Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.31 no.3 Bogotá jul./set. 2016
Frequency of Antibodies to Hepatitis E In Blood Donors in the Municipality of Yarumal, Antioquia
Alejandra Duque Jaramillo Biol. (1), Luisa F. Restrepo, Ing. Biomédica (1), Carolina Mantilla-Rojas, Biol. (1), Mónica Toro, Biol. (1), Juan Camilo Olarte, Bact. Esp. (2), W. Alfredo Ríos Ocampo, Biol. MSc (1), Fabián Cortés-Mancera, Bact., MSc (1,3), María Cristina Navas, Bact., MSc, PhD. (1)
(1) Gastrohepatology Group of the Faculty of Medicine at the Universidad de Antioquia in Medellín, Colombia.
(2) Antioquia branch of the Red Cross Blood Bank in Medellín, Colombia.
(3) GI2B Biomedical Research and Innovation Group in the Faculty of Sciences of the Instituto Tecnológico Metropolitano in Medellín, Colombia.
Received: 26-02-16 Accepted: 25-07-16
Abstract
Introduction: The hepatitis E virus (HEV) is transmitted via the fecal-oral route and causes acute liver disease. In Colombia there have been some studies of patients who have been diagnosed with viral hepatitis, of swine farm workers and in environmental samples.
Objective: The objective of this study was evaluate samples from blood donors in the municipality of Yarumal in the department of Antioquia for the presence of anti-HEV antibodies.
Methods: Serum samples were obtained from blood donated to the Colombian Red Cross by blood donors on a voluntary basis in a campaign in the municipality of Yarumal. Samples in the presence of anti-HEV IgM and IgG ELISA using commercial kit was determined.
Results: Forty-two serum samples were analyzed: 19 (45.2%) were positive for anti-HEV IgG. None of the samples were positive for anti-HEV IgM.
Conclusions: This is the first report of anti-HEV antibodies in blood donors in Colombia. The frequency of anti-HEV (45.2%) is higher than previously reported in other studies in this country and in blood donors in other Latin American countries. This frequency may be linked to contact with infected pigs and to exposure to water contaminated with the virus. However, additional studies should be conducted in similar populations in the country to confirm this finding.
Keywords
Hepatitis E virus, antibodies, ELISA, blood donors, rural populations.
INTRODUCTION
The Hepatitis E Virus (HEV) is an enterically transmitted etiological agent of acute viral hepatitis. The World Health Organization estimates that there are 20 million HEV infections, more than 3 million symptomatic cases, and 56,600 virus-related deaths each year. (1) HEV (Orthohepevirus A) belongs to the Orthohepevirus genus of the Hepeviridae family which also includes other viruses that infect mammals (humans, pigs, boars, deer) and fowl. (2) It is a non-enveloped virus whose 7.3 kb single-stranded RNA genome has positive polarity and contains three open reading frames (ORF) and two highly conserved noncoding regions, 5' and 3'-UTR (untranslated region). (3)
Four genotypes of HEV have been identified based on phylogenetic analysis of ORF2. (4) Genotypes 1 and 2 infect only humans and have been associated with epidemics in developing countries while genotypes 3 and 4 can infect humans, pigs and other animal species and have been reported in sporadic cases of infection, mainly in industrialized countries. (5, 6) In Latin America, the circulation of genotypes 1 and 3 has been described. Genotype 2 has been described in a single epidemic event in Mexico. (7)
The fecal-oral route is the main route of transmission and occurs consumption of water and food contaminated with feces. Nevertheless, occur zoonotic transmission through consumption of undercooked pork organ meats, particularly liver, and exposure to feces of infected animals also occurs. (8) Other less frequent transmission routes are blood transfusions and vertical transmission. (9, 10) HEV infections present no clinical features that distinguish it from other types of viral hepatitis that would allow diagnosis. Consequently, diagnosis is based on detection of anti-HEV IgG and IgM by ELISA and/or detection of the viral genome by RT-PCR. (5)
The first studies of HEV infections in Colombia were conducted among patients who had been diagnosed with viral hepatitis. Their serological and molecular markers were described and genotype 3 HEV was identified. (11,12)
Then, studies of pig farm workers demonstrated the presence of anti-HEV IgG, (13,14) and tests of pigs at pig farms found HEV IgM and IgG antibodies. Viral genomes were detected in livers, and the virus was also found in stool samples. (15-17)
Four genotypes and 24 subtypes of HEV have been identified. The presence of viral strains in both humans and in other animal species suggests that there are HEV animal reservoirs, including in pigs, that play an important role.
