Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.31 no.2 Bogotá abr./jun. 2016
Liver Biopsies in Transplant Pathology: Histopathological Diagnosis and Clinicopathological Correlation in the Early Post-Transplant Period
Rocío del Pilar López Panqueva MD. (1)
(1) Pathologist at the Hospital Universitario Fundación Santa Fe de Bogotá and the Faculty of Medicine at the Universidad de Los Andes. Bogotá, Colombia.
Received: 29-04-16 Accepted: 03-05-16
Abstract
Biopsies of liver allografts are still considered to be the gold standard. They play an important and integral role in the interpretation and explanation of changes that may occur in response to alterations in function tests, in the interpretation and explanation of liver biochemistry, in the interpretation and explanation of functional abnormalities, and in the interpretation and explanation of diagnostic images (whether or not accompanied by symptoms). Biopsies are also useful for monitoring and are often part of the protocol (1-3). The evaluation of biopsy samples after transplantation can be difficult especially because of the very broad spectrum of complications that may arise in the post-transplant period. Many of them require immediate diagnosis and treatment despite this difficulty. Although the most common condition is acute rejection, many other conditions and disorders can be observed. They include perfusion/reperfusion alterations, functional impairment, recurrence of underlying diseases, injury to the bile duct, vascular lesions, opportunistic infections, de novo pathologies such as autoimmune hepatitis, post-transplant idiopathic chronic hepatitis, drug toxicity, and tumors (4). This is the second article about the pathology of liver transplantation. It discusses the most common pathologies in the early post-transplant period and provides a histopathological approach towards difficulties and controversies for adequate clinicopathological correlation.
Keywords
Liver transplantation, liver biopsy, graft dysfunction, perfusion ischemia, reperfusion injury, allograft rejection, acute humoral rejection, C4d, acute cellular rejection, portal inflammation, ductulitis, endotheliitis, endothelialitis
INTRODUCTION
Traditionally, post liver transplantation pathologies have been divided according to time of onset into early or late. Some of these are summarized in Table 1. The questions we most often face depend on the time at which the event occurs. If there is worsening liver function during the first week after transplant, or no tests have normal results, we face primary or secondary graft dysfunction and should consider the perfusion/reperfusion injury, immune problems such as acute cellular rejection, ABO incompatibility, antibody-mediated rejection; donor-dependent factors or technical problems, especially vascular anastomosis due to failure or thrombosis.
EARLY POSTOPERATIVE COMPLICATIONS FOLLOWING LIVER TRANSPLANTATION
Graft Dysfunction
Although there is no clear definition of the term "graft dysfunction", its main feature is poor functioning of the graft in the first weeks after transplantation. This portends poor graft survival and thus a poor prognosis for patient survival. It is clinically reflected high levels of aminotransferase and prolonged clotting times. When poor graft functioning is life-threatening there is very severe elevation of transaminases, usually above 2,000 U/L, accompanied by hyperbilirubinemia of over 10 mg/dL.
The patient's life is at risk when transaminase values are above 5000 U/L, the international normalized ratio (INR) stays at three or more times its normal value, and there is metabolic acidosis. In this situation we speak of primary graft failure: this is considered an emergency that requires retransplantation. Without one, the probability of death is very high. (5, 6) Some studies have shown associations between early dysfunction and the preoperative condition of the recipient, especially if this has required use of assisted ventilation, donation after cardiac death, donor age, graft size, the degree of steatosis, operating time and the need for intraoperative transfusion. (7, 8)
Ischemia and Reperfusion Injury
