Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.31 no.2 Bogotá Apr./June 2016
Descriptive Analysis of Transactional Database Date on the Use of Gastroprotective Drugs in Patients With Polypharmacy in a Colombian Population
Álvaro Vallejos N. MD Msc (1), Laura Maldonado C. (2), Juan Camilo Calvache V. MD (3), William Hernandez D. (4), Sandra Torres R. (5), Dieric Diaz S. MD Esp. (6)
(1) Surgeon, MSc in Pharmacology, MSc in Medical Education, Specialist in Epidemiology, Member of the Colombian Association of Clinical Pharmacology and Vice President of Servicios Clínicos de Mc21 Colombia S.A.S. in Bogotá, Colombia.
(2) Pharmaceutical Chemist at the National University of Colombia. Clinical Pharmacist at Servicios Clínicos de Mc21 Colombia S.A.S. in Bogotá, Colombia. Mail: lmaldonado@mc21colombia.com
(3) Surgeon at the National University of Colombia, Member of the pharmaceutical epidemiology research group of Servicios Clínicos de Mc21 Colombia S.A.S. in Bogotá, Colombia.
(4) Pharmaceutical Chemist at the National University of Colombia and Clinical Pharmacist at Servicios Clínicos de Mc21 Colombia S.A.S. in Bogotá, Colombia.
(5) Pharmaceutical Chemist at the National University of Colombia, MSc in Pharmaceutical Attention, Candidate for specialization in Epidemiology, Senior Clinical Pharmacist at Servicios Clínicos de Mc21 Colombia S.A.S. in Bogotá, Colombia.
(6) Specialist in Epidemiology, Candidate for MSc in Pharmacology, Physician at Servicios Clínicos de Mc21 Colombia S.A.S. in Bogotá, Colombia.
This study was presented as a poster in the XV Colombian Congress of Pharmacology and Therapeutics held in Pereira, Colombia from August 14 to August 16, 2015.
Received: 24-09-15 Accepted: 18-04-16
Abstract
Objective: The objective of this study was to describe and analyze patterns of use of prescription ulcer drugs, factors associated with prescriptions by physicians, and costs for outpatients With polypharmacy at a Colombian healthcare promotion entity (EPS) over a six-month period.
Methodology: This is a retrospective and descriptive cross-sectional study based on electronic records of drugs prescribed to 2,458,447 outpatient members. Patients who took five or more prescription drugs per month were included. Patient were excluded if their records in the transactional data were not complete enough for analysis. Associations between anti-ulcer drugs and factors justifying their prescription were evaluated by the odds ratio (OR) which was calculated from a logistic regression model.
Results: Of the 2,458,447 affiliates of the EPS, on average 60,671 patients had polypharmacy each month: 40% used anti-ulcer drugs and 70% used gastro-damaging drugs. Of the gastroprotected patients, 47% were elderly and 12% had associated diagnoses of upper gastrointestinal risk. Gastroprotection was not justified in 35% of patients with polypharmacy. This represents 75 million Colombian pesos every month. There was no statistical association between prescription of anti-ulcer drugs and factors that justify their prescription (OR: 1.13; 95% CI: 1.00 to 1.27).
Conclusion: Given the lack of association between prescription of anti-ulcer drugs and factors which justify such prescriptions, it is likely that prescriptions contribute to polypharmacy per se. We recommended optimizing gastroprotection by reserving it for patients with more than one gastrointestinal lesion and with upper gastrointestinal risks whether or not they have polypharmacy.
Keywords
Polypharmacy, antiulcer, proton-pump inhibitor, NSAIDs, elderly.