Given that the department of Antioquia is the leading producer and consumer of pork in Colombia, the pigs at various farms were tested for anti-HEV and porcine fecal was tested for viral RNA. In addition, HEV genotype 3 was detected in the water supplies and waste of several municipalities in Antioquia (Zaragoza, San Pedro de los Milagros, Granada, Puerto Berrio, Frontino, Girardota, Venice and Cisneros). (18) However, no data has been collected on the frequency of anti-HEV in blood donors in Colombia.
This study evaluates the presence of anti-HEV in samples from blood donors in the municipality of Yarumal, Antioquia department.
MATERIALS AND METHODS
Type of Study
Descriptive cross-section study
Study Population
The municipality of Yarumal is located in the north of Antioquia department, 120 kilometers from Medellin, the capital of the department (Figure 1). Its urban center has 27 neighborhoods, and its rural area has 45 rural districts within which are seven unincorporated residential and commercial areas. (19) According to 2006 data from the National Administrative Department of Statistics (DANE), the municipality had 31,816 inhabitants of whom 26,716 (83.9%) resided in the urban center and 5,100 (16.02%) in the rural districts. (20) Eighty percent of rural households are engaged in agriculture. Of these, 95.2% work raising cattle and pigs. (20) In 2006, 87.6% of the population had access to piped water and 84.4% had access to sewer service. (20) The municipality of Yarumal has had a water treatment plant for drinking water since 2007. (21)
Samples
Fifty serum samples from blood donors that had been obtained by the Colombian Red Cross in the municipality of Yarumal, during the second half of 2012 were evaluated. Consent was obtained from all study participants. The serum obtained was frozen at -20 ° C until analysis at Gastrohepatology Laboratory at the University of Antioquia. Data on donors' ages, genders and occupations were obtained from the donation form. This study was approved by the ethics committee of the Metropolitan Technological Institute (ITM).
Detection of anti-HEV
Anti-HEV IgM and IgG found in serum samples were evaluated using commercial ELISA kits (HEV Dia.pro, Bioprobes Diagnostic, Milan, Italy). This kit uses HEV-specific synthetic antigens that are derived from strains from Mexico and Myanmar and that code for conserved and immunodominant determinants. (22) The sensitivity and specificity of the kit is 99% according to the manufacturer. Of the 50 serum samples, 42 were evaluated; the remaining 8 samples were not analyzed due to insufficient volume. The 42 samples were analyzed in duplicate and the results analyzed according to the manufacturer's instructions. The optical density/cut-off point was determined: samples whose results were greater than 1.1 were considered to be positive, samples whose results were between 0.9 and 1.1 were considered to be indeterminate, and samples whose results were less than 0.9were considered to be negative.
