined auxiliary liver-kidney transplantation? Transplantation. 2009;88(7):911-9. [ Links ]
19. Hanish SI, Samaniego M, Mezrich JD, Foley DP, Leverson GE, Lorentzen DF, et al. Outcomes of simultaneous liver/kidney transplants are equivalent to kidney transplant alone: a preliminary report. Transplantation. 2010;90(1):52-60. [ Links ]
20. Francis JM, Palmer MR, Donohoe K, Curry M, Johnson SR, Karp SJ, et al. Evaluation of native kidney recovery after simultaneous liver-kidney transplantation. Transplantation. 2012;93(5):530-5. [ Links ]
21. Levitsky J, Baker T, Ahya SN, Levin ML, Friedewald J, Gallon L, et al. Outcomes and native renal recovery following simultaneous liver-kidney transplantation. Am J Transplant. 2012;12(11):2949-57. [ Links ]











 text in
text in