Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.31 no.2 Bogotá abr./jun. 2016
Diagnostic Methods in Portal Hypertension
Juan Sebastián Pareja Q. (1), Juan Carlos Restrepo G. MD (2)
(1) Medical Student at the School of Medicine of the University of Antioquia, Medellín, Colombia.
(2) Professor in the Department of Internal Medicine and Gastrohepatology Group at the University of Antioquia and Hepatologist at Hospital Pablo Tobon Uribe in Medellín, Colombia. Mail: jcrestrepo@hptu.org.co
Received: 15-03-15 Accepted: 18-04-16
Abstract
Portal hypertension is one of the most frequent complications in the natural course of liver disease. It results from increased hepatic vascular resistance and determines the development of other events responsible for increased mortality in patients with liver disease. Consequently, knowledge of the pathophysiology of portal hypertension and its causes is an important factor for handling it and related complications proper. Explanation of the various diagnostic methods for early and appropriate detection is one of the objectives of this review which will take a look at diagnostic methods available and in use for the detection of portal hypertension.
Keywords
Cirrhosis, portal hypertension, hepatic venous pressure gradient, elastography, Acoustic Radiation Force Impulse (ARFI) Imaging, magnetic resonance imaging, biomarkers.
INTRODUCTION
Portal hypertension is the most common complication of cirrhosis and the leading cause of mortality associated with this disease. Reciprocally, cirrhosis is the leading cause of portal hypertension and responsible for a large number of deaths: it is the fourteenth cause of death worldwide. Increased portal pressure results from blockage of flow through the venous system as a result of chronic liver disease and portal vein thrombosis. However, there are disorders of origins than cirrhosis that can cause portal hypertension through compromising the portal vascular system. Once portal hypertension develops, intrahepatic compromise influences extrahepatic vascular beds that increase pressure and cause hyperdynamic circulation. Esophageal varices and other complications develop as consequences of processes of vascular and blood dynamics. All of which helps explain why liver cirrhosis is a leading cause of hospitalization, death and liver transplantation in the world. (1)
Currently, a number of diagnostic methods for the evaluation of portal hypertension are available. Diagnosis of portal hypertension is not only part of the comprehensive evaluation and monitoring of patients with chronic liver disease, it ultimately promises to improve the prognosis and quality of life for these patients.
This article aims to review currently available methods for measurement of portal hypertension that are described in the medical literature.
DEFINITION
Portal hypertension is a common complication of chronic liver diseases, especially cirrhosis. It is defined as an increase in the specific portal blood pressure as measured by the Hepatic Venous Pressure Gradient (HVPG). This corresponds to the pressure difference between the portal vein and the inferior vena cava and ranges from 1 to 5 mm Hg. Portal hypertension is defined as the pressure represented by HVPG values greater than 5 mm Hg. Typically, measurements between 5 and 9 mm Hg represent a subclinical state of hypertension and pressures greater than 10 mm Hg are clinically significant because of risks such as esophageal varices and variceal hemorrhaging. (1, 2)
DEMOGRAPHIC DATA
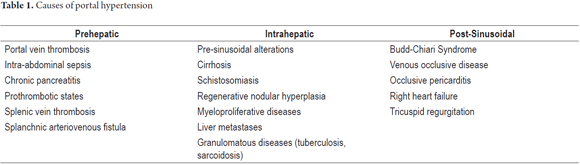
In chronic liver disease, portal hypertension is a common and very important consequence that leads to complications which cause large numbers of hospital admissions, transplants and death. (1, 3) The most important causes of portal hypertension are alcoholic liver cirrhosis and because hepatotropic virus infections. Nevertheless, better understanding of non-cirrhotic causes can be obtained by classifying them as extrahepatic, intrahepatic (presinusoidal, sinusoidal or post-sinusoidal) and post-hepatic. (4)
Most deaths from cirrhosis are the result of complications related to portal hypertension. Bleeding gastroesophageal varices has the highest mortality rate. (5) Other complications include hepatic encephalopathy, ascites, spontaneous bacterial peritonitis (SBP), hepatorenal syndrome, hypertensive gastropathy, portal-pulmonary hypertension, and hepatopulmonary syndrome. Any of these can be responsible for morbidity, mortality and decreased quality of life for patients with chronic liver disease. (4)
At least 80% of the patients with liver cirrhosis have portal hypertension. Of those who do, about 40% develop esophageal varices. Factors such as the grade of varices, signs of bleeding, decreased liver functioning and increased portal pressure correlate with the appearance and rates of variceal bleeding which range from 10% to 30% in two years. Finally, it is important to consider that the 6 week mortality rate for bleeding esophageal varices is 12% to 20%, and that, if a patient does not receive effective treatment, about two-thirds of these patients will have recurrences over the next two years. (1)
PATHOPHYSIOLOGY OF PORTAL HYPERTENSION
Portal hypertension is a local phenomenon with systemic consequences. It is the result of a series of molecular and cellular events that lead to increased portal blood flow and vascular resistance. Its causes have been extensively studied and compromises of hepatic systems have been classified into extrahepatic portal hypertension, intrahepatic portal hypertension and post- hepatic portal hypertension (Table 1). (6) Cirrhosis is the most common cause of portal hypertension and the main indication for liver transplantation everywhere in the world. (1) Structural and functional alterations in cirrhosis lead to endothelial dysfunction that increases vascular tone and resistance and explains the appearance of intrahepatic portal hypertension. Such alterations determine 25% of increases in vascular resistance. (7, 8)
Changes in intrahepatic and extrahepatic circulation are essential elements of the pathophysiology of portal hypertension. Intrahepatic circulation is affected by events resulting from endothelial dysfunction such as disturbances of vasomotor regulatory mechanisms and inflammation of the damaged liver which lead to the development of fibrosis and development of regenerative nodules that compromise the intrahepatic architecture. (8)
In addition, decreasing endogenous production of vasodilator substances, especially nitric oxide (NO), explains part of the increase of vascular resistance in portal hypertension. Caveolin is an integral membrane protein which is involved in inhibiting the activity of the nitric oxide synthase enzyme which is responsible for synthesizing nitric oxide. (1) Added to this, the small amount of NO that is synthesized reacts with excess oxygen free radicals produced by inflammatory activity. This ultimately produces peroxynitrite and results in a significant reduction of the remaining nitric oxide molecules and hence of their functioning. (3). Additionally, current evidence supports the existence of increases in vasoconstrictors at the expense of production of thromboxane A2 (TXA2) and cyclooxygenase-1 (COX-1). When this process is combined with the presence of endothelin, hepatic vascular resistance is amplified. (9)
Activation of hepatic stellate cells (HSC) and the occurrence of hepatic angiogenesis are additional factors that are responsible for intrahepatic portal hypertension. Cytokines such as TGF-β, the extracellular matrix and other inflammatory factors mediate activation of stellate cell differentiation into myofibroblasts through an increase in the concentration of molecules of the cytoskeleton. Such cytological transformation generates increasing cell contraction, profibrotic activity and decreased response to vasodilators. Ultimately, this process promotes intrahepatic vascular resistance (7,10,11).