INTRODUCTION
More than 50% of all patients over 65 have two or more chronic diseases. (1) Chronicity and multimorbidity lead to polypharmacy which is defined as the use of 5 or more drugs simultaneously. (2) This increases the likelihood of visits to the doctor for adverse drug events (ADEs) by 88% and increases costs for patients and health systems. (3, 4) Gastrointestinal, psychotropic and cardiovascular medications are often associated with polypharmacy. (5) Drugs that are most frequently prescribed for chronic patients include antihypertensives (82.6%), anti-ulcer (73.8%), antithrombotics (70.3%) and nonsteroidal anti-inflammatory drugs (NSAIDs) (36.5%). (6 ) In Korea in 2002, 81.6% of internists included antiulcer medications in their prescriptions. (7, 8) Prescription of antiulcer medications is a predictor for polypharmacy. (9)
In 2014, it was clarified that only certain patients require gastroprotective medication. They include patients being treated with more than one gastrolesive drug, patients who have risk factors such as previous histories of upper gastrointestinal disease, diabetes mellitus and hypertension, patients who are 65 years of age or older, patients undergoing concomitant therapy with other gastro-damaging drugs, patients with severe comorbidities and/or patients undergoing long-term treatment at maximum doses of gastro-damaging drugs. (10, 11) Not all patients with gastro-damaging drugs or polypharmacy need gastric protection. (12) Therefore, the objective of this study was to describe and analyze patterns of prescriptions of ulcer drugs, factors associated with prescribing and outpatient costs of polypharmacy over a 6 month period throughout Colombia at the facilities of one Healthcare Promotion Entity (EPS).
MATERIALS AND METHODS
This is a descriptive retrospective cross-sectional study about patterns of prescriptions of anti-ulcer drugs to the patients of an EPS with polypharmacy. The EPS' average monthly membership is 2,458,447, it has 420 health institutions (IPS) in 136 municipalities in Colombia. The study looked at electronic records of outpatient prescriptions at the EPS over the six month period from September 2014 to February 2015. We included patients who received five or more prescription drugs per month and excluded those whose transaction records were not complete enough for analysis. Electronic prescription records were obtained from MC21 Colombia Pharmacy Benefit Management. Relations of this data to age, medical diagnosis, medication prescribed by month, anti-ulcer drugs, gastrolesive drugs and costs were then analyzed.
Patients were grouped into the four age categories established by the National Health Statistics Group in the United States: pediatric patients (0-12 years), young adult patients (13-44 years), mature adult patient (45-64 years) and elderly patients (over 65 years). (13) They were also grouped into five categories based on medical diagnoses and study objectives: gastrointestinal pathologies (gastritis; duodenitis; esophagitis; gastric, duodenal, peptic and gastric-jejunal ulcers; gastroesophageal reflux disease (GERD); esophageal, gastric and duodenal varices, and cancers of the upper gastrointestinal tract); serious pathologies (sepsis, other cancers, hepatitis and other liver diseases, chronic renal failure and other renal dysfunctions; severe trauma and severe burns); hypertension; diabetes mellitus and others. The tenth edition of the International Classification of Diseases of the World Health Organization (WHO) used to definie the five categories.
Anti-ulcer and gastrolesive drugs were grouped by pharmacological groups according to the Anatomical, Therapeutic and Chemical Classification System (ATC) of the WHO. The anti-ulcer drugs were classified as antacids (A02A), H2 antagonists (A02BA), prostaglandins (A02BB), proton-pump inhibitors (PPI) (A02BC) and direct protectors of the gastric mucosa (A02BX). Gastrolesive drugs were grouped into nonsteroidal anti-inflammatory drugs (NSAIDs) (M01A), antiplatelet agents (B01AC), anticoagulants (B01AA, B01AB, B01AE, B01AF, B01AX), selective reuptake inhibitors (SSRIs) (N06AB ) and systemic corticosteroids (H02AB).
Univariate and multivariate descriptive analyses were performed. Pearson and Phi Correlation coefficients were calculated to measure the correlation between prescription anti-ulcer medications and factors that justify prescriptions. Odds ratios (OR) were calculated with a logistic regression model. The specificity and sensitivity of the model was verified by the ROC curve (AUC: 0.62, the model predicted actual behavior at 62%). The analysis was performed using R statistical software. (14) Manipulation of variables that were dates was carried out with the Lubridate package. (15) Transformation, extraction and aggregation processes were done with the sqldf package (16) and goodness of fit was found with the pROC package. (17)
RESULTS
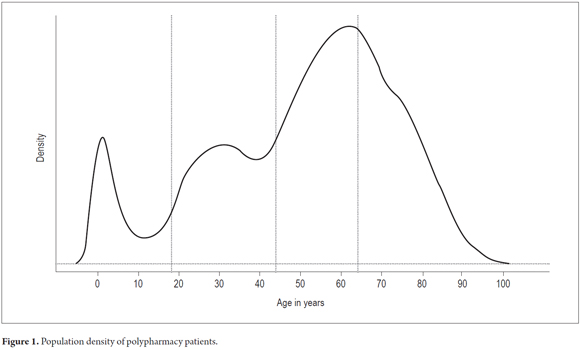
Of the 2,458,447 members per month, 13% (321,184) received medications. Of these, 18% (60,671) were cases of polypharmacy: 11% (6,644) had 10 or more prescriptions drugs and 49 patients had had 20 to 30 drugs prescribed in the same month. 31% of the polypharmacy patients (18,808) were elderly, 35% (21,235) were mature adults, 24% (14, 561) were young adults and 10% (6,067) were pediatric patients. Most patients were between 50 and 70 years (Figure 1). Nationally, the polypharmacy index (PI - polypharmacy patients/patients receiving drugs x 100) increased from 16% in November 2014 to 22% in February 2015. In that month, 22 of 100 patients who received drugs had had five or more drugs prescribed to them, and three out of 100 patients had had 10 or more active ingredients prescribed to them in the same month.