RESULTS
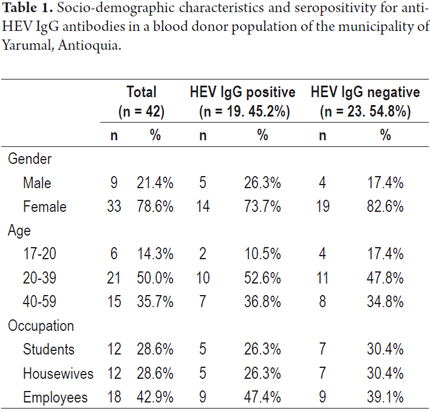
The donors' age range was 17 to 58 years with an average of 33.4 years. Of the samples analyzed, 78.6% came from women and 21.4% from men. The population was divided according to occupations into students, housewives and employees. The information available from donors does not indicate whether a donor lives in the urban area of the municipality or in a village or rural area. Nineteen serum samples (45.2%) were positive for anti-HEV IgG, but none presented HEV IgM antibodies. Samples with an indeterminate result were categorized as negative. Seropositivity according to gender, age and occupation is summarized in Table 1.
DISCUSSION
This is the first study of HEV in blood donors ever to be conducted in Colombia. When serum samples from Yarumal were analyzed, anti-HEV IgG was detected in 45.2% of samples, but IgM antibodies were not. This high seroprevalence may be related to contact with infected pigs because pig farming is part of the economic activity of the municipality of Yarumal. (20) It may also be related to exposure to water contaminated with the virus prior to start of operations of the water treatment plant in 2007. (21)
The blood donation campaigns are conducted in the main park and usually occur between 9:00 am and 4:00 pm. This may explain the high percentage of female donors, considering that most women donors declared themselves to be either students or housewives while all of the male donors reported being employed in different trades.
It is noteworthy that seropositivity for anti-HEV IgG found in this study is significantly higher than those described in previous studies elsewhere in Colombia and Latin America. Samples from patients with clinical diagnoses of viral hepatitis from 15 of the 32 departments of the country have a of 6.3% frequency (16/253) for anti-HEV IgG, and patient samples from Medellin have a frequency for IgG antibody of 10.9% (10/91). (11) In contrast, Betancur et al. reported that workers on pig farms in Caldas, San Pedro and Guarne (Antioquia) had an IgG antibody frequency of 11.2% (11/98), (13) and Gutierrez et al. reported a similar frequency of 15.7% (25/159) for IgG/IgM in a study of people with occupational exposure to pigs conducted in 10 municipalities of Antioquia department (Barbosa, Bello, Caldas, Medellin, Entrerríos, Don Matias, San Pedro, Santa Rosa de Osos, Jericho and Rionegro). (14) These people constitute a population that is at risk for HEV infection. Neverhteless, in all cases seropositivity was lower than that found in this study.
Gutierrez et al. also report an anti-HEV seropositivity of 5.9% (2/34) in individuals cohabiting with people with occupational exposure to pigs, and 7.2% (71/983) in the general population of the urban areas of the municipalities they studied. (14). A recent report that evaluated coinfections of Hepatitis E virus and other etiological agents of viral hepatitis in serum samples of patients from several regions of the country found a 25.3% frequency of IgG anti-HEV (342/1097), a 5.6% frequency of IgM anti-HEV (126/1097) type, and a 5.8% frequency of the presence of both markers (64/1097). (23) This seroprevalence of IgG antibodies is the closest to that reported in our study although the populations are not comparable since the study population of Gutierrez et al. consisted of individuals who had tested positive for markers of Hepatitis A, Hepatitis B and/or Hepatitis C infections, a group which has high risk of being infected with HEV. It should be noted that the frequency of antibodies differs from those previously reported in individuals with clinical diagnoses of viral hepatitis in this country. (11)
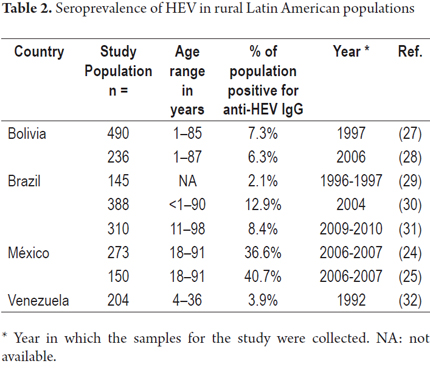
A study in the rural town of Durango, Mexico found an IgG anti-HEV frequency of 36.6% (100/273) and an IgM anti-HEV frequency of 40.7% (61/150) which are similar to those described in this study. (24, 25) In this same population, the frequency of anti-HEV markers was 5.7% among pregnant women (25/439) and 6.7% among Mennonites (10/150). (25, 26) Studies in rural populations of Bolivia, (27, 28), Brazil (29-31) and Venezuela (30) have reported frequencies of anti-HEV IgG of between 3.9% and 12.9% (Table 2). Differences among risk factors areas could explain this range of seroprevalences. Risk factors in rural areas can include access to drinking water, sewerage infrastructure, economic activities and population hygiene habits. The sensitivity and specificity of the methodologies used in these studies should also be considered. In addition, it should be noted that most of these studies are not recent and may not reflect the current state of affairs.