Under normal conditions, there are portosystemic collateral veins that act as part of the abdominal venous system without any major impact on body hemodynamics. However, once portal hypertension has been established, circulation of blood in these vessels increases abnormally as a compensatory mechanism. In fact, the presence of abnormal collateral vessels is a key sign in the sonographic diagnosis of portal hypertension. Its sensitivity ranges from 70% to 83%. (12)
It is important to note that the extrahepatic mechanisms that contribute to portal hypertension are nearly identical, but opposite to those seen in intrahepatic circulation. In response to the appearance of collateral circulation, there is also an increase in splanchnic circulation. This consists of increased portal circulation to compensate for the decrease in hepatic blood flow which ultimately worsens portal hypertension. This increased compensatory flow of collateral circulation originates in the endothelial response induced in response to increased portal pressure that elevates the activity of nitric oxide synthase and consequently also elevates levels of nitric oxide in the portal system. (l7, 13)
For many authors, this is called hyperdynamic circulation syndrome or progressive vasodilation syndrome due to the vasodilator component that favors increased systemic circulation and vascular changes seen in the chronic liver diseases that occur with portal hypertension. (14) In addition to nitric oxide which is the primary substance associated with splanchnic vasodilatation, some authors have also identified carbon monoxide and substances such as endocannabinoids as mediators in the pathophysiology of portal hypertension. (14)
Finally, other mechanisms involved in splanchnic and systemic vasodilation that worsen portal hypertension include arterial hypocontractility and thinning of the arterial walls. These factors change splanchnic hemodynamics in favor of portal hypertension. Arterial hypocontractility is the result of decreased production of vasoconstrictor molecules and impaired neural activity (sympathetic atrophy). (7, 13) This means that portal hypertension can be understood as the result of interactions among multiple mechanisms, many of which have been described, but many others which may yet be described, and whose main cause is liver cirrhosis.
DIAGNOSTIC TESTS FOR PORTAL HYPERTENSION
Currently there are several diagnostic methods for measuring portal pressure. Generally speaking, one can calculate portal pressure as the difference between the pressures of the hepatic vein and inferior vena cava (hepatic venous pressure gradient) which can be determined directly or indirectly. Direct determination requires an invasive procedure which uses either a transvenous or transhepatic catheter which can lead to complications such as intraperitoneal bleeding. (1) Listed below are the various methods for measuring portal pressure classified into invasive and non-invasive procedures.
Invasive Methods
Hepatic venous pressure gradient (HVPG)
The hepatic venous pressure gradient is now recognized as the standard method for measuring portal pressure. However, since it is an invasive method that is not routinely available, it is not frequently used. (15) Despite this, HVPG is a safe and method. HVPG is the difference between hepatic venous wedge pressure (HVWP) and free hepatic venous pressure (FHVP). (16) Technical issues involved with recording HVPG include consideration of the use of local anesthesia, monitoring of vital signs and continuous electrocardiogram of the patient.
Initially, a catheter is introduced into the jugular, femoral or cubital vein. It is then fluoroscopically guided to the main hepatic vein. Once it has arrived it is positioned at a distance of two to four centimeters from the inferior vena cava. The catheter measures FHVP. Then, a balloon carried by the catheter is inflated at the same location. The balloon creates an obstruction in the blood flow which is confirmed by injection of contrast medium which should show a wedge pattern. Finally, after pressure stability has been achieved in the hepatic vein, pressure is measured within the wedge. (1, 17) Although the HVPG is the most commonly used method for measuring portal pressure, many studies have tried to find alternative measurement methods that are equally accurate or more accurate to determine the portal pressure, compared to obtained through the hepatic venous pressure gradient. To date, many of these studies have failed to obtain reliable results.