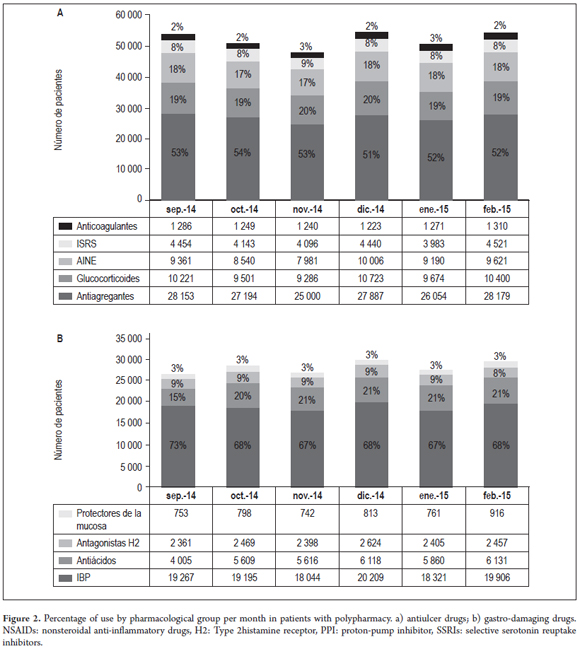
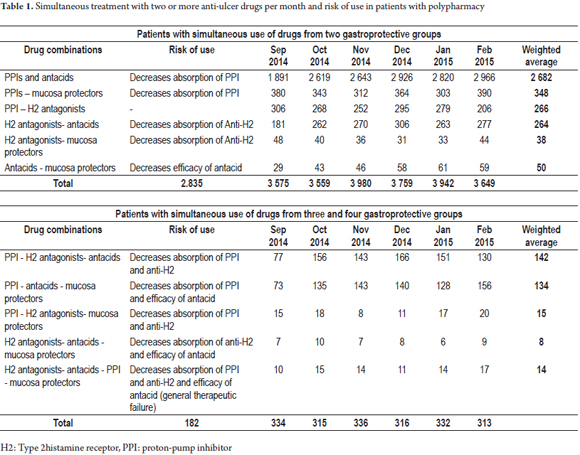
Forty percent of the polypharmacy patients (23,738) received anti-ulcer medications. PPIs were the most frequently used (19,157), followed by antacids (Figure 2a). Of the total patients receiving gastroprotection medications, 17% (4,060) had had two or more types of antiulcer drugs prescribed simultaneously. The most frequent association, PPIs with antacids, occurred in 1644 patients (65%). PPIs, antacids and sucralfate were prescribed simultaneously to 156 patients (6%) and 17 patients had prescriptions for anti-ulcer drugs from four drug groups simultaneously (PPIs, antacids, mucosal protectants and H2 antagonists) (Table 1). 70% of polypharmacy patients (42,461) had at least one gastrolesive prescription drug, and 17% (10,140) had simultaneous prescriptions for two or more gastrolesive drugs within the same month. Antiplatelet drugs were taken by 52% (27, 078) and were the most frequently prescribed gastrolesive drugs. They were followed by glucocorticoids and NSAIDs (Figure 2b).