The process of organ preservation and subsequent reperfusion invariably leads to cell injury. The main etiological factor in primary graft dysfunction (DPI) is associated with ischemia/reperfusion injury which occurs immediately upon the return of oxygen to circulation in an ischemic organ. Ischemia decreases nutrients and energy and disrupts homeostasis. It is initiated through the generation of reactive oxygen species (ROS) by hepatocytes during ischemia. This activates signaling pathways involved in hypoxic response while an inflammatory immune response activates Kupffer cells, CD4 + and polymorphonuclear neutrophils to migrate to the site of injury and activate cytokines, chemokines and complement proteins where previously large amounts of ROS had been generated. This process leads to cellular apoptosis and cell death with ischemic necrosis after reperfusion. Moreover, endothelial lesions damage microcirculation and promote development of thrombosis which result in necrosis of liver tissue. (9-11) This is an important cause of graft failure in the early postoperative period. Up to 50% of allografts show some sign of ischemic damage, and ischemia accounts for 10% of early organ failures. It is more likely to occur if there is preexisting damage such as macrovesicular steatosis or siderosis in the donor organ. (12)
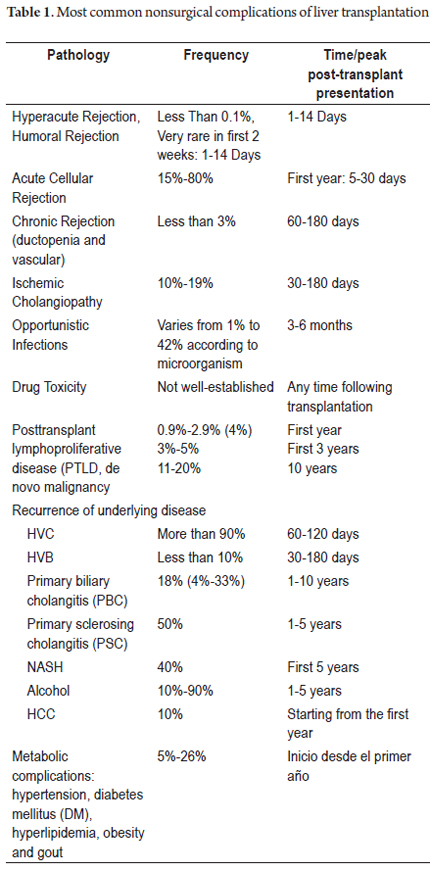
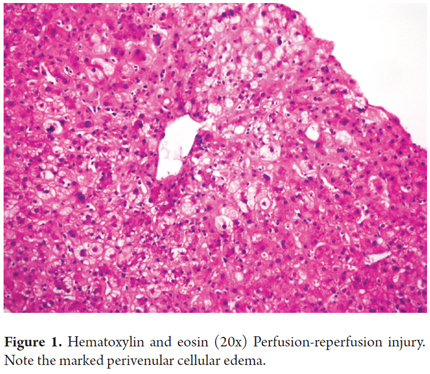
Histologically, ischemia/reperfusion injury is characterized by cell ballooning and either isolated necrosis with formation of acidophilic bodies or massive necrosis accompanied by aggregates of neutrophils and hepatocanalicular cholestasis in perivenular locations (zone 3). Given the increased susceptibility of this region to ischemic events, changes become evident within the first 48 hours after transplantation (Figures 1 and 2). When biopsies are taken at time zero or post-reperfusion, aggregates of inflammatory polymorphonuclear neutrophils are more prominent. In severe cases, inflammation decreases and necrosis increases, which compromises the acini. (13)
Periportal necrosis and subcapsular infarcts are less frequently observed. When the donor liver is fatty, reperfusion injury promotes the breakdown of hepatocytes. This frees fat globules which are caught in the cords of hepatocytes and cause obstruction. This resembles dilated sinusoids or lipopeliosis. They can migrate to the lungs causing fat embolism. The prognosis for this condition depends directly on the percentage of parenchymal necrosis. (14) Differential diagnosis must consider humoral rejection, cellular rejection and surgical complications, especially vascular and bile duct anastomosis. Very early cholestasis can result from small-for-size donor grafts. In these cases cell edema is very prominent and diffuse rather than only in perivenular locations.
ALLOGRAFT REJECTION
Criteria for histopathological diagnosis of rejection in transplantation have been defined and characterized, by the introduction of the 1995 Banff classification by international consensus and then, in 1997 by a panel of experts. To date, the humoral rejection, acute cellular rejection and chronic rejection have been accepted. (15,16). In this article we focus on the first two.
Humoral Rejection
Acute humoral rejection is very rare among ABO compatible liver transplants. Initially, it was seen as an issue of incompatible liver grafts, but it is now recognized as a very likely cause of injury, both early and late. Its true incidence has been difficult to establish because of the lack of studies to correlate the presence of C4d with specific anti-donor antibodies. (17) The primary rejection is antibody mediated and/or complemented. It occurs immediately (within hours or days) and is called hyperacute rejection. If rejection occurs a few days after or during the first week, it is called acute humoral rejection, or post-transplant acute rejection mediated by antibodies. The secondary form occurs when antibodies develop de novo. The antibodies may have formed previously or may be specific anti-donor antibodies that develop after transplantation. The antibodies react with antigens of the donor and have various effects within the allograft that range from destruction of the allograft to promoting its survival and including causing no change in its synthetic function. The class, antibody titer, and specificity of anti-donor antibodies as well as the density and distribution of the target antigens in the recipient organ influence the outcome. In particular, antibodies directed against the major antigens of blood groups ABO and MHC antigens have been shown to be capable of causing allograft rejection. (18, 19) Rejection is characterized by progressive elevation of bilirubin and severe thrombocytopenia associated with signs of liver failure.