The hepatic atrial pressure gradient (HAPG) is an alternative method that uses right atrial pressure instead of free hepatic venous pressure. This method is similar to HVPG, but was hoped to be more effective. However, a study that compared HAPG to HVPG showed that HAPG was not feasible. The study was conducted with a cohort of 154 cirrhotic patients with portal pressure over 12 mm Hg. It revealed that the HAPG measurement was always less than or equal to that reported by free hepatic venous pressure (baseline right atrial pressure: 4.9 ± 2.8 mm Hg versus basal free hepatic venous pressure: 8.1 ± 3.9). Consequently HAPG will always be higher than HVPG (3.2 mm Hg, 95% CI: 2.8-3.7 mm Hg; p <0.001). This demonstrates that measurement of right atrial pressure is not suitable for calculating the hepatic venous pressure gradient. (17)
The concept of clinically significant portal pressure arises as the result of the knowledge that pressures greater than 10-12 mm Hg are at the lower limits for increased risk of bleeding varices. Consequently, HVPG has great prognostic value in chronic liver disease, especially in cirrhosis. It is a b predictor of patient survival and also of complications associated with portal hypertension and hepatic encephalopathy. These complications include ascites, spontaneous bacterial peritonitis and even the development of hepatocellular carcinoma. (16) On the other hand, HVPG is also valued as an indicator of the effectiveness of treatment of portal hypertension. (18) In fact, an observational study of 103 patients with cirrhosis who had developed bleeding varices between 2001 and 2010 has shown that maintenance of HVPG below 2 mm Hg or reductions 20% or more from the initial level of HVPG is useful for prophylaxis of renewed bleeding with chronic liver disease. (19)
Various studies have shown the feasibility of measuring portal pressure through hepatic venous pressure gradient. A retrospective study published in 2013 searched the database at the Hospital for Sick Children for children who underwent the HVPG procedure in the department of interventional radiology hepatic venous gradient between 2000 and 2011. There were 49 children whose records met the selection criteria (25 children with a mean age of 5.6 to 8.2 years). The study indicated that this technique is safe for infants with severe liver disease since none of the patient in the study had any complications related to the procedure. (20) In conclusion, the hepatic venous pressure gradient continues to be a b marker of liver diseases that occur with portal hypertension. It is probably still the best prognostic measurement for the evolution of these diseases over time. (16)
Noninvasive Methods
Transient Elastography
Transient elastography (TE) is a noninvasive diagnostic method for portal hypertension that measures stiffness of the liver with ultrasound. TE has good sensitivity and specificity for assessing the degree of fibrosis and cirrhosis: for stage F4 its sensitivity is 83% (95% CI: 79-86), and its specificity is 89% (95% CI: 87-91); for stage F3 its sensitivity is 82% (95% CI: 78-86), and its specificity is 86% (95% CI: 82-89); for stage F2 its sensitivity is 79% (95% CI: 74-82), and its specificity is 78 % (95: 72-83); and for stage F1 its sensitivity is 78% (95% CI: 73-83), and its specificity is 83% (95% CI: 72-90). (15, 21) This technique is based on a generalization of Hooke's Law that states that deformation of a material is proportional to the stress applied to it. (22)
Transient elastography technique for measuring stiffness of the liver requires several conditions of the patient and the instruments used. The measurement is made in the right hepatic lobe with the patient supine with her right arm in maximum abduction. Then the physician must locate an area of the liver that is at least 6 cm thick free and is free of large vessels. Finally, the test is performed to acquire data. However, 10 valid ultrasound shots must be obtained to get accurate and reliable information. The test is considered a failure if no valid shots are acquired and there can be little confidence placed in the results if less than 10 valid shots have been obtained. (22)
Several studies of the validity and reliability of the use of TE for the evaluation of portal hypertension are available. A study conducted between January 2004 and September 2006 sought to assess the relationship between the measurement of liver stiffness and HVPG in patients with cirrhosis related to HCV or alcohol in order to assess the performance of transient elastography and to define the best cut-off point its use in diagnosis of portal hypertension. The 92 eligible patients included in the study had to have a Child-Pugh A score and undergo simultaneous transient elastography and liver biopsy. TE was bly correlated with HVPG (R2 = 0.53, p <0.0001) and the ROC for predicting significant portal hypertension, defined as HVPG over 10 mm Hg, was 0.84 ± 0.04 with a 95% CI. (23)
Another similar study was conducted between November 2005 and October 2006 and published in 2011. It sought to compare the prognostic effectiveness of HVPG, the standard method for the measurement of portal hypertension, and transient elastography. That investigation included 100 patients with compensated chronic liver disease without any antiretroviral treatment or change in portal pressure. Patients were followed for two years, until their disease decompensated, until they underwent liver transplantation, or until the patient passed away. All patients had their portal pressure measured by HVPG and simultaneous transient elastography at the beginning of the study and again during the study until they fulfilled the conditions for termination of monitoring. The study showed no significant differences in predictions of complications between the two diagnostic methods. When they considered only complications related to portal hypertension, similar levels of accuracy were found with an ROC of 0.830 (0.751 to 0.910) for HVPG and an ROC of 0.845 [.767-.823] for TE. This demonstrates that transient elastography is as effective as the hepatic venous pressure gradient as a predictor of clinical decompensation and complications related to portal hypertension in patients with chronic liver disease. (24)