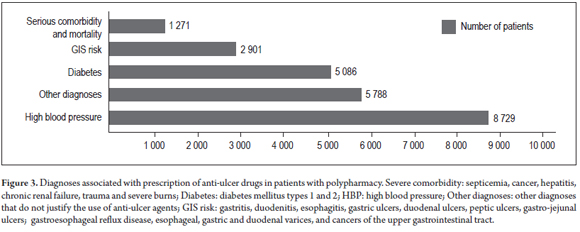
On average, every month 280 patients took four gastrolesive prescription drugs simultaneously, twenty-eight patients took five gastrolesive prescription drugs simultaneously, and one 77 year old patient had been simultaneously prescribed with seven gastrolesive drugs (3 glucocorticoids, 2 NSAIDs, and two SSRIs). Of the total number of polypharmacy patients receiving gastroprotective drugs, 47% (11,157) were elderly, 35% (8,308) were mature adults, 13% (3,086) were young adults and 5% (1,187) were pediatric patients. Only 12% (2,901) had had diagnoses of upper gastrointestinal risk and 24% (5,788) had no diagnosis that justified the use of anti-ulcer drugs (Figure 3). 48% of patients (11,182) taking gastroprotective medications did not take gastrolesive medications. Use of two or more gastrolesive agents was justified in only 13% (3,370) of the case. Patients taking gastroprotective agents included 88% of the patients using anticoagulants (1,116), 35% of those taking antiplatelet medication (8,420), 17% of those taking glucocorticoids (1,645), 11% of those taking SSRIs (452) and 10% of those using NSAIDs (924).
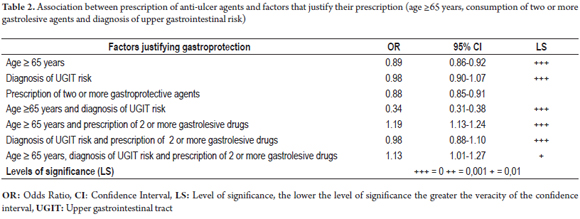
According to the transactional patient information, and pre-established criteria for prescription of gastroprotection, (10, 11), gastric protection was not justified in 35% of these patients (8,364). This represents approximately 75 million Colombian pesos (COP) every month in unjustified anti-ulcer drugs. Proper and justified prescriptions for these patients with polypharmacy would lead to an estimated savings of 450 million Colombian pesos over six months. Calculations of the Pearson and Phi correlation coefficients showed no correlation between the prescription of anti-ulcer drugs and some of the factors that justify their prescription. Correlation coefficients were between zero and 30% for age ≥65 years, consumption of 2 or more gastrolesive drugs and/or diagnosis of gastrointestinal risk. The OR estimates confirmed this result (Table 2). No single factor alone increased the likelihood of prescription of ulcer drugs in patients with polypharmacy although the use of two or more gastro-damaging drugs in patients 65 years and older increased the likelihood of an anti-ulcer drug prescription by 1.2 times (OR: 1.19, 95% CI: 1.13 to 1.24) and the use of two or more gastro-damaging drugs in patients 65 years and older who had been diagnosed with upper gastrointestinal risks increased the likelihood of prescription of anti-ulcer drugs by 1.1 times (OR: 1.13, 95% CI: 1.01 to 1.27).
DISCUSSION
B. Guthrie et al. have shown that in the United Kingdom between 1995 and 2010, patients taking 5 to 9 drugs increased from 9.7% to 16.3%, patients taking 10 to 14 drugs from 1.5% to 4.7 %, and patients taking 15 or more drugs increased from 0.2% to 1.1%. (18) These figures correlate well with the PI found in this study: 18.9% for five or more drugs, 2.1% for ten or more drugs, and 0. 2% for 15 or more drugs. As in this study, D. Qato et al. found that the ages of 30% of the patients with polypharmacy were 65 years or over. (19) One out of five drugs used by these patients may be inappropriate. (20) In addition, A Eiras et al. have shown that patients with polypharmacy are four times more likely to use inappropriate medications than are other patients. (21) this puts them at increased risk of adverse drug reactions. (22) The population of this study has a high probability of polypharmacy and of inappropriate drug use between the ages of 50 and 70 years.