Latin American studies of seropositivity for anti- HEV IgG in blood donors have described frequencies between 1.2% and 16.7%. These included reported frequencies of 1.8% and 16.7% in Argentina, (34, 33), 16.2% in Bolivia, (35) 2% to 10% in Brazil, (9, 29, 36, 37), 8% in Chile, (38) 1.4% in Cuba, (39) and 1.2% in Uruguay. (40). The frequency of 45.2% found in this study is much higher than any previously reported in blood donors in Latin America. This could be because blood donors in other studies usually come from larger urban areas in which exposure risk factors for HEV infection is lower.
Since the sample analyzed in this study and is very limited and is not representative, we cannot compare the results with other studies of rural populations and blood donors in Latin America. With respect to the technique used for detection of anti-HEV antibodies, most commercial immunoassays for the detection of anti-HEV IgM and anti-HEV IgG are based on synthetic antigens of HEV derived from ORF2 and ORF3 strains from Mexico (genotype 2) and Myanmar (genotype 1). (41) Schnegg et al. have evaluated the sensitivity of the kit used in this study with a panel of 20 serum samples, 15 from patients with acute HEV infections and five from patients who had recovered from-acute infection (4 to 14 weeks after the acute phase). (42) Eighty percent of the acute phase samples and 20% of the post-acute samples were positive for IgG for an overall sensitivity of 65%. Avellón and colleagues evaluated the sensitivity of various commercial kits for detection of anti-HEV genotype 3 antibodies and found that the commercial kit used in this study (Dia.pro) had an anti-HEV IgG sensitivity of 59.6% and an anti-HEV IgM sensitivity of 77.5%. (43) These results contrast 99% sensitivity reported by the manufacturer. No other authors have reported problems with sensitivity for detecting HEV genotype 3. (43)
CONCLUSIONS
It is important to consider that all studies conducted to date in Colombia have been used Dia.pro immunoassay commercial kits for detection of anti-HEV antibodies.
Despite the limited sample size, this study is the first description of anti-HEV antibodies in blood donors in Colombia. This study provides evidence of a seroprevalence of 45.2% for HEV infection in the population of the municipality of Yarumal, Antioquia. The presence of pig farming activity and limited access to clean water until 2007 could be considered risk factors for this population, although no cases of acute infection (anti-HEV IgM 0%) were detected.
The results of this study contribute to the understanding of the epidemiology of HEV infection in Colombia and Latin America despite the study's small sample size. It is necessary to expand the number of samples in this population to confirm these findings and clearly identify risk factors. In addition, studies are needed in blood donors from other parts of Colombia to establish the clinical and epidemiological importance of HEV infection in this country.
Acknowledgements
The authors wish to express their thanks to the Metropolitan Technological Institute and to the Sustainability Project of the Office of the Vice-Rector for Research at the University of Antioquia.
Financing
The Metropolitan Technological Institute (ITM) and the Sustainability Project of the Office of the Vice-Rector for Research at the University of Antioquia financed this study.
Conflict of interests
The authors declare that they have no conflicts of interests.