Despite the good results of these studies, other studies only provide partially support for the usefulness of TE as a diagnostic method of portal hypertension in patients with compensated cirrhosis and individuals with liver tumors. Transient elastography is an adequate method for only half of the patients with liver tumors with the possibility of undergoing resection. (25) One study that tried to determine the correlation between TE and HVPG assessed the feasibility of using elastography as a method for the diagnosis of significant portal hypertension (VHPG> 10 mm Hg) in patients co-infected with HCV and HIV. This study included 38 patients who underwent simultaneous HVPG and transient elastography from 2007 to 2009. The study found that there was a b correlation between the values obtained with TE and those from HVPG, but the authors emphasized the need for further studies given the small number of patients studied. (26)
Despite the advantages of elastography, its applicability is conditioned by its limitations since one out of every five TEs cannot be interpreted or are difficult to complete. This is mainly the result of patient obesity and lack of operator experience. In addition, since the liver is incased in a distensible but not elastic capsule, abnormalities such as edema, inflammation, cholestasis and congestion that occupy space may interfere with the measurement of liver stiffness regardless of whether or not there is any fibrosis. (27)
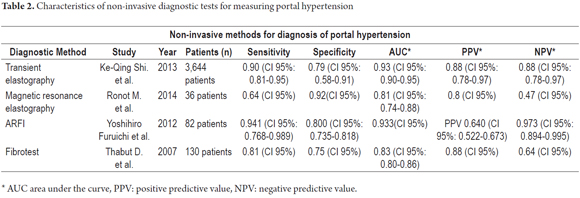
Finally, beyond those studies that bet on TE and those that indicate limits on its use for portal hypertension, we have found more studies in favor of this technique than against it. A meta-analysis conducted in 2013 evaluated 18 studies involving 3644 patients. It found that transient elastography is highly accurate and useful for detecting significant portal hypertension since it has a sensitivity of 90% and a specificity of 79%. Nevertheless, the diagnostic capacity of TE does have a significant limit because it requires significant pressure when the pretest probability of portal hypertension is better than 25%. (15)
Magnetic Resonance Elastography
The liver is considered to be a biphasic system consisting of a solid tissue and fluid filled vascular tree. The liver's volume is 25% to 30% blood while the rest is liver tissue. Consequently, interactions occurring between the two types of tissue phases generate a mechanical response which can be measured and quantified by in vivo methods such as elastography. (28) Elastography mechanically stimulates the tissue being studied by static compression through focused acoustic radiation or low frequency vibrations. The low frequency vibrations are scanned by magnetic resonance elastography (MRE - Magnetic resonance elastography) which evaluates the wave fields in two or three dimensions. In fact, the MRE is the most accurate elastography technique currently in use for staging the degree of hepatic fibrosis. (29)
Current evidence shows that magnetic resonance elastography is an appropriate means for evaluation of portal hypertension and its relationship to the development of esophageal varices. (30) An experimental study in an animal model has looked at the relationship between splenic MREs and portal venous pressure gradient. The animals common bile ducts were ligated to induce cholestatic disease. Portal hypertension was found to already be present at stage F1 of liver fibrosis (HVPG: 11.0 ± 5.1 mm Hg). Even though fibrosis was increasing, HVPG measurements did not increase (HVPG: 11.3 ± 3.2 mm Hg). In the fourth week of the experiment, there was a 100% increase in splenic rigidity. It stabilized after the eighth week. This study concluded that there is a temporal relationship between portal hypertension and development of liver fibrosis, so that the results for splenic stiffness from MRE and HVPG can be extrapolated for diagnosis and screening of portal hypertension in patients with chronic liver diseases. (31)
Other studies have been conducted to assess MREs ability to measure the degree of liver fibrosis its correlation with the development of portal hypertension and esophageal varices. Between August 2010 and October 2011, 1,358 patients with chronic liver disease or suspected focal liver lesions had MRIs, MREs and upper digestive endoscopies were considered for a study that evaluated the usefulness of MREs as non-invasive method to predict the occurrence of esophageal varices. Of the 1,358, 126 patients met the inclusion criteria for the study and continued in it. It was found that the average parenchymal stiffness of the liver measured by MRE correlated with the degree of esophageal varices, with an area under the curve of 0.859 (p <0.0001, CI: 0.786 to 0.915) for prediction of varices (regardless of grade) and 0.810 (p <0.0001, CI: 0.730 to 0.874) for prediction of high risk varices at Stage 2 or higher. Measurement of liver stiffness using MREs may be a useful noninvasive method for identifying esophageal varices and high risk varices and their correlation with HVPG in patients with cirrhosis. (32)
In 2014 another study assessed the measurement of hepatic and splenic viscoelasticity by magnetic resonance elastography to determine the degree of portal hypertension and the presence of varices at high risk of bleeding. This research involved 36 patients with cirrhosis who underwent HVPGs, upper digestive tract endoscopies and magnetic resonance elastography. A statistically significant relationship was found between the loss modulus in liver and spleen magnetic resonance and HVPG values corresponding to severe portal hypertension greater than 12 mm Hg. For MREs of the liver the values were r = 0.44 and p = 0.017 while for MREs the spleen they were r = 0.5, p = 0.002. So the splenic loss module was the best parameter for identification of portal hypertension (p = 0.019, AUC = 0.81). This study concluded that the assessment can be performed with MREs of the liver and spleen and HVPG since the loss modulus of the spleen correlates with severe portal hypertension. (33)
Finally, another study conducted between November 2010 and March 2012 and published in 2014 has shown a significant correlation between liver and spleen stiffness, plus the length of the spleen and the degree of esophageal varices (r = 0.46, r = 0.48, r = 0.36, respectively; p = 0.0001). The study included 533 patients. This reinforces the idea of using MREs to evaluate liver and spleen stiffness which are associated with esophageal varices and are in turn closely related to HVPG values above 10 mm Hg. (34)
ARFI (acoustic radiation force impulse imaging)
ARFI (acoustic radiation force impulse imaging) is an emerging technology that provides information on the elasticity of tissue in real time and which is incorporated into a conventional ultrasound unit. Acoustic pulses of approximately 262 microseconds are generated at a frequency of 2.67 MHz. This generates shear waves in the target tissue of the study. Then, the velocities of shear waves in the tissue are measured in a small area of parenchyma in which a small displacement is generated. (35, 36)