B. Guthrie et al. also evaluated the association between the number of drugs taken and the probability of serious drug interactions. They found that 80.8% of patients taking 15 or more drugs had potential for serious drug interactions. (18) Other studies have found an association between polypharmacy and risk of hospitalization but have found that survival rates are no higher than those for patients who use fewer drugs. (2, 23 - 25) For this reason, the 49 patients in our study who were taking 20 drugs of more had high probabilities of serious drug interactions and hospitalization. The PI found by A. Eiras of 23.9% was very close to the 22.3% PI found in this study. (23) Antiulcer drugs were prescribed to 31.8% of those patients. (23) Despite similar PIs, our study showed higher levels of anti-ulcer drug use (40%). PPIs were the most frequently employed. This follows recommendations for use of omeprazole as first-line prophylaxis for drug induced ulcers to reduce the risk by 50%. (10, 11)
Highlighting just how debatable the treatment received by 300 patients of three or more prescriptions of anti-ulcer medications, there are no guidelines or studies that advocate or justify the prophylactic use of various anti-ulcer medications at the same time. This treatment does not involve an additional benefit to the patient, but does result in increased risk of serious drug interactions, adverse drug reaction, hospitalizations and other therapeutic failures from modification of the absorption of other drugs and increased gastric pH. (26) The interactions of PPIs or H2-blockers may persist even after use of the drugs is suspended because of the long half-lives of these drugs (6-8 hours for ranitidine and more than 72 hours for omeprazole). (26) In addition, simultaneous use of aluminum-containing antacids with sucralfate, which also contains aluminum, has the potential for increased toxicity. (27)
Gastroprotection reduces morbidity and mortality from long-term ASA and NSAID use. (28) L. Ibanez et al. have reported that 14.5% of cases of upper gastrointestinal tract bleeding occur in patients taking antiplatelet agents. Incidence of acetylsalicylic acid (ASA) usage at low doses is 50.6 cases/million/year. (29) The reported incidence of upper gastrointestinal tract bleeding due to usage of NSAIDs significantly greater: 152 cases/million/year. (30) In our study, 31% of the patients on antiplatelet therapy but only 10% of the patients using NSAIDs received gastroprotective agents. This shows possible under-utilization and is consistent with other studies that have observed underutilization of gastric protection in patients using NSAIDs. (11, 31) There were justifications for the use of anti-ulcer drugs for only 12% of patients using gastrolesive drugs according to the existing transactional information and established prescriptin criteria. (10, 11) This could support the conclusion that those who need gastroprotection do not receive it while those who do not need it, do receive it.
Twenty-four percent of patients with polypharmacy who were gastroprotected had no diagnoses that justified the use of gastroprotective drugs in their transactional data, and only 12% had diagnoses of upper gastrointestinal pathologies in the database. This percentage is well below those reported in epidemiological studies which show a prevalence of acid-peptic disease of 41% and a prevalence of gastroesophageal reflux of 23%. (32, 33) This finding may be associated with the improper registration of diagnoses by the prescribing physicians. Clearly, the quality of the results of this study depends on the quality of the transactional records. Therefore, it may well be that the number of patients with diagnoses or histories of upper gastrointestinal pathologies that justify the use of gastroprotective agents may have been underestimated due to the absence of diagnoses in the database.
P. Santiestevea et al. found that 28.2% of patients who had been prescribed NSAIDs in Barcelona were not receiving adequate gastroprotection. (34) This is similar to the observation in our study of 35% inadequate gastroprotection. This result represents 75 million Colombian pesos monthly. No significant associations between prescription of gastroprotection and factors that justify their prescription were found, leaving open the possibility that anti-ulcer medications are explained by polypharmacy per se and not upper gastrointestinal risk factors. It is therefore recommended that patients using more than one gastrolesive agent who have gastrointestinal risk factors be given gastroprotection regardless of whether or not there is polypharmacy.
There are several tools that support the optimization of medication and reduction of prescriptions. These include the STOPP and Beers criteria (35) within the framework of pharmaceutical care and interventions. They have been shown to reduce the average number of drugs prescribed by 53% (RR: 0.47) with improved clinical results and of patient quality of life. (36)
The main limitation of this study is the quality of the source of information. There is the possibility that medical diagnoses and patient histories have been poorly recorded and do not show underlying diseases and chronic patients. This may have led to underestimation of the number of patients who require gastroprotection. In addition, the lack of information about adverse drug reactions and therapeutic failures as well as of clinical outcomes of patients whose gastric protection was not adequate, either by excess or defect, are other limitations of this study. Nevertheless, the findings here open the door to future research regarding the association between polypharmacy and gastric protection as well as research into the consequences of inadequate gastric protection for patient quality of life, healthcare costs, and the financial impact on the health care system.
