Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.31 no.2 Bogotá abr./jun. 2016
Pathophysiology, Diagnosis and Management of Hepatorenal Syndrome
Juan Ricardo Ospina T. (1), Juan Carlos Restrepo G. MD (2)
(1) XIII semester medical student in the Gastrohepatology Group at the University of Antioquia in Medellin Colombia
(2) Professor in the School of Medicine and Gastrohepatology Group of the University of Antioquia and the Hepatology and Liver Transplantation Unit of the Hospital Pablo Tobon Uribe in Medellín, Colombia. Mail:jcrestrepo@hptu.org.co
Received: 01-05-15 Accepted: 06-06-15
Abstract
Hepatorenal syndrome is a form of renal dysfunction develops as a complication in patients with advanced liver disease and in patients with acute liver failure. Significant alterations in renal blood flow lead to progressively increasing levels of creatinine and to ascites. Currently, the best treatment option is liver transplantation because options for treatment with drugs are very limited.
Keywords
Hepatorenal syndrome, cirrhosis, diagnosis, treatment.
INTRODUCTION
The liver and kidney are two organs that play a major role in corporal hemostasis. The first is the cornerstone of the body's metabolic activities. The second is responsible for removing waste products generated in the various metabolic cycles of the organism, as well as for controlling the volume and composition of the liquids in the different compartments. (1) In some pathologies, functions of both organs deteriorate together. One of these is hepatorenal syndrome (HRS) which is defined as reversible functional renal failure in the absence of parenchymal renal disease. (1-4) It is known that the development of HRS significantly increases the mortality rate and the rate of complications after liver transplantation. For this reason, the MELD (Model for Endstage Liver Disease) scale was developed, and began to be implemented in 2002, to assess the severity of cirrhosis and prioritize patients on the liver transplant list. (3, 4)
The term hepatorenal syndrome was first used in 1939 to describe renal dysfunction that followed biliary surgery and liver trauma. Then, between 1994 and 1996 a series of meetings in Chicago standardized nomenclature and proposed diagnostic criteria for refractory ascites and HRS. In 2007, at the fifty-sixth convention of the American Association for the Study of Liver Diseases, a study group on to review previously established diagnostic criteria for HRS was created. (5)
EPIDEMIOLOGY AND RISK FACTORS
Epidemiology
HRS occurs in about 10% of patients with advanced cirrhosis and acute liver failure. (1-9) However, it has been established that 18% of the patients with cirrhosis and ascites develop the syndrome within one year and that 39% of these patients develop it within five years. (3) The risk increases even more among patients with refractory ascites. (10)
Risk Factors
- Spontaneous bacterial peritonitis (SBP): Prophylactic antibiotic therapy has been shown to be important for preventing kidney damage secondary to cirrhosis. Recent studies have shown that administration of norfloxacin reduces the one year probability of HRS (28% treatedg group versus 41% control group). (6) Similarly, albumin management can be preventive element for these patients. Studies have shown that SBP patients who receive an albumin dose of 1.5 g/kg at the time they are diagnosed with SBP and 1 g/kg on the third day have 66% less incidence of HRS. (5, 7)
- Alcoholic hepatitis increases the risk of HRS, although there is evidence that this can be reduced by using drugs with hematologic effects rather than steroids. A study in India has found that administration of pentoxifylline 400 mg three times a day in patients with severe acute alcoholic hepatitis was associated with decreased incidence of HRS. (8)
- Fulminant hepatic failure (9)
- Excessive diuresis (9)
- Large volume of paracentesis in the absence of albumin type volume expanders (9)
- Gastrointestinal hemorrhaging (9)
- Administration of contrast media (10)
- Nephrotoxic antibiotics (10)
- Nonsteroidal anti-inflammatory drugs (NSAIDs) (10).
PHYSIOPATHOLOGY
To understand HRS it is necessary to understand cirrhotic disease and its key elements including the changes typical of patients with cirrhosis, ascites, and portal hypertension. (11)
Cirrhosis is the final stage of all chronic liver diseases. It is characterized by progressive distortion in hepatic architecture and microcirculation. (12) It is a disease with many kinds of etiologies including metabolic, toxic, infectious, autoimmune, pharmacological, genetic, biliary, vascular and cryptogenic etiologies. Nevertheless, the three main causes are alcohol abuse, NAFLD and chronic viral hepatitis. (12-14)
Cirrhotic pathology begins with an asymptomatic phase in which patients who have no other signs or symptoms may develop portal hypertension and esophageal varices. (12,14-16) As the disease progresses, portal pressure increases causing symptoms specific to this condition to develop. These include complications like ascites, variceal bleeding, HRS and/or hepatopulmonary syndrome, hepatic encephalopathy and SBP. (12-18) Given the context of cirrhosis, we can focus on the factors which have important roles.
