Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.31 no.3 Bogotá July/Sept. 2016
Acute-On-Chronic Liver Failure
Verónica Pérez Guerra MD (1), Laura Ramírez Cardona MD (1), Oscar Mauricio Yepes Grajales MD (1), Juan David Vélez Rivera MD (2), Juan Ignacio Marín Zuluaga MD (3)
(1) Gastrohepatology Group of the School of Medicine at the Universidad de Antioquia in Medellín, Colombia.
(2) Internist and clinical hepatologist at IPS Universitaria in Medellín, Colombia.
(3) Internist - clinical hepatologist at the Hospital Pablo Tobón Uribe and member of the Liver Transplant Group and Gastrohepatology Group at the Universidad de Antioquia, Professor at the Universidad Pontificia Bolivariana in Medellín, Colombia.
Received: 29-09-15 Accepted: 25-07-16
Abstract
Acute-on-chronic liver failure (ACLF) includes acute deterioration of liver functions in patients with either chronic de novo liver disease or already diagnosed liver disease. ACLF can be triggered by various hepatic or extrahepatic precipitating factors. Among its clinical manifestations are renal dysfunction, hepatic encephalopathy, and multisystem organ failure. If not treated promptly translate the prognosis can be poor. Several scoring systems have been used to assess liver function and patient prognosis. Although multisystem organ failure contraindicates liver transplantation, it remains the treatment of choice for this patients.
Keywords
Acute-on-chronic liver failure, MARS, hepatorenal syndrome, hepatic encephalopathy, multisystem organ failure, immune paralysis, SOFA scores.
INTRODUCTION
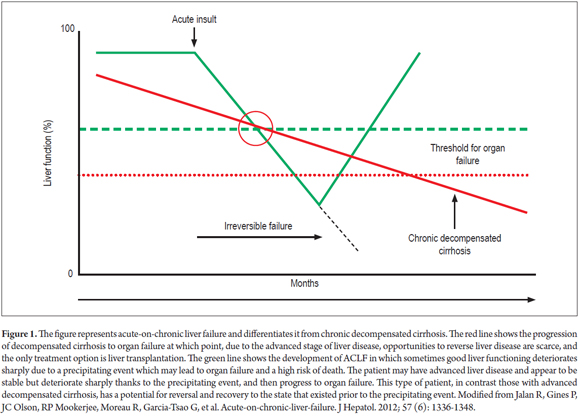
Acute on chronic liver failure (ACLF) is an entity that has increasingly gained recognition but that still generates controversy because of the lack of a consensus definition since it covers acute deterioration of liver function in patients with known chronic liver disease as well as newly diagnosed diseases. (1) The clinical concept of ACLF seeks to differentiate this condition from decompensated liver cirrhosis as shown in Figure 1. The hepatocellular functioning of patients