Acknowledgments
The authors wish to thank MC21 Colombia for providing the information used in this study and to thank Camilo Yate, the clinical department's statistician, who performed data analysis and provided input and feedback for this article.
Source of financial support
Funding and transactional databases were provided by MC21 Colombia SAS. Nevertheless, the authors declare that they have no conflicts of personal interests or financing related to this work.
REFERENCES
1. Hung WW, Ross JS, Boockvar KS, Siu AL. Recent trends in chronic disease, impairment and disability among older adults in the United States. BMC Geriatr. 2011;11:47. [ Links ]
2. Jokanovic N, Tan ECK, Dooley MJ, Kirkpatrick CM, Bell JS. Prevalence and factors associated with polypharmacy in long-term care facilities: a systematic review. J Am Med Dir Assoc. 2015;16(6):535.e1-12. [ Links ]
3. Bourgeois FT, Shannon MW, Valim C, Mandl KD. Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiol Drug Saf. 2010;19(9):901-10. [ Links ]
4. Hovstadius B, Petersson G. The impact of increasing polypharmacy on prescribed drug expenditure-a register-based study in Sweden 2005-2009. Health Policy. 2013;109(2):166-74. [ Links ]
5. Fulton MM, Allen ER. Polypharmacy in the elderly: a literature review. J Am Acad Nurse Pract. 2005;17(4):123-32. [ Links ]
6. Ramírez-Duque N, Rivas-Cobas C, Bernabeu-Wittel M, Ruiz-Cantero A, Murcia-Zaragoza J, Oliver M, et al. [Drug prescription profile in patients with advanced chronic diseases]. Rev Esp Geriatr Gerontol. 2014;49(6):255-9. [ Links ]
7. Cho HJ, Kim CB. Prescription behaviours of office-based doctors to standardized common cold patients in Korea. Pharmacoepidemiol Drug Saf. 2002;11(5):401-5. [ Links ]
8. Kim S. An analysis of effects by delisting non-ethical gastrointestinal drugs. In College of Public Health. Seoul National University: Seoul, 2008. [ Links ]
9. Cho E, Sukyeong K. Prescribing Superfluous Gastroprotective Agents: an Indicator of Polypharmacy. Kor J Clin Pharm. 2011;21(2):156-60. [ Links ]
10. Vallès Fernández R, Franzi Sisó A, Ferro Rivera J. Condiciones clínicas y terapéuticas que requiere gastroprotección. FMC. 2014;21(9):528-33. [ Links ]
11. Grupo de Trabajo del Sector Zaragoza I SALUD. Guía de Práctica Clínica de empleo de los inhibidores de la bomba de protones en la prevención de gastropatías secundarias a fármacos. Unidad docente de medicina familiar y comunitaria. Zaragoza. 2012. [ Links ]
12. Información Farmacoterapéutica de la Comarca. Inhibidores de la bomba de protones: ¿se puede vivir sin ellos? 2010; 18(3). [ Links ]
13. Martin AB, Lassman D, Washington B, Catlin A, Team NHEA, others. Growth in US health spending remained slow in 2010; health share of gross domestic product was unchanged from 2009. Health Affairs. 2012;31(1):208-19. [ Links ]
14. R Core Team. R: A language and environment for statistical computing. R. 2015. http://www.R-project.org/. [ Links ]
15. Dates and Times Made Easy with lubridate, Grolemund, Journal of Statistical Software (Internet). citado 20 de mayo de 2016. Recuperado a partir de: https://www.jstatsoft.org/article/view/v040i03 [ Links ]
16. Grothendieck G. sqldf: Perform SQL Selects on R Data Frames (Internet). 2014 citado 20 de mayo de 2016. Recuperado a partir de: https://www.cran.r-project.org/web/packages/sqldf/index.html [ Links ]
17. Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. [ Links ]
18. Guthrie B, Makubate B, Hernandez-Santiago V, Dreischulte T. The rising tide of polypharmacy and drug-drug interactions: population database analysis 1995-2010. BMC Med. 2015;13:74. [ Links ]
19. Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA. 2008;300(24):2867-78. [ Links ]
20. Roughead EE, Anderson B, Gilbert AL. Potentially inappropriate prescribing among Australian veterans and war widows/widowers. Intern Med J. 2007;37(6):402-5. [ Links ]
21. Eiras A, Teixeira MA, González-Montalvo JI, Castell M-V, Queipo R, Otero Á. Consumo de medicamentos en mayores de 65 años en Oporto (Portugal) y riesgo de prescripción de medicamentos potencialmente inapropiados. Atención Primaria. 2016;48(2):110-20. [ Links ]
22. Atkin PA, Veitch PC, Veitch EM, Ogle SJ. The epidemiology of serious adverse drug reactions among the elderly. Drugs Aging. 1999;14(2):141-52. [ Links ]
23. Cherubini A, Eusebi P, Dell'Aquila G, Landi F, Gasperini B, Bacuccoli R, et al. Predictors of hospitalization in Italian nursing home residents: the U.L.I.S.S.E. project. J Am Med Dir Assoc. 2012;13(1):84.e5-10. [ Links ]
24. Nguyen JK, Fouts MM, Kotabe SE, Lo E. Polypharmacy as a risk factor for adverse drug reactions in geriatric nursing home residents. Am J Geriatr Pharmacother. 2006;4(1):36-41. [ Links ]
25. Díez-Manglano J, Giménez-López M, Garcés-Horna V, Sevil-Puras M, Castellar-Otín E, González-García P, et al. Excessive polypharmacy and survival in polypathological patients. Eur J Clin Pharmacol. 2015;71(6):733-9. [ Links ]
26. De las Salas-Martínez RB,Villarreal-Cantillo E. Interacciones en el uso de antiácidos, protectores de la mucosa y antisecretores gástricos. Salud Uninorte. Barranquilla (Col.) 2013; 29 (3): 441-57. [ Links ]
27. Ritschel WA, Banerjee PS, Koch HP, Czeijka M. Cimetidine-sucralfate drug interaction. Methods Find Exp Clin Pharmacol. 1984;6(5):261-3. [ Links ]
28. Rostom A, Dube C, Wells G, Tugwell P, Welch V, Jolicoeur E, et al. Prevention of NSAID-induced gastroduodenal ulcers. Cochrane Database Syst Rev. 2002;(4):CD002296. [ Links ]
29. Ibáñez L, Vidal X, Vendrell L, Moretti U, Laporte JR, Spanish-Italian Collaborative Group for the Epidemiology of Gastrointestinal Bleeding. Upper gastrointestinal bleeding associated with antiplatelet drugs. Aliment Pharmacol Ther. 2006;23(2):235-42. [ Links ]
30. Laporte J-R, Ibáñez L, Vidal X, Vendrell L, Leone R. Upper gastrointestinal bleeding associated with the use of NSAIDs: newer versus older agents. Drug Saf. 2004;27(6):411-20. [ Links ]
31. Rostom A, Wells G, Tugwell P, Welch V, Dube C, McGowan J. Prevention of NSAID-induced gastroduodenal ulcers. Cochrane Database Syst Rev. 2000;(4):CD002296. [ Links ]
32. Morini S, Zullo A, Oliveti D, Chiriatti A, Marmo R, Chiuri DAE, et al. A very high rate of inappropriate use of gastroprotection for nonsteroidal anti-inflammatory drug therapy in primary care: a cross-sectional study. J Clin Gastroenterol. 2011;45(9):780-4. [ Links ]
33. Verdejo-Bravo C, Calleja-Panero JL, Guillén-Llera F, Ribera-Casado JM. Estudio epidemiológico sobre la enfermedad ácido-péptica en pacientes ancianos ambulatorios. Rev Esp Geriatr Gerontol. 2006;41(1):21-8. [ Links ]
34. Del Piano M, Bianco MA, Cipolletta L, Zambelli A, Chilovi F, Di Matteo G, et al. The «Prometeo» study: online collection of clinical data and outcome of Italian patients with acute nonvariceal upper gastrointestinal bleeding. J Clin Gastroenterol. 2013;47(4):e33-37. [ Links ]
35. Carrillo Santiesteve P, Amado Guirado E, de la Fuente Cadenas JA, Pujol Ribera E, Tajada C, Calvet S, et al. [Prescribing non-steroidal antiinflammatory drugs and gastrointestinal protection in primary care]. Aten Primaria. 2008;40(11):559-64. [ Links ]
36. Wilcock M, Hughes P. GP perceptions of medicines optimisation principles. Prescriber. 2014;25(18):13-7. [ Links ]











 text in
text in