Portal Hypertension and Ascites
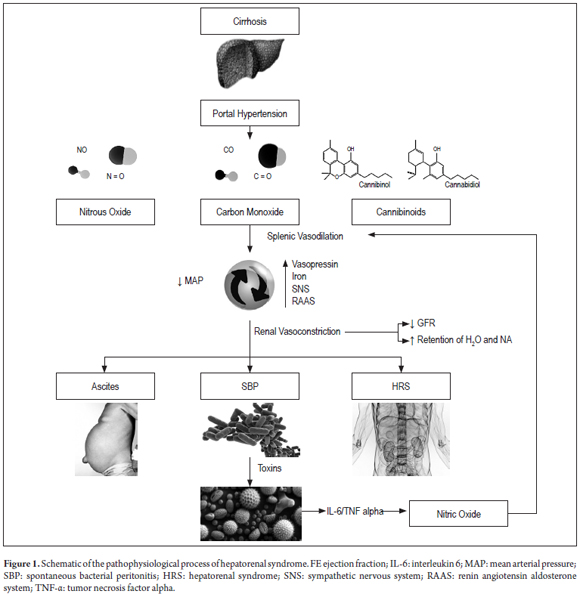
Increased portal pressure leads to increasing production of endogenous vasodilators such as nitric oxide (NO), carbon monoxide (CO) and cannabinoids which are released into splenic blood circulation where they in turn increase local plasma volume. The end result is a rapid decrease of mean arterial pressure. (19) To counter this, the body increases the ejection fraction, releases vasopressin, activates the sympathetic nervous system, and activates endogenous vasoconstriction systems. These include the renin-angiotensin-aldosterone system which causes vascular contraction in the kidney which decreases the glomerular filtration rate (GFR) and causes retention of both water and sodium. (20) This explains the development of ascites which favors bacterial translocation and promotes SBP and HRS. (21,22). It is noteworthy that this translocation and the resulting endotoxemia are responsible for stimulating a proinflammatory response characterized by production of cytokines such as tumor necrosis factor-alpha and interleukin-6 which facilitate production of nitric oxide which worsens vasodilation of the splanchnic bed. (3, 4)
Structural and Functional Changes in Cirrhotic Patients
There are many structural and functional changes that occur in cirrhotic patients. They include cardiac hypertrophy, diastolic dysfunction and decreased response to ionotropic and chronotropic stimuli all of which are associated with lower ejection fractions and thus with renal failure. (23-25)
Activation of vasoconstrictor systems to counteract vasodilator stimulus is the main mediator of tubular dysfunction and renal hemodynamics. (26). This powerful effect leads to renal ischemia which stimulates the release of endothelin-1 leading to hyperactivity of the sympathetic nervous system. All this creates a cycle that ends up aggravating the GFR. (27,28) Decreased GFR is a late event which occurs after other symptoms of HRS have developed. These include hyponatremia and ascites which have been considered predictive markers that are independent of creatinine (Figure 1). (29-32)
DIAGNOSIS
Until 2015, HRS diagnosis was established when a patient met six criteria that had been revised in 2007. (3, 33)
1. Cirrhosis + ascites
2. Serum creatinine > 1.5 mg/dL
3. Lack of improvement in serum creatinine (<1.5 mg/dL) after at least 2 days without diuretics and volume expansion with 1 g/kg/day of albumin (maximum dose 100 g/day)
4. No shock
5. No current or recent treatment with nephrotoxic agents
6. Absence of renal parenchymal disease (proteinuria < 500 mg/day, microhematuria < 50 erythrocytes/high-power field, normal renal ultrasound)
Once the diagnosis is made, the type of HRS must be established. (3, 7, 33, 35)
Type 1 is rapidly progressive. Initial serum creatinine doubles to more than 2.5 mg/dL in less than two weeks. This generates profound circulatory dysfunction and leads to dilutional hyponatremia. The one month survival rate is only 25% and the three month survival rate is only 10% if the patient does not receive treatment. Type 1 HRS can be precipitated by severe alcoholic hepatitis, gastrointestinal bleeding, sepsis or infections such as SBP.