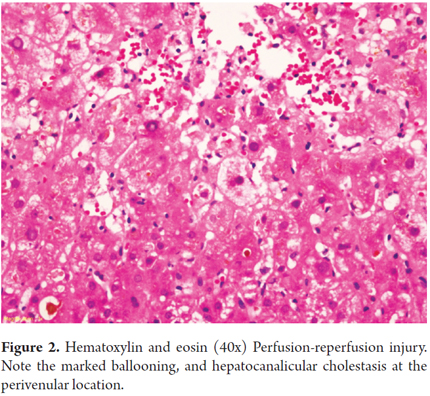
Pathologic examination in the initial stages shows nonspecific changes resembling those observed in a preservation injury with hepatocyte ballooning and hepatocyte necrosis in zone 3. Endothelial cells are directly damaged, so microvascular damage that occurs in the first few hours leads to focal hemorrhaging with sinusoidal aggregates of inflammatory polymorphonuclear neutrophils and platelets with small vessel thrombosis. This progresses to portal and periportal edema with cholangiolar or ductal proliferation which resembles obstruction of a bile duct. Hypertrophy of endothelial cells compromises venous portal vessels and capillaries, accompanied by adhesion of lymphocytes and eosinophils to the endothelium. (Figure 3)
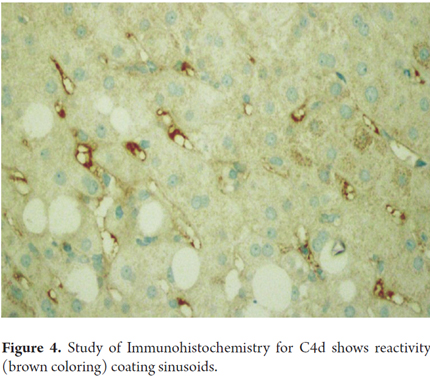
Thrombosis of the intrahepatic branches of the portal vein can develop in severe cases with venous infarction and extensive ischemia with geographic infarcts. Hepatic arteries and veins as well as the inferior vena cava can be compromised. To make this diagnosis, it is essential to exclude thrombosis of the hepatic artery and biliary obstruction. C4d reactivity tested by immunohistochemistry (in paraffin-embedded tissue) is difficult to interpret in isolation. It has several patterns: it must be intense in at least 50% of the portal veins or in capillaries of the portal stroma, or it must have sinusoidal distribution (Figure 4). If fresh or frozen tissue is available, the method that is considered to be most reliable is immunofluorescence with sinusoidal reactivity for C4d. Linear reactivity for IgG, IgM, fractions the complements of C3, C4, C1q, fibrinogen in the walls of arteries and the presence of anti HLA has also been demonstrated (17.20 to 24). It is important to emphasize that the focal presence of C4d has been described in many allografts which have no evidence of humoral antibody-mediated rejection. Therefore, accurate diagnosis of humoral rejection may be useful for determining and indicating whether or not plasmapheresis should be used in the immediate postoperative period. It could be useful for evaluating current protocols for case with the possibility of ABO-incompatible transplants. (24)
Keys to the diagnosis of humoral rejection
- Graft dysfunction diagnosed by clinical evaluation and laboratory tests
- Liver biopsy with evidence of active graft damage that suggests humoral rejection
- Any pattern of intense reactivity for C4d
- Circulation of anti-donor specific antibodies
Acute Cellular Rejection