Several studies have described the relationship between ARFI elastography findings and HVPG as predictors of the degree of portal hypertension in patients with chronic liver disease. A study published in 2012 looked at the relationship between liver and spleen stiffness, the ratio between these two values measured by ARFI, and diagnosis of idiopathic portal hypertension. This study included 82 patients, of whom 20 were healthy. The others suffered from various liver ailments including idiopathic portal hypertension (17 patients), cirrhosis (25 patients) and hepatocellular carcinoma (20 patients). The study found that patients with liver cirrhosis and hepatocellular carcinoma did not have high degrees of liver/spleen stiffness, but that liver/spleen stiffness was greater in patients with idiopathic portal hypertension (p <0.001). In addition, comparisons of ROC curves for liver/spleen stiffness ratios between the group with idiopathic portal hypertension and to the other groups found an area under the curve with a sensitivity of 0.933 to 0.941, specificity of 0.800 and accuracy of 0.839. Consequently, the measurement of liver/spleen stiffness using ARFI may be a noninvasive technique with high sensitivity and accuracy for studying idiopathic portal hypertension. (37)
A retrospective study of 46 pediatric patients who had biliary atresia after undergoing portoenterostomy was published in 2015. It revealed that the degree of splenic rigidity measured by ARFI elastography can be used to predict the severity of portal hypertension in these patients. The reason for this conclusion is that the ratio of stiffness, splenic diameter (r = 0.320, p = 0.043) and the diameter of the portal vein (r = -0.409, p = 0.009) correlates with the presence of portal hypertension. These findings suggest when ARFI elastography is performed serially to evaluate splenic stiffness in patients with biliary atresia, it can be a non-invasive method for monitoring the severity of portal hypertension. (38)
In 2012 another study assessed the use of ARFI for evaluation of portal hypertension through the correlation between liver elasticity as measured by ARFI and hemodynamic indices evaluated by Doppler. This study included 154 patients with cirrhosis (91 men, 63 women) who underwent ARFI and hepatosplenic Doppler to determine the velocity of the portal vein, splenic index and splenic-portal index. Of these, 47 patients had esophageal varices and 74 did not. The other patients did not undergo endoscopy of the upper digestive tract. Similar increases were found in the velocity of shear waves, the splenic index and the portal-splenic index (p = 0.01 and r = 0.451; p = 0.01, r = 0.409, respectively). Furthermore, in the group of patients who did not have varices, there was a correlation between shear wave velocity and splenic parameters (splenic index: r = 0.447, portal-splenic index: r = 0.552; p = 0.01) while in the group that had esophageal varices, there was no correlation between the values of ARFI and splenic indices. Therefore, despite the limited value of ARFI with respect to Doppler, given the variability of Doppler data, measurement of the speed of shear waves could be a complementary tool for noninvasive prediction of portal hypertension. (39 )
There is evidence that supports the use of ARFI as a diagnostic method and for monitoring of portal hypertension, but its use is not generally widespread because few data directly assess ARFI and portal hypertension beyond comparing it to the existence of esophageal varices. Consequently, ARFI needs more study to clarify and reinforce the usefulness of this technique for the evaluation of portal hypertension.
Fibrotest
Numerous markers of liver fibrosis have been studied to evaluate the portal hypertension. Fibrotest is a panel of five serum markers, α2-macroglobulin, apolipoprotein A-1, haptoglobin, γ-glutamyl transferase, and serum bilirubin which are related by an algorithm. It has been widely validated in terms of its relationship to severe portal hypertension and generates a score between 0 and 135 in the context of HVPG as the standard method for detection of portal hypertension. (40) In 2007, an eight month long study of patients with liver disease was conducted. The patients had transjugular liver biopsies taken measurement of HVPG as well as blood samples. They were used to assess the relationship between FibroTest and the presence and degree of hypertension portal and to determine whether Fibrotest can diagnose severe portal hypertension (≥ 12 mm Hg) in patients with cirrhosis. Only 130 of the initial 147 patients were include in the study. Of the total number of patients, 12% had mild fibrosis, 17% had moderate fibrosis and the remaining 71% had severe fibrosis according to liver histopathology. There was a significant correlation between FibroTest and HVPG (Pearson correlation coefficient = 0.58, p <0.0001) although it was weaker in cirrhotic patients (Pearson correlation coefficient = 0.24 and p = 0.02 ). In cirrhotic patients, FibroTest had a significantly higher value when portal hypertension was more severe (0.87 ± 0.15, p = 0.02). The AUC for Fibrotest was 0.79 ± 0.07. FibroTest correlates with the presence and degree of portal hypertension, but the relationship is weak in patients with cirrhosis. Although this test is a significant advance in the detection of this complication in patients with liver disease, further studies are needed to confirm the early study results, especially in patients with compensated cirrhosis. (40-42)
Table 2 summarizes the characteristics of non-invasive methods used for diagnosis of portal hypertension.
Other Methods
Finding new diagnostic techniques to determine portal pressure that are accurate, highly sensitive and specific, and continuously non-invasive remains a challenge faced by medicine. Various investigations have attempted to find non-invasive methods.
One of them, a study published in 2014, sought to determine whether measurement of inflammatory biomarkers with clinical laboratory parameters and demographic data could be used as a predictive paradigm for HVPG. This study that included 213 patients found that IL-1b, IL-1Ra, Fas-R, VCAM-1, TNFb and HSP-70 are biomarkers associated with HVPG. Their sensitivity was 86% and their specificity was 87% with respect to the prediction portal pressure when this was significantly high (HVPG> 12 mm Hg). Nevertheless, despite the results of this study this biomarker test is still not widely used for determining portal pressure, given the constraints that the measurement of such biomarkers can have. (43).