1. World Health Organization (WHO). Hepatitis E Fact sheet #280 Internet. 2015 citado el 15 de septiembre de 2015. Disponible en: http://www.who.int/mediacentre/factsheets/fs280/en [ Links ]
2. Smith DB, Simmonds P, Jameel S, Emerson SU, Harrison TJ, Meng X-J, et al. Consensus proposals for classification of the family Hepeviridae. J Gen Virol. 2014;95(Pt 10):2223–32. [ Links ]
3. Sánchez Partidas DA, Gutiérrez García C del R. Virus de la hepatitis E: Características biológicas y epidemiológicas. Rev Soc Venez Microbiol. 2012;32(1):6–12. [ Links ]
4. Lu L, Li C, Hagedorn CH. Phylogenetic analysis of global hepatitis E virus sequences: genetic diversity, subtypes and zoonosis. Rev Med Virol. 2006;16(1):5–36. [ Links ]
5. Pérez-Gracia MT, García M, Suay B, Mateos-Lindemann ML. Current Knowledge on Hepatitis E. J Clin Transl Hepatol. 2015;3(2):117–26. [ Links ]
6. Khuroo MS, Khuroo MS. Hepatitis E: an emerging global disease – from discovery towards control and cure. J Viral Hepat. 2016;23(2):68-79. [ Links ]
7. Echevarría J m., González J e., Lewis-Ximenez L l., dos Santos DRL, Munné M s., Pinto M a., et al. Hepatitis E virus infection in Latin America: A review. J Med Virol. 2013;85(6):1037–45. [ Links ]
8. Yugo DM, Meng X-J. Hepatitis E Virus: Foodborne, Waterborne and Zoonotic Transmission. Int J Environ Res Public Health. 2013;10(10):4507–33. [ Links ]
9. Bortoliero AL, Bonametti AM, Morimoto HK, Matsuo T, Reiche EMV. Seroprevalence for hepatitis E virus (HEV) infection among volunteer blood donors of the Regional Blood Bank of Londrina, State of Paraná , Brazil. Rev Inst Med Trop São Paulo. 2006;48(2):87–92. [ Links ]
10. El Sayed Zaki M, El Razek MMA, El Razek HMA. Maternal-Fetal Hepatitis E Transmission: Is It Underestimated? J Clin Transl Hepatol. 2014;2(2):117–23. [ Links ]
11. Peláez D, Hoyos MC, Rendón JC, Mantilla C, Ospina MC, Cortés-Mancera F, et al. Infección por el virus de la hepatitis E en pacientes con diagnóstico clínico de hepatitis viral en Colombia. Biomédica. 2014;34(3):354–65. [ Links ]
12. Rendón J, Hoyos MC, di Filippo D, Cortes-Mancera F, Mantilla C, Velasquez MM, et al. Hepatitis E Virus Genotype 3 in Colombia: Survey in Patients with Clinical Diagnosis of Viral Hepatitis. PLoS ONE. 2016;11(2):e0148417. [ Links ]
13. Betancur CA, Mejía MV, Portillo S. Seroprevalencia de hepatitis E en trabajadores de fincas porcícolas del Valle de Aburrá 2011-2012. Acta Médica Colomb. 2013;38(2):68–70. [ Links ]
14. Gutiérrez-Vergara CC, Rodríguez B, Parra-Suescún J, Correa-Londoño G, López-López L, López-Herrera A, et al. Determinación de anticuerpos totales (IgG/IgM) y específicos (IgM) para el virus de la hepatitis E y detección molecular del virus en heces de humanos con o sin exposición ocupacional a porcinos en 10 municipios de Antioquia. Iatreia. 2015;28(3):248–58. [ Links ]
15. Gutiérrez Vergara CC, Ospina Vélez DA, Forero Duarte JE, Rodríguez BDJ, Gutiérrez Builes LA, Correa Londoño G, et al. Detección serológica y molecular del virus de la Hepatitis E en cerdos de granjas antioqueñas. Rev CES Med Vet Zootec. 2014;9(2):158–68. [ Links ]