Acute cellular rejection is an immune-mediated lymphocytic inflammatory response which is dependent on T cells. Today, the risk of acute cellular rejection has been reduced by appropriate immunosuppression and mortality and morbidity due to this cause are rare. Nevertheless, it is still the leading cause of graft dysfunction, occurring in 50% -75% of all transplant patients. It can occur in the first weeks after transplantation, particularly during the first 5 to 30 days, and 60% of all cases occur in the first 6 months after transplantation. However, acute rejection can occur at any time after transplantation, even several years after. It occurs in patients with poor adherence to immunosuppressive treatment, interactions with drugs such as calcineurin inhibitors and other factors that alter serum levels such as autoimmune diseases. A biopsy plays a key role in the diagnosis and management of acute cellular rejection. (3, 4, 25)
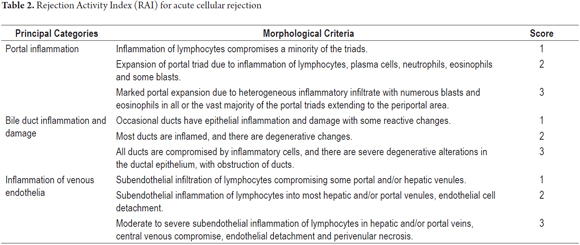
The Banff rating system assigns a numerical weight to each criterion or morphological parameter according to severity. The well-known Rejection Activity Index (RAI) for acute cellular rejection assigns a score from 1 to 3 with maximum possible score of 9. Table 2 shows an approach to pathological evaluation using a semi-quantitative index while Table 3 shows the scoring and terminology used by the Banff consensus. The application of the Banff scheme assumes that the histological diagnosis has already been established. (16, 26) The classic triad includes portal inflammation, portal bile duct injury and inflammatory vascular lesions. Portal inflammation is essential for histopathological diagnosis of acute cellular rejection, but at least two of these three characteristics must be present to make the diagnosis. (16) Histopathological criteria taken into account are evaluated in terms of inflammation density and percentage of compromised portal spaces represented in the biopsy, or of the compromised ductal or vascular structures. Some authors recommend at that least seven portal tracts be included.
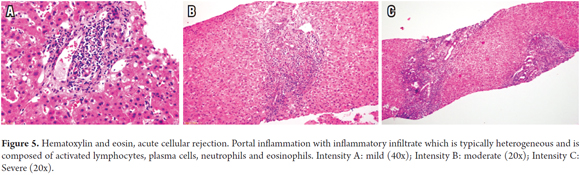
Portal Inflammation is defined as any degree of portal inflammation with the presence of a heterogeneous population of inflammatory cells, such as activated T lymphocytes, eosinophils, immunoblasts accompanied by plasma cells and polymorphonuclear neutrophils. There may be mild or minimal patches of lobular inflammation (Figure 5).
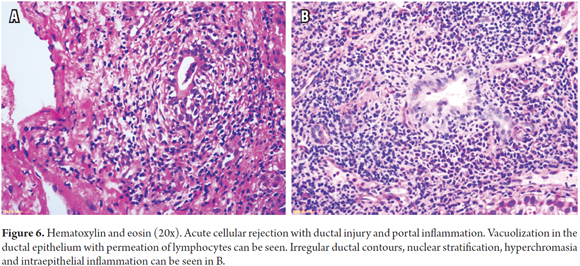
Inflammatory Bile Duct Injuries result from inflammatory lymphoid cells in the epithelium of small ducts (<30 microns) and degenerative changes caused by increases in the nucleus/cytoplasm ratio, hyperchromasia, nuclear pleomorphism, nuclear pseudostratification, alteration of polarity, mitosis, and/or paranuclear vacuolization of the ductal epithelium. There may be ruptures in the basal membrane (Figure 6).
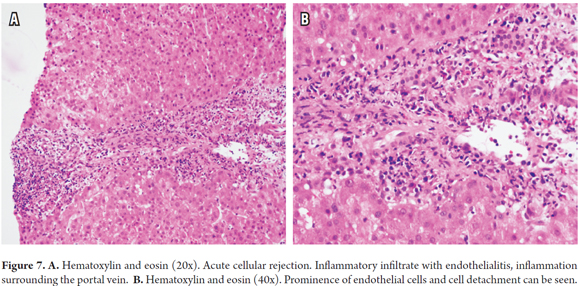
Inflammation of the vascular endothelium is not present in all cases, but when it is, it is located in the portal region and/or the terminal or central hepatic vein. It presents with subendothelial lymphocyte inflammation, prominence and endothelial detachment. Sinusoidal endothelialitis is also very occasionally present (Figure 7).
Necrotizing arteritis may be present in cases of severe rejection, although it is not common to find it in biopsies, as it usually involves hilar vessels.
Other Patterns of Acute Cellular Rejection
Central Perivenulitis and Central Venulitis
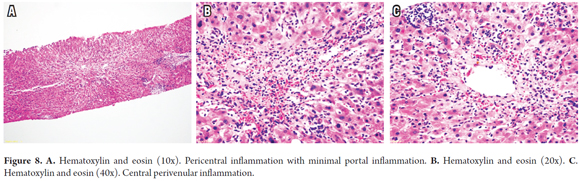
Central Perivenulitis (previously known as Central Venulitis) is observed in up to 30% of cases. It is more frequent among late acute rejections (after day 100) and pediatric rejections. It is characterized by inflammation endotheliitis or terminal venules, sometimes accompanied by congestion, extravasation of red blood cells and sinusoidal dilation, with mild or minimal inflammation and with isolated apoptotic hepatocytes in the immediate vicinity (zone 3) (Figure 8). Unlike classic acute cellular rejection, there is usually no endothelialitis, and in most cases the endothelium appears to be essentially normal. It may be accompanied by acute cellular rejection with inflammatory changes in the portal area or present as isolated central perivenulitis. (27,28)
Rejection Based On Lobular Inflammation Or Lobular Pattern
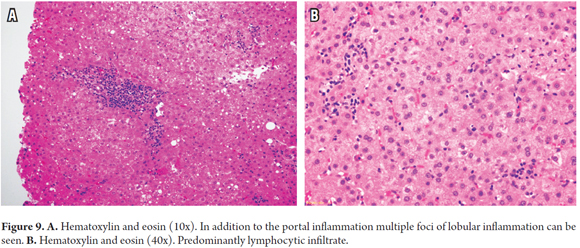
Sometimes inflammation of the lobule or lobular hepatitis dominates the morphological picture. Changes in the portal area and non-specific changes may be minimal. This pattern is especially seen in children, but also occurs in adults who have immunosuppression abruptly suspended after complying fully with immunosuppression. The lobule shows mild or patchy infiltration of lymphocytes (lymphocytic hepatitis) with scattered apoptotic bodies, hyperplastic Kupffer cells, minimal portal inflammation, mild ductulitis, but no evidence of endothelial inflammation nor compromised central veins (Figure 9). The main objects of differential diagnosis are early recurrence of hepatitis C virus infections, Epstein-Barr virus (EBV) and drug toxicity (26).
Infiltrative (Sinusoidal) Pattern and Hepatic Pattern
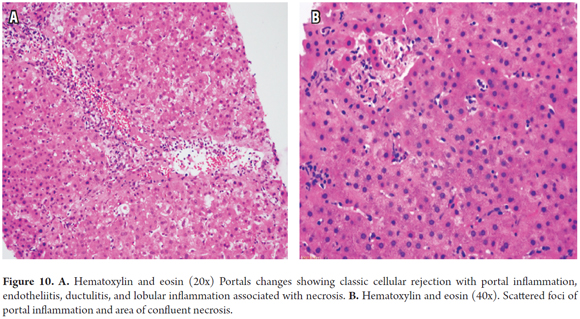
The infiltrative (sinusoidal) pattern and hepatic pattern of cellular rejection have been observed during the first year after transplantation. The infiltrative pattern shows prominent sinusoidal infiltration of activated lymphocytes CD3 (+) accompanying the classic triad of rejection. In the hepatic pattern, the typical features of acute cellular rejection overlap with necrotizing parenchymal inflammation or confluent foci of necrosis in varying locations (Figure 10). Clinically, there is more systemic involvement with elevated AST. These patters respond well to treatment, but have greater potential for chronicity. They are not included in the Banff score for which reason use of the RAI score is recommended to indicate the presence of these patterns. This will require closer monitoring to prevent progression to chronic rejection. The most important differential diagnosis is for viral infections. (29)
Chronic Hepatitis Pattern and Idiopathic Post-Transplant Hepatitis
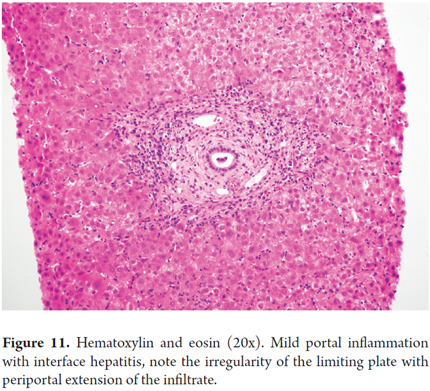
Characteristically, in chronic hepatitis pattern and idiopathic post-transplant hepatitis portal inflammation is mild to moderate and ductal lesions are mild or absent although there is interface hepatitis activity (Figure 11). This pattern can only be recognized in transplant patients that have no base necroinflammatory disease and no potential for recurrence of diseases such as hepatitis C. Differential diagnosis must consider drug reactions and other causes of nonspecific hepatitis. In severe rejections, the limiting plate may be compromised by extension of infiltrate to the lobule and a moth-eaten appearing image where there is interface hepatitis. This image is mostly seen in late acute rejection (over 100 days) and is very difficult to distinguish from chronic hepatitis. This pattern has been seen sixth months or more after transplantation and is associated with progression to fibrosis. (26,30)
Predominance Of Plasma Cells Pattern
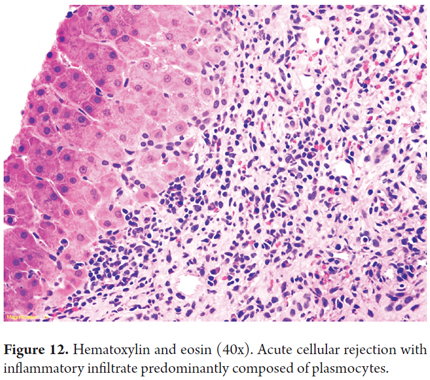
This pattern is seen within the clinical and morphological context of acute cellular rejection, but with predominantly plasma cell infiltrate (Figure 12). Liver transplantation data on its true meaning are limited, but proportions of plasma cells of more than 30% of the population of inflammatory cells have been associated with greater histological severity raising the possibility this measurement might be used as a marker of severity. The main problem is differentiating between a diagnosis of recurrent autoimmune hepatitis (AIH) and de novo hepatitis. Ductulitis and endotheliitis, key features of classic acute cellular rejection, are absent or minimal in the AIH. It is also important to note the absence of interface hepatitis because recurrent AIH usually occurs more than two years after transplantation, while the plasma cell rejection of variant occurs during the first 2 years. On rare occasions, low titers of autoantibodies are found in these patients. It should be noted that patients treated with interferon for hepatitis C may have this variant of acute cellular rejection, and many cases are associated with previous episodes of typical acute cellular rejection and with under-dosing of immunosuppressants. (31)