A 2012 article has published the results of research conducted between May 2006 and November 2008 about the association between clinical parameters and direct measurement of portal venous pressure. Forty patients with some form of liver tumor were included. Portal pressure was determined through direct percutaneous transhepatic puncture. Blood samples were taken and laboratory and clinical parameters including patient age, platelet count, AST, ALT, serum albumin, serum bilirubin, serum bile acid levels of hyaluronic acid, NH3, prothrombin activity, ICGR15 , APRI and spleen volume were recorded. The results showed that the values of AUC were significantly correlated with serum levels of bile acids and spleen volume (AUC 0.792 and 0.926, respectively). This suggests that these measurements are sensitive predictors of early and advanced portal hypertension. (44)
A prospective study of 202 patients with chronic liver disease has added more support to the argument for the broad possibilities for non-invasive diagnosis of portal hypertension. All patients had clinical or laboratory evidence consistent with portal hypertension and cirrhosis. These patients underwent assessments of HVPG, serum tests (single or combined scores) and measurements of liver stiffness. The study evaluated the accuracy of 6 different serum scoring systems, artificial models of neural connection and measurement of liver stiffness by transient elastography for diagnosis of cirrhosis, portal hypertension and esophageal varices. The results showed that transient elastography was the best method for diagnosis. Of the serum scoring systems evaluated, the Fibrosis-4 (FIB-4) was identified as the most accurate for diagnosis of cirrhosis, and the Lok Score was identified as the most accurate for diagnosis of portal hypertension and esophageal varices. (45)
It is notable that the FIB-4 score is a non-invasive test to determine the degree of liver fibrosis based on a combination of biochemical values and age. (46) In 2014, a study revealed the association between FIB-4 score and the presence of collagen degradation products with the status of liver fibrosis and portal hypertension. This study involved 58 patients coinfected with HIV and HCV. Forty-three of these patients had HVPG measured to evaluate portal pressure and FIB-4 score was calculated to determine the status of liver fibrosis. Additional degradation products of extracellular matrix were measured by ELISA in peripheral blood. Among the most important results was the finding of a b correlation between FIB-4 index values and those of HVPG ( p = 0.0000007, r = 0.628). In addition, a b relationship was also found between PRO-C3, a product of extracellular matrix degradation, and HVPG (p = 0.0354, r = 0.02). The study concluded that PRO-C3 levels reflect liver damage, fibrosis status and degree of portal hypertension in patients with HIV/HCV coinfection. (47)
The Lok Score combines constant variables with biochemical values such as AST, ALT and INR. (48) This score that has been used to predict complications of liver cirrhosis and portal hypertension. (49) A study published in February 2015 evaluated the use of combined scores for the evaluation of portal hypertension and cirrhosis and their abilities to predict development of esophageal varices. This study assessed the combination of the Lok score with liver and splenic stiffness for diagnosing high risks of developing esophageal varices. It was closely related to the value of portal pressure and compared well with other non-invasive methods available today.
This study has shown that any of the above methods including the Lok Score and measurement of liver and spleen stiffness can identify patients at high risk of developing esophageal varices with moderate accuracy. This suggests that they can be used to evaluate patients with low risk of developing esophageal varices. (50) Consistent with previous studies, many new non-invasive techniques are being developed for diagnosis of portal hypertension, cirrhosis and esophageal varices. Despite the good results that they have been obtained, they are not widely used due to the lack of sufficient evidence to support them and give them validity.
CONCLUSION
Portal hypertension is the main complication of chronic liver disease, including of cirrhosis, and is one of the most common causes of morbidity and mortality in patients with these diseases. Various molecular, vasomotor and blood flow mechanisms are actively involved in the development of portal hypertension. Many of them have already been described which may favor the reduction of portal hypertension through the development of treatments aimed at eliminating or reducing it. Currently, there are several diagnostic methods used for initial evaluation and follow-up of patients with cirrhosis and portal hypertension that ensure better prognosis and disease management. The gold standard continues to be the hepatic venous pressure gradient which, despite being invasive, is the most sensitive and specific for the evaluation of portal hypertension.
Nevertheless, transient elastography, which is safe and non-invasive, has emerged as an effective, sensitive and specific method for evaluation of portal hypertension. It has yet to come into wide usage on a day to day basis because of the need for significant values of portal pressure in order for it to be used to assess the degree of hypertension and to be used for follow-up. The lack of sufficient numbers of studies that compare TE and HVPG is also a factor inhibiting its day to day usage by physicians. On the other hand, magnetic resonance elastography, Fibrotest and ARFI are also non-invasive methods whose sensitivity and specificity have been increasing for evaluation of portal hypertension. Their availability and their capacity for relating with severe portal hypertension values over12 mm Hg potentially limit their daily use for evaluation of patients with chronic liver disease.
Finally, alternative methods that are characteristically noninvasive such as the use of biomarkers using scores based on blood tests and the evaluation of splenic stiffness have shown some degree of relationship with the HVPG for monitoring patients with cirrhosis and portal hypertension. However, additional studies are required before these methods can be used routinely for evaluation of portal hypertension security.
Acknowledgements
The authors wish to thank the Sustainability Project of the Office of the Vice-Rector for Research, at the University of Antioquia.