Type 2 is characterized by being slowly progressive. Initial serum creatinine increases to 1.5-2.5 mg/dL. Patients survive for six to twelve months. Type 2 is often associated with refractory ascites.
This year the International Club of Ascites (ICA) published new criteria for diagnosis of acute kidney injury (AKI) in cirrhotic patients. The new criteria modify the standard threshold for serum creatinine of 1.5 mg/dL which has been used in recent years. Changes are related to two issues:
Serum creatinine of 1.5 mg / dL requires a markedly decreased GFR
A fixed threshold does not take into account dynamic changes in serum creatinine levels occurring days or weeks prior to reaching the threshold which are necessary for distinguishing between acute and chronic renal damage. (34)
These issues were considered in the formulation of the new diagnostic criteria for AKI in cirrhotic patients. Three stages of disease progression were established, progression, response to treatment and recommendations for early pharmacological management of this condition were given. (34)
Diagnosis: HRS should be diagnosed when there is an increase in serum creatinine ≥0.3 mg/dL over a period of 48 hours or an increase of more than 50% of the initial serum creatinine (known or suspected) within a period of 7 days.
Stages:
- Stage 1: Serum creatinine increases to 0.3 mg/dL or more or to more than 1.5 to 2 times baseline level.
- Stage 2: Serum creatinine increases to 2 or 3 times baseline level.
- Stage 3: Serum creatinine increases to more than 3 times baseline level or to more than 4 mg/dL with acute increase of at least 0.3 mg/dL or initiation of kidney replacement therapy.
Progression-Regression: Progression is defined as increases in patient stages and/or need for renal replacement therapy. Regression occurs when the patient's stage decreases.
Treatment Response:
- No response: No regression of acute renal failure.
- Partial response: Stage regression with serum creatinine reduction ≥0.3 mg/dL of baseline.
- Total response: Creatinine returns to within 0.3 mg/dL of baseline.
- It was determined that any measurement of serum creatinine for the patient within the last 3 months may be used as the baseline, but that if there are several measurements, the most recent prior to hospital admission should be used. If the patient does not have prior baseline measurements, serum creatinine measured at hospital admission should be used.
- In this way, the previously used criteria of a fixed value of serum creatinine levels over 1.5 mg/dL for diagnosis of AKI in patients with HRS has been replaced in the new criteria established by the ICA. On the other hand, the ICA suggests that the old serum creatinine threshold of 1.5 mg/dL should still be used to establish the prognosis of cirrhotic patients with AKI who are classified as stage 1. They should be classified further into two subgroups. (34)
- Patients whose serum creatinine levels upon hospitalization are below 1.5 mg/dL. The short-term mortality rate for these patients is similar to that of patients without AKI. They are also more likely to regress to a lower stage of renal disorder.
- Patients with serum creatinine levels above 1.5 mg/dL. The short-term mortality rates is higher than that of patients without AKI.
TREATMENT
Despite advances of knowledge about HRS, the range of therapeutic possibilities remains very limited. For the moment, they are based on general recommendations, pharmacological treatments, transjugular intrahepatic portosystemic shunts (TIPS), liver transplantation and following the directions proposed by the ICA.
General Recommendations (35)
- Restricting salt intake to 80-120 mmol/day
- Do not restrict fluid intake
- Remove identified nephrotoxic agents. If the patient is using diuretics, it must be remembered that among their adverse effects are renal failure, encephalopathy and hyponatremia. If the latter occurs, the drug must always be discontinued if serum sodium is less than 120 mEq/L.
- For Grade 1 or 2 ascites, doses of diuretics can be increased to control the condition. (36) Clonidine can be added to decrease the requirement for the diuretic and to reduce the accumulation of ascitic fluid. (37)
- For Grade 3 ascites and refractory ascites (no improvement of ascites in response to high doses of diuretics), the first line treatment is large-volume paracentesis (X> 5 liters). The problem with this procedure is the risk of HRS, so prevention using albumin infusion therapy to replace 8 g/L extracted is required to increase renal perfusion pressure, improve creatinine levels and facilitate the fractional excretion of sodium. (38)
Recommendations proposed by the ICA for control of AKI in Cirrhotic Patients
Stage 1 Cirrhotic Patients (34)
- Assess medications used by patient and remove nephrotoxic drugs such as NSAIDs, ACE inhibitors, and A2RBs vasodilators. Diuretics can be reduced or removed depending on the patient's condition.