Biochemical evidence of liver damage is provided by increases in liver function tests and aminotransferase alkaline phosphatase and γ-glutamyl activity. In some cases these are accompanied by leukocytosis and peripheral eosinophilia. Clinical triggers include any changes in immunosuppression and conditions that alter the immune system, for example an infectious process. Other risk factors that should be considered are an elderly donor to a very young receiver, or preexisting conditions that alter immunity. A biopsy is done when there is clinical suspicion of acute rejection or graft dysfunction without an obvious explanation. Acute rejection is only found infrequently in biopsies conducted according to a protocol. (14,26).
It is important to note that if a biopsy is performed after start of immunosuppressive treatment, classification and scoring can be difficult to establish, especially because of the rapid modification of inflammation. Mild acute rejection in the Banff schema is the most common grade. It accounts for 60% -80% of total episodes, and most of these respond to increased dosages of immunosuppressants.
Moderate acute rejection with scores between 5 and 7 occurs in 10% to 20% of all episodes of acute cellular rejection. This is more common in patients who have risk factors and show clinical signs or symptoms such as fever or leukocytosis and impaired liver function tests.
Severe rejection accounts for less than 5% of cases. Most often it manifests itself in patients who have not been adequately immunosuppressed, patients with high lymphocytotoxic crossmatch titers, patients with histories of autoimmune diseases, and patients who have undergone retransplantation. Invariably, these patients have symptoms and signs that mimic an infectious systemic process. (29, 32)
Many of the studies that have evaluated clinical value and the Banff classification are contradictory. Even though it has not being fully validated, and it has not been shown to predict graft survival and even response to steroids with certainty, it is still widely accepted. It is regarded as a useful marker of the severity of rejection. Nevertheless, it is important to note that neither the total score nor any of the individual histopathological criteria correspond to the response to steroids or to graft survival. The results of a biopsy for a case of rejection has clinical significance and becomes the guide for treatment of acute rejection episodes, as it does also for asymptomatic patients (protocol biopsies) and for those with mild symptoms and low scores or mild rejection. Although, it is better if you can avoid the use of high-dose steroids and even if you can avoid minimal modification of treatment, patients usually have good responses to treatment with increases from baseline dosages of immunosuppressants or with corticosteroid therapy pulses. This is not the case when most cases of moderate or severe acute rejection are steroid-resistant and sometimes require immediate treatment with higher doses or pulsed steroids or monoclonal antibodies which are useful for preventing progression to chronic ductopenic rejection. (32)
Patients with centrilobular changes that do not meet Banff criteria for rejection respond well to immunosuppressive treatment similar to that used for usual rejection. The long-term outcome of patients with isolated centrilobular injuries is similar to that of patients with centrilobular changes associated with rejection in the classic triad. This finding has been reported as a harbinger of future episodes of acute rejection and chronic rejection. (27, 28) Patients with severe perivenular injuries have also developed perivenular fibrosis, Budd-Chiari syndrome and veno-occlusive disease. (33) The main problem that arises in the differential diagnosis of acute cellular rejection is differentiating between hepatitis B and recurrent hepatitis C because they can share criteria and both pathologies can be present simultaneously. For acute cellular rejection with heterogeneous infiltrate, periductal location, and without limiting plate injuries, clinical and serological parameters in addition to morphological criteria may be useful for diagnostic support or to rule out viral infection. In the next article we will go into this topic more deeply.