16. Forero JE, Parra JE, López A. Detección del genoma del virus de la hepatitis E en muestras de heces de cerdos en plantas de beneficio en Antioquia, Colombia. Rev Fac Med Vet Zootec. 2014;61(3):221–7. [ Links ]
17. Gutiérrez-Vergara C, Quintero J, Duarte JF, Suescún JP, López-Herrera A. Detection of hepatitis E virus genome in pig livers in Antioquia, Colombia. Genet Mol Res GMR. 2015;14(1):2890–9. [ Links ]
18. Báez P, Jaramillo CM, Arismendi L, Rendón JC, Cortes-Mancera A, González MM, et al. Evidencia molecular de virus de la hepatits E en fuentes de agua en Antioquia. Biomédica. 2015;35(1):40–1. [ Links ]
19. Alcaldía de Yarumal. Información General del Municipio Internet. Citado el 15 de septiembre de 2015. Disponible en: http://www.yarumal.gov.co/alcaldia/presentacion. [ Links ]
20. Departamento Administrativo Nacional de Estadísticas (DANE). Censo General 2005. Perfil Yarumal, Antioquia Internet. 2010. Disponible en: http://www.dane.gov.co/files/censo2005/PERFIL_PDF_CG2005/05887T7T000.PDF. [ Links ]
21. Aguas del Norte Antioqueño. Servicio de Acueducto Internet. citado el 15 de septiembre de 2015. Disponible en: http://www.yarumal.gov.co/aguas/. [ Links ]
22. DIA.PRO. ELISA : HEV Ab Internet. Citado el 14 de septiembre de 2015. Disponible en: https://www.diapro.it/index.php/products/elisa/hev-ab-detail. [ Links ]
23. Peláez-Carvajal D, Martínez-Vargas D, Escalante-Mora M, Palacios-Vivero M, Contreras-Gómez, Lady. Coinfección del virus de la hepatitis E con otras hepatitis virales en Colombia y su caracterización genotípica. Biomédica. 2016;36(1).(Nota: falta proporcionar datos). [ Links ]
24. Alvarado-Esquivel C, Sánchez-Anguiano LF, Hernández-Tinoco J. Seroepidemiology of hepatitis E virus infection in general population in rural durango, México. Hepat Mon. 2014;14(6):e16876. [ Links ]
25. Alvarado-Esquivel C, Sánchez-Anguiano LF, Hernández-Tinoco J. Seroepidemiology of hepatitis E virus infection in mennonites in Mexico. J Clin Med Res. 2015;7(2):103–8. [ Links ]
26. Alvarado-Esquivel C, Sánchez-Anguiano LF, Hernández-Tinoco J. Hepatitis E virus exposure in pregnant women in rural Durango, Mexico. Ann Hepatol. 2014;13(5):510–7. [ Links ]
27. Bartoloni A, Bartalesi F, Roselli M, Mantella A, Arce CC, Paradisi F, et al. Prevalence of antibodies against hepatitis A and E viruses among rural populations of the Chaco region, south-eastern Bolivia. Trop Med Int Health TM IH. 1999;4(9):596–601. [ Links ]
28. DellAmico MC, Cavallo A, Gonzales JL, Bonelli SI, Valda Y, Pieri A, et al. Hepatitis E Virus Genotype 3 in Humans and Swine, Bolivia. Emerg Infect Dis. 2011;17(8):1488–90. [ Links ]
29. Trinta K, Liberto M. Hepatitis E virus infection in selected Brazilian populations. Mem Inst Oswaldo Cruz. 2001;96(1):25–9. [ Links ]
30. Vitral CL, da Silva-Nunes M, Pinto MA, de Oliveira JM, Gaspar AMC, Pereira RCC, et al. Hepatitis A and E seroprevalence and associated risk factors: a community-based cross-sectional survey in rural Amazonia. BMC Infect Dis. 2014;14:458. [ Links ]