REFERENCES
1. Berzigotti A, Seijo S, Reverter E, Bosch J. Assessing portal hypertension in liver diseases. Expert Rev Gastroenterol Hepatol. 2013;7(2):141-55. [ Links ]
2. García Buey L, González Mateos F, Moreno-Otero R. Cirrosis hepática. Medicine - Programa de Formación Médica Continuada Acreditado. 2012;11(11):625-33. [ Links ]
3. Hu LS, George J, Wang JH. Current concepts on the role of nitric oxide in portal hypertension. World J Gastroenterol. 2013;19(11):1707-17. [ Links ]
4. Sarin SK, Khanna R. Non-cirrhotic portal hypertension. Clin Liver Dis. 2014;18(2):451-76. [ Links ]
5. Carey W. Portal hypertension: diagnosis and management with particular reference to variceal hemorrhage. J Dig Dis. 2011;12(1):25-32. [ Links ]
6. Palaniyappan N, Aithal GP. Portal hypertension and ascites. Surgery (Oxford). 2011;29(12):640-6. [ Links ]
7. Iwakiri Y. Pathophysiology of portal hypertension. Clin Liver Dis. 2014;18(2):281-91. [ Links ]
8. Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383(9930):1749-61. [ Links ]
9. Bosch J, Abraldes JG, Fernandez M, Garcia-Pagan JC. Hepatic endothelial dysfunction and abnormal angiogenesis: new targets in the treatment of portal hypertension. J Hepatol. 53. England: 2010 European Association for the Study of the Liver. Published by Elsevier B.V; 2010. p. 558-67. [ Links ]
10. Kim MY, Baik SK, Lee SS. Hemodynamic alterations in cirrhosis and portal hypertension. Korean J Hepatol. Korea South. 2010. p. 347-52. [ Links ]
11. Iwakiri Y, Shah V, Rockey DC. Vascular pathobiology in chronic liver disease and cirrhosis - current status and future directions. J Hepatol. 2014;61(4):912-24. [ Links ]
12. Sharma M, Rameshbabu CS. Collateral Pathways in portal hypertension.J Clin Exp Hepatol. 2012;2(4):338-52. [ Links ]
13. Thabut D, Shah V. Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension? J Hepatol. 53. England: 2010 European Association for the Study of the Liver. Published by Elsevier B.V; 2010. p. 976-80. [ Links ]
14. Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology. 2006;43(2 Suppl 1):S121-31. [ Links ]
15. Shi KQ, Fan YC, Pan ZZ, Lin XF, Liu WY, Chen YP, et al. Transient elastography: a meta-analysis of diagnostic accuracy in evaluation of portal hypertension in chronic liver disease. Liver Int. 2013;33(1):62-71. [ Links ]
16. Merkel C, Montagnese S. Hepatic venous pressure gradient measurement in clinical hepatology. Dig Liver Dis. 43. Netherlands: 2011 Editrice Gastroenterologica Italiana S.r.l. Published by Elsevier Ltd; 2011. p. 762-7. [ Links ]
17. La Mura V, Abraldes JG, Berzigotti A, Erice E, Flores-Arroyo A, Garcia-Pagan JC, et al. Right atrial pressure is not adequate to calculate portal pressure gradient in cirrhosis: a clinical-hemodynamic correlation study. Hepatology. 2010;51(6):2108-16. [ Links ]
18. Procopeţ B, Tantau M, Bureau C. Are there any alternative methods to hepatic venous pressure gradient in portal hypertension assessment? J Gastrointestin Liver Dis. 2013;22(1):73-8. [ Links ]
19. Augustin S, González A, Badia L, Millán L, Gelabert A, Romero A, et al. Long-term follow-up of hemodynamic responders to pharmacological therapy after variceal bleeding. Hepatology. 2012;56(2):706-14. [ Links ]
20. Woolfson J, John P, Kamath B, Ng VL, Ling SC. Measurement of hepatic venous pressure gradient is feasible and safe in children. J Pediatr Gastroenterol Nutr. 2013;57(5):634-7. [ Links ]
21. Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011;54(4):650-9. [ Links ]
22. Şirli R, Sporea I, Bota S, Raţiu I. Liver elastography for the diagnosis of portal hypertension in patients with liver cirrhosis. Med Ultrason. 2012;14(3):225-30. [ Links ]
23. Lemoine M, Katsahian S, Ziol M, Nahon P, Ganne-Carrie N, Kazemi F, et al. Liver stiffness measurement as a predictive tool of clinically significant portal hypertension in patients with compensated hepatitis C virus or alcohol-related cirrhosis. Aliment Pharmacol Ther. 2008;28(9):1102-10. [ Links ]
24. Robic MA, Procopet B, Métivier S, Péron JM, Selves J, Vinel JP, et al. Liver stiffness accurately predicts portal hypertension related complications in patients with chronic liver disease: a prospective study. J Hepatol. 2011;55(5):1017-24. [ Links ]
25. Llop E, Berzigotti A, Reig M, Erice E, Reverter E, Seijo S, et al. Assessment of portal hypertension by transient elastography in patients with compensated cirrhosis and potentially resectable liver tumors. J Hepatol. 2012;56(1):103-8. [ Links ]
26. Sánchez-Conde M, Miralles P, Bellón JM, Rincón D, Ramírez M, Gutiérrez I, et al. Use of transient elastography (FibroScan®) for the noninvasive assessment of portal hypertension in HIV/HCV-coinfected patients. J Viral Hepat. 2011;18(10):685-91. [ Links ]
27. Castera L. Invasive and non-invasive methods for the assessment of fibrosis and disease progression in chronic liver disease. Best Pract Res Clin Gastroenterol. 2011;25(2):291-303. [ Links ]