Keys to the diagnosis of acute cellular rejection
- Portal inflammation: heterogeneous infiltrate with activated lymphocytes and eosinophils
- Ductal injury: ductulitis of lymphocytes and degeneration of ducts
- Endotheliitis: portal and/or central subendothelial inflammation
CONCLUSION
The pathologist plays an important role in defining the complications that can occur in liver transplantation, especially because the clinical picture and patterns of abnormal liver enzymes, and other clinical and imaging parameters are unclear. More than a few times, they indicate diametrically opposed interventions, leading to the need for a liver biopsy to resolve a diagnostic question and get a more accurate diagnosis. Nevertheless, correlation with a complete medical history is essential for interpretation of histopathological findings.
REFERENCES
1. Yu YY, Ji J, Zhou GW, Shen BY, Chen H, Yan JQ, et al. Liver biopsy in evaluation of complications following liver transplantation. World journal of gastroenterology. 2004;10(11):1678-81. [ Links ]
2. Coffin CS, Burak KW, Hart J, Gao ZH. The impact of pathologist experience on liver transplant biopsy interpretation. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2006;19(6):832-8. [ Links ]
3. Adeyi O, Fischer SE, Guindi M. Liver allograft pathology: approach to interpretation of needle biopsies with clinicopathological correlation. Journal of clinical pathology. 2010;63(1):47-74. [ Links ]
4. Lucey MR, Terrault N, Ojo L, Hay JE, Neuberger J, Blumberg E, et al. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2013;19(1):3-26. [ Links ]
5. Stockmann M, Lock JF, Malinowski M, Seehofer D, Puhl G, Pratschke J, et al. How to define initial poor graft function after liver transplantation? - a new functional definition by the LiMAx test. Transplant international: official journal of the European Society for Organ Transplantation. 2010;23(10):1023-32. [ Links ]
6. Uemura T, Randall HB, Sanchez EQ, Ikegami T, Narasimhan G, McKenna GJ, et al. Liver retransplantation for primary nonfunction: analysis of a 20-year single-center experience. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2007;13(2):227-33. [ Links ]
7. Lee DD, Croome KP, Shalev JA, Musto KR, Sharma M, Keaveny AP, et al. Early allograft dysfunction after liver transplantation: an intermediate outcome measure for targeted improvements. Annals of hepatology. 2016;15(1):53-60. [ Links ]
8. Lee DD, Singh A, Burns JM, Perry DK, Nguyen JH, Taner CB. Early allograft dysfunction in liver transplantation with donation after cardiac death donors results in inferior survival. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2014;20(12):1447-53. [ Links ]
9. Quesnelle KM, Bystrom PV, Toledo-Pereyra LH. Molecular responses to ischemia and reperfusion in the liver. Archives of toxicology. 2015;89(5):651-7. [ Links ]
10. Abu-Amara M, Yang SY, Tapuria N, Fuller B, Davidson B, Seifalian A. Liver ischemia/reperfusion injury: processes in inflammatory networks: a review. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2010;16(9):1016-32. [ Links ]
11. Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125(4):1246-57. [ Links ]
12. Bruinsma BG, Wu W, Ozer S, Farmer A, Markmann JF, Yeh H, et al. Warm ischemic injury is reflected in the release of injury markers during cold preservation of the human liver. PloS one. 2015;10(3):e0123421. [ Links ]
13. Hubscher SG. Transplantation pathology. Seminars in diagnostic pathology. 2006;23(3-4):170-81. [ Links ]
14. Lefkowitch JH. Scheuer´s Liver biopsy interpretation. Ninth Edition. Elsevier, 2016. [ Links ]
15. Terminology for hepatic allograft rejection. International Working Party. Hepatology (Baltimore, Md). 1995;22(2):648-54. [ Links ]
16. Banff schema for grading liver allograft rejection: an international consensus document. Hepatology (Baltimore, Md). 1997;25(3):658-63. [ Links ]
17. Neil DA, Hubscher SG. Current views on rejection pathology in liver transplantation. Transplant international: official journal of the European Society for Organ Transplantation. 2010;23(10):971-83. [ Links ]
18. Demetris AJ, Murase N, Nakamura K, Iwaki Y, Yagihashi A, Valdivia L, et al. Immunopathology of antibodies as effectors of orthotopic liver allograft rejection. Seminars in liver disease. 1992;12(1):51-9. [ Links ]
19. Hubscher SG. Antibody-mediated rejection in the liver allograft. Current opinion in organ transplantation. 2012;17(3):280-6. [ Links ]
20. Della-Guardia B, Almeida MD, Meira-Filho SP, Torres MA, Venco F, Afonso RC, et al. Antibody-mediated rejection: hyperacute rejection reality in liver transplantation? A case report. Transplantation proceedings. 2008;40(3):870-1. [ Links ]
21. Haga H, Egawa H, Shirase T, Miyagawa A, Sakurai T, Minamiguchi S, et al. Periportal edema and necrosis as diagnostic histological features of early humoral rejection in ABO-incompatible liver transplantation. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2004;10(1):16-27. [ Links ]
22. Taner T, Stegall MD, Heimbach JK. Antibody-mediated rejection in liver transplantation: current controversies and future directions. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2014;20(5):514-27. [ Links ]
23. O'Leary JG, Michelle Shiller S, Bellamy C, Nalesnik MA, Kaneku H, Jennings LW, et al. Acute liver allograft antibody-mediated rejection: an inter-institutional study of significant histopathological features. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2014;20(10):1244-55. [ Links ]
24. Kozlowski T, Andreoni K, Schmitz J, Hayashi PH, Nickeleit V. Sinusoidal C4d deposits in liver allografts indicate an antibody-mediated response: diagnostic considerations in the evaluation of liver allografts. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2012;18(6):641-58. [ Links ]
25. Song AT, Avelino-Silva VI, Pecora RA, Pugliese V, D'Albuquerque LA, Abdala E. Liver transplantation: fifty years of experience. World journal of gastroenterology. 2014;20(18):5363-74. [ Links ]
26. Torbenson, MS. Biopsy Interpretation of the Liver. Series Editor Jonathan I. Epstein Wolteers Kluwer. Chapter 14th. Transplant Pathology, 2015. [ Links ]
27. Khettry U, Backer A, Ayata G, Lewis WD, Jenkins RL, Gordon FD. Centrilobular histopathologic changes in liver transplant biopsies. Human pathology. 2002;33(3):270-6. [ Links ]
28. Sundaram SS, Melin-Aldana H, Neighbors K, Alonso EM. Histologic characteristics of late cellular rejection, significance of centrilobular injury, and long-term outcome in pediatric liver transplant recipients. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2006;12(1):58-64. [ Links ]
29. Siddiqui I, Selzner N, Hafezi-Bakhtiari S, Marquez MA, Adeyi OA. Infiltrative (sinusoidal) and hepatitic patterns of injury in acute cellular rejection in liver allograft with clinical implications. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2015;28(9):1275-81. [ Links ]
30. Syn WK, Nightingale P, Gunson B, Hubscher SG, Neuberger JM. Natural history of unexplained chronic hepatitis after liver transplantation. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2007;13(7):984-9. [ Links ]
31. Alexander J, Chu W, Swanson PE, Yeh MM. The significance of plasma cell infiltrate in acute cellular rejection of liver allografts. Human pathology. 2012;43(10):1645-50. [ Links ]
32. Horoldt BS, Burattin M, Gunson BK, Bramhall SR, Nightingale P, Hubscher SG, et al. Does the Banff rejection activity index predict outcome in patients with early acute cellular rejection following liver transplantation? Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2006;12(7):1144-51. [ Links ]
33. Sebagh M, Debette M, Samuel D, Emile JF, Falissard B, Cailliez V, et al. «Silent» presentation of veno-occlusive disease after liver transplantation as part of the process of cellular rejection with endothelial predilection. Hepatology (Baltimore, Md). 1999;30(5):1144-50. [ Links ]











 texto en
texto en