31. Silva SMT da, Oliveira JM de, Vitral CL, Vieira K de A, Pinto MA, Souto FJD. Prevalence of hepatitis E virus antibodies in individuals exposed to swine in Mato Grosso, Brazil. Mem Inst Oswaldo Cruz. 2012;107(3):338–41. [ Links ]
32. Pujol FH, Favorov MO, Marcano T, Esté JA, Magris M, Liprandi F, et al. Prevalence of antibodies against hepatitis E virus among urban and rural populations in Venezuela. J Med Virol. 1994;42(3):234–6. [ Links ]
33. H. Fainboim, J. González, E. Fassio, A. Martínez, L. Otegui, M. Eposto, P. Cahn RM, G. Landeira, G. Suaya, E. Gancedo, 1 R. Castro LB and HL. Prevalence of hepatitis viruses in an anti-human immunodeficiency virus-positive population from Argentina. A multicentre study. J Viral Hepat 1. 1999;6(1):53–7. [ Links ]
34. Munné MS, Altabert NR, Otegui M LO, Vladimirsky SN, Moreiro R, Espul MP, et al. Updating the knowledge of hepatitis E: new variants and higher prevalence of anti-HEV in Argentina. Ann Hepatol. 2014;13(5):496–502. [ Links ]
35. Konomi N, Miyoshi C, La Fuente Zerain C, Li T-C, Arakawa Y, Abe K. Epidemiology of Hepatitis B, C, E, and G Virus Infections and Molecular Analysis of Hepatitis G Virus Isolates in Bolivia. J Clin Microbiol. 1999;37(10):3291–5. [ Links ]
36. Parana R, Cotrim HP, Cortey-Boennec ML, Trepo C, Lyra L. Prevalence of hepatitis E virus IgG antibodies in patients from a referral unit of liver diseases in Salvador, Bahia, Brazil. Am J Trop Med Hyg. 1997;57(1):60–1. [ Links ]
37. Passos-Castilho AM, de Sena A, Geraldo A, Spada C, Granato CFH. High prevalence of hepatitis E virus antibodies among blood donors in Southern Brazil. J Med Virol. 2016;88(2):361-4 [ Links ]
38. Ibarra H, Riedemann S, Reinhardt G, Frieck P, Siegel F, Toledo C, et al. Prevalence of hepatitis E virus antibodies in blood donors and other population groups in southern Chile. Rev Médica Chile. 1997;125(3):275–8. [ Links ]
39. Lemos G, Jameel S, Panda S, Rivera L, Rodríguez L, Gavilondo JV. Hepatitis E virus in Cuba. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2000;16(1):71–5. [ Links ]
40. Cruells MR, Mescia G, Gaibisso R, Ramírez M, Gutiérrez M, Kohen S, et al. [Epidemiological study of hepatitis A and E viruses in different populations in Uruguay]. Gastroenterol Hepatol. 1997;20(6):295–8. [ Links ]
41. Pas SD, Streefkerk RHRA, Pronk M, de Man RA, Beersma MF, Osterhaus ADME, et al. Diagnostic performance of selected commercial HEV IgM and IgG ELISAs for immunocompromised and immunocompetent patients. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2013;58(4):629–34. [ Links ]
42. Schnegg A, Bürgisser P, André C, Kenfak-Foguena A, Canellini G, Moradpour D, et al. An Analysis of the Benefit of Using HEV Genotype 3 Antigens in Detecting Anti-HEV IgG in a European Population. PLoS ONE. 2013;8(5):e62980 [ Links ]
43. Avellon A, Morago L, García-Galera del Carmen M, Muñoz M, Echevarría J-M. Comparative sensitivity of commercial tests for hepatitis E genotype 3 virus antibody detection. J Med Virol. 2015;87(11):1934–9. [ Links ]











 texto em
texto em