28. Hirsch S, Guo J, Reiter R, Schott E, Büning C, Somasundaram R, et al. Towards compression-sensitive magnetic resonance elastography of the liver: sensitivity of harmonic volumetric strain to portal hypertension. J Magn Reson Imaging. 2014;39(2):298-306. [ Links ]
29. Hirsch S, Guo J, Reiter R, Papazoglou S, Kroencke T, Braun J, et al. MR elastography of the liver and the spleen using a piezoelectric driver, single-shot wave-field acquisition, and multifrequency dual parameter reconstruction. Magn Reson Med. 2014;71(1):267-77. [ Links ]
30. Talwalkar JA, Yin M, Venkatesh S, Rossman PJ, Grimm RC, Manduca A, et al. Feasibility of in vivo MR elastographic splenic stiffness measurements in the assessment of portal hypertension. AJR Am J Roentgenol. 2009;193(1):122-7. [ Links ]
31. Nedredal GI, Yin M, McKenzie T, Lillegard J, Luebke-Wheeler J, Talwalkar J, et al. Portal hypertension correlates with splenic stiffness as measured with MR elastography. J Magn Reson Imaging. 2011;34(1):79-87. [ Links ]
32. Sun HY, Lee JM, Han JK, Choi BI. Usefulness of MR elastography for predicting esophageal varices in cirrhotic patients. J Magn Reson Imaging. 2014;39(3):559-66. [ Links ]
33. Ronot M, Lambert S, Elkrief L, Doblas S, Rautou P-E, Castera L, et al. Assessment of portal hypertension and high-risk oesophageal varices with liver and spleen three-dimensional multifrequency MR elastography in liver cirrhosis. Eur Radiol. 2014;24(6):1394-402. [ Links ]
34. Shin SU, Lee J-M, Yu MH, Yoon JH, Han JK, Choi B-I, et al. Prediction of esophageal varices in patients with cirrhosis: usefulness of three-dimensional MR elastography with echo-planar imaging technique. Radiology. 2014;272(1):143-53. [ Links ]
35. Berzigotti A, Ashkenazi E, Reverter E, Abraldes JG, Bosch J. Non-invasive diagnostic and prognostic evaluation of liver cirrhosis and portal hypertension. Dis Markers. 2011;31(3):129-38. [ Links ]
36. Gerstenmaier JF, Gibson RN. Ultrasound in chronic liver disease. Insights Imaging. 2014;5(4):441-55. [ Links ]
37. Furuichi Y, Moriyasu F, Taira J, Sugimoto K, Sano T, Ichimura S, et al. Noninvasive diagnostic method for idiopathic portal hypertension based on measurements of liver and spleen stiffness by ARFI elastography. J Gastroenterol. 2013;48(9):1061-8. [ Links ]
38. Uchida H, Sakamoto S, Kobayashi M, Shigeta T, Matsunami M, Sasaki K, et al. The degree of spleen stiffness measured on acoustic radiation force impulse elastography predicts the severity of portal hypertension in patients with biliary atresia after portoenterostomy. J Pediatr Surg. 2015;50(4):559-64. [ Links ]
39. Han J-Y, Cho JH, Kwon HJ, Nam KJ. Predicting portal hypertension as assessed by acoustic radiation force impulse: correlations with the Doppler ultrasound. Br J Radiol. 2012;85(1016):e404-409. [ Links ]
40. Friedrich-Rust M, Rosenberg W, Parkes J, Herrmann E, Zeuzem S, Sarrazin C. Comparison of ELF, FibroTest and FibroScan for the non-invasive assessment of liver fibrosis. BMC Gastroenterol. 2010;10:103. [ Links ]
41. Thabut D, Imbert-Bismut F, Cazals-Hatem D, Messous D, Muntenau M, Valla DC, et al. Relationship between the Fibrotest and portal hypertension in patients with liver disease. Aliment Pharmacol Ther. 2007;26(3):359-68. [ Links ]
42. Poca M, Puente A, Graupera I, Villanueva C. Prognostic markers in patients with cirrhosis and portal hypertension who have not bled. Dis Markers. 2011;31(3):147-54. [ Links ]
43. Buck M, Garcia-Tsao G, Groszmann RJ, Stalling C, Grace ND, Burroughs AK, et al. Novel inflammatory biomarkers of portal pressure in compensated cirrhosis patients. Hepatology. 2014;59(3):1052-9. [ Links ]
44. Hayashi H, Beppu T, Okabe H, Nitta H, Imai K, Doi K, et al. Combined measurements of serum bile acid level and splenic volume may be useful to noninvasively assess portal venous pressure. J Gastroenterol. 2012;47(12):1336-41. [ Links ]
45. Procopet B, Cristea VM, Robic MA, Grigorescu M, Agachi PS, Metivier S, et al. Serum tests, liver stiffness and artificial neural networks for diagnosing cirrhosis and portal hypertension. Dig Liver Dis. 2015;47(5):411-6. [ Links ]
46. Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32-6. [ Links ]
47. Jansen C, Leeming DJ, Mandorfer M, Byrjalsen I, Schierwagen R, Schwabl P, et al. PRO-C3-levels in patients with HIV/HCV-Co-infection reflect fibrosis stage and degree of portal hypertension. PLoS ONE. 2014;9(9):e108544. [ Links ]
48. Hassan EM, Omran DA, El Beshlawey ML, Abdo M, El Askary A. Can transient elastography, Fib-4, Forns Index, and Lok Score predict esophageal varices in HCV-related cirrhotic patients? Gastroenterol Hepatol. 2014;37(2):58-65. [ Links ]
49. Stefanescu H, Procopet B. Noninvasive assessment of portal hypertension in cirrhosis: liver stiffness and beyond. World J Gastroenterol. 2014;20(45):16811-9. [ Links ]
50. Stefanescu H, Radu C, Procopet B, Lupsor-Platon M, Habic A, Tantau M, et al. Non-invasive ménage à trois for the prediction of high-risk varices: stepwise algorithm using lok score, liver and spleen stiffness. Liver Int. febrero de 2015;35(2):317-25. [ Links ]











 texto en
texto en