- Hypovolemia should be addressed by expanding plasma volume with crystalloid or blood albumin. The latter is used in cases of AKI secondary to gastrointestinal bleeding.
- Control bacterial infections. If there are signs of SBP, antibiotic treatment should be started, ideally together with albumin infusions. (37, 38)
- Patients whose response is total should be followed up by measurement of serum creatinine every two to four while hospitalized and every two to four weeks for the first 6 months after discharge in order to identify any new AKI episode. However, for those patients who do not progress or have only partial response to treatment, there is no consensus on management, so more studies are needed.
Stages 2 and 3 Patient and Non-responding Stage 1 Patients (34)
- Diuretics must suspended if they have not already been suspended. Plasma volume must be expanded with 1g/kg of intravenous albumin for two consecutive days (maximum dose 100 g/day) to treat pre-renal AKI.
- Differential diagnosis is necessary especially if the patient meets the diagnostic criteria for HRS. If the patient does, treatment with albumin and a vasoconstrictor (terlipressin ideally) should be initiated independent of the serum creatinine level. If the patient does not, seek the cause of AKI.
Liver Transplantation
Liver transplantation should be considered for all cirrhotic patients with ascites, but especially for patients with refractory ascites since 50% of them die within 6 months and 75% within one year. Similarly, it should be considered for patients with SBP and/or HRS when there is no currently associated alcohol issue or malignant disease.
Transplantation has been shown to increase survival times of patients. Currently MELD prioritizes patients on the transplant list. (3 - 5, 11). Although transplantation is the treatment of choice for patients with HRS because it reverses the disease, physicians should be aware that various studies have shown that transplant patients with HRS prior to the procedure have higher morbidity rates after surgery that transplant patients who did not have HRS. Similarly, these studies show that over a the five year period following transplantation, the mortality rate was 32% for patients with advanced liver disease and pre-renal dysfunction. (5, 11) The current recommendation is to perform simultaneous kidney transplant for all cirrhotic patients with AKI 3 classifications, patients with chronic kidney disease with GFR less than 35 mL/min for more than 4 weeks, and patients with AKI who have required dialysis for more than 8 weeks. (11-38)
Terlipressin
Terlipressin is an analogue of vasopressin which induces vasoconstriction. It is believed to improve renal perfusion in HRS because it reduces splenic vasodilation thus decreasing renal flow. (39) Several randomized controlled clinical trials have shown that it reverses HRS Type 1 resulting in a slight reduction in short-term mortality. (39) In contrast, renal failure returns in patients with Type 2 HRS once the drug is stopped. For this reason, it has been proposed that it be used in these patients only as a bridge to liver transplantation. (36) Recent studies have shown that terlipressin can be used in conjunction with albumin in patients with HRS type 1 and associated sepsis, as this increases arterial blood volume and preload. (40)
The ICA recommends that treatment with terlipressin be started as soon as HRS is diagnosed. The recommended initial dose is 1 mg every four to six hours. If serum creatinine does not decrease by more than 25% after two days of treatment, the dosage should be doubled every 48 hours to a maximum of 12 mg/day. The drug should be discontinued serum creatinine does not decrease at least 50% after 7 days of using the drug at high doses, or if serum creatinine levels do not fall within the first 3 days. For patients who respond immediately, treatment should be continued until reversal of the syndrome or until a maximum of 14 days. (2) Up to 30% of patients present adverse effects from this drug, but treatment should be discontinued for only the 4% of patients with acute myocardial infarction or malignant arrhythmias, both of which are absolute contraindications. (11)
Norepinephrine
Norepinephrine is a catecholamine that acts on adrenergic receptors. It has proven to be as effective as terlipressin for controlling HRS Type 1. A pilot study of treatment with an infused dose of 0.5-3 mg/hour together with albumin expansion has found results similar to those obtained with terlipressin. (2) Nevertheless, this drug has more disadvantages than does terlipressin which is why its use is reserved for moments when terlipressin is not available. (11, 35)
Midodrine and Octreotide
Midodrine is a prodrug that acts as a selective agonist at α-1 generating peripheral vasoconstriction and thereby increasing blood pressure. Octreotide is a somatostatin analog that acts as an inhibitor of secretion of peptides synthesized by the gastroenteropancreatic endocrine system which reduces splenic blood flow. Two pilot studies have evaluated treatment with octreotide and midodrine + albumin infusion for managing HRS Type 1. The results show that these drugs produce effects similar to those of terlipressin but that the response is slower. It has been proposed that this treatment be used for HRS Type 1 when terlipressin is not available, but there is no consensus regarding the dosage. (11) There has been less study of treatment of Type 2 HRS with vasoconstrictors. All that is known from studies that have been done is that, for most patients use of terlipressin normalizes serum creatinine, but renal failure returns when the medication is suspended. Further studies are required. (11)
TIPS
Transjugular intrahepatic portosystemic shunt (TIPS) is a procedure that creates a shunt between the portal and systemic circulations by placing a stent between hepatic vein and portal vein. This decreases portal pressure and events that induce ascites. (11) This procedure is recommended for patients with Type 2 HRS who are refractory to treatment with diuretics. Studies have shown that its use decreases the rate of recurrence of ascites and improves survival more than paracentesis to remove fluid. The problem is that TIPS can cause heart failure and hepatic encephalopathy, so its use is not free of complications. (11) currently, TIPS is contraindicated in patients with chronic encephalopathy, patients with Child-Pugh scores over 12, patients over 70 years of age, and in patients with cardiac dysfunction with ejection fractions below 50%. (11)
CONCLUSION
Day by day, cirrhosis has established itself as one of the most prevalent pathologies in the world. Its many complications claim the lives of increasing numbers of people every year. One of these complications is HRS, which is observed in 10% of these patients and which indicates poor prognosis. This year, the international association of ascites published new criteria for diagnosis of acute renal failure in cirrhotic patients. Publication of guidelines for management of these patients that explain the pathology's impact on morbidity and mortality is expected. Despite the amount of research in this area, the range of therapeutic options is still very small, so new studies are needed to develop other therapeutic options to offer these patients.
Acknowledgements
We would like to thank the Gastrohepatology Research Group and Dr. Juan Carlos Restrepo Gutierrez to whom we especially direct our immense recognition for his academic work, human quality and clinical teaching. The authors also thank the Sustainability Project of the Office of the Vice-Rector for Research of the University of Antioquia.
REFERENCES
1. Longo DL, Fauci AS, Kasper DL, Hauster SL, Jamenson JL, Harrison JL. Principios de Medicina Interna. 18.a edición. México, D.F. McGRAW-HILL. 2012. p. 2601. [ Links ]
2. Toranzo Labrada R, González Castilla R, García Medina J. Síndrome hepatorrenal: diagnóstico y tratamiento. Medisan. 2012;16(5):787. [ Links ]
3. Higuera-de la Tijera MF et al. Conceptos actuales en síndrome Hepatorrenal. Rev Med Hosp Gen Méx. 2011;74(1):42-9. [ Links ]
4. Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361(13):1279-90. [ Links ]
5. Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. septiembre de 2007;56(9):1310-8. [ Links ]
6. Muñoz SJ. The hepatorenal syndrome. Med Clin N Am 2008;92:813–837 [ Links ]
7. Ginés P, Cárdenas A, Arroyo V, Rodés J. Management of cirrhosis and ascites. N Engl J Med 2004;350:1646-54. [ Links ]
8. De BK, Gangopadhyay S, Dutta D, et al. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: A randomized controlled trial. World J Gastroenterol. 2009;15:1613-9. [ Links ]
9. Craig E. Gordon. Hepatorenal Syndrome. HospMedClin. 2012;1:e62-e68. [ Links ]
10. Fowler C. Management of patients with complications of cirrhosis. Nurse Pract. 2013;38(4):14-21-23. [ Links ]
11. Egerod Israelsen M, Gluud LL, Krag A. Acute kidney injury and hepatorenal syndrome in cirrhosis. J Gastroenterol Hepatol. febrero de 2015;30(2):236-43. [ Links ]
12. García Bueya, F. González Mateosb y R. Moreno-Oteroa. Cirrosis hepática. Medicine. 2012;11(11):625-33. [ Links ]
13. Lefton HB, Rosa A, Cohen M. Diagnosis and epidemiology of cirrhosis. Med Clin North Am. 2009;93(4):787-799, vii. [ Links ]
14. de Franchis R, Baveno V Faculty. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53(4):762-8. [ Links ]
15. Benvegnù L, Gios M, Boccato S, Alberti A. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut. 2004;53:744-9. [ Links ]
16. D'Amico G, García-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-31. [ Links ]
17. Nagula S, Jain D, Groszmann RJ, García-Tsao G. Histological-hemodynamic correlation in cirrhosis-a histological classification of the severity of cirrhosis. J Hepatol. 2006;44:111-7. [ Links ]
18. De Franchis R, Baveno V Faculty. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762-8. [ Links ]
19. Brinch K, Moller S, Bendtsen F, Becker U, Henriksen JH. Plasma volume expansion by albumin in cirrhosis. Relation to blood volume distribution, arterial compliance and severity of disease. J. Hepatol. 2003;39:24-31. [ Links ]
20. Gines P, Schrier RW. Renal failure in cirrhosis. N. Engl. J. Med. 2009;361:1279-90. [ Links ]
21. Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J. Hepatol. 2013; 60:197-209. [ Links ]
22. Madsen BS, Havelund T, Krag A. Targeting the gut-liver axis in cirrhosis: antibiotics and non-selective β-blockers. Adv Ther. 2013;30(7):659-70. [ Links ]
23. Ruiz-del-Arbol L, Urman J, Fernández J, González M, Navasa M, Monescillo A, et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2003;38(5):1210-8. [ Links ]
24. Ruiz-del-Arbol L, Urman J, Fernández J, González M, Navasa M, Monescillo A, et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2003;38(5):1210-8. [ Links ]
25. Mackelaite L, Alsauskas ZC, Ranganna K. Renal failure in patients with cirrhosis. Med Clin North Am. 2009;93(4):855-869, viii. [ Links ]
26. Guevara M, Bru C, Ginès P, Fernández-Esparrach G, Sort P, Bataller R, et al. Increased cerebrovascular resistance in cirrhotic patients with ascites. Hepatology. 1998;28(1):39-44. [ Links ]
27. Arroyo V, Colmenero J. Ascites and hepatorenal syndrome in cirrhosis: pathophysiological basis of therapy and current management. J Hepatol. 2003;38 Suppl 1:S69-89. [ Links ]
28. Faber JE, Brody MJ. Afferent renal nerve-dependent hypertension following acute renal artery stenosis in the conscious rat. Circ Res. 1985;57(5):676-88. [ Links ]
29. Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359(10):1018-26. [ Links ]
30. Heuman DM, Abou-Assi SG, Habib A, Williams LM, Stravitz RT, Sanyal AJ, et al. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology. 2004;40(4):802-10. [ Links ]
31. Hasper D, Jörres A. New insights into the management of hepato-renal syndrome. Liver Int. 2011;31 Suppl 3:27-30. [ Links ]
32. Arroyo V, Colmenero J. Ascites and hepatorenal syndrome in cirrhosis: pathophysiological basis of therapy and current management. J Hepatol. 2003;38 Suppl 1:S69-89. [ Links ]
33. Pérez RM. Síndrome hepatorrenal: Enfoque actual. GastrLatinoam. 2006;17:211-217. [ Links ]
34. Angeli P, Ginès P, Wong F, Bernardi M, Boyer TD, Gerbes A, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol. 2015;62(4):968-74. [ Links ]
35. Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56(9):1310-8. [ Links ]
36. European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53(3):397-417. [ Links ]
37. Lenaerts A, Codden T, Meunier J-C, Henry J-P, Ligny G. Effects of clonidine on diuretic response in ascitic patients with cirrhosis and activation of sympathetic nervous system. Hepatology. 2006;44(4):844-9. [ Links ]
38. Leithead JA, Hayes PC, Ferguson JW. Review article: advances in the management of patients with cirrhosis and portal hypertension-related renal dysfunction. Aliment Pharmacol Ther. 2014;39(7):699-711. [ Links ]
39. Francoz C, Durand F. Type-1 hepatorenal syndrome in patients with cirrhosis and infection vs. sepsis-induced acute kidney injury: what matters? J Hepatol. mayo de 2014;60(5):907-9. [ Links ]
40. Rodríguez E, Elia C, Solà E, Barreto R, Graupera I, Andrealli A, et al. Terlipressin and albumin for type-1 hepatorenal syndrome associated with sepsis. J Hepatol. 2014;60(5):955-61. [ Links ]











 texto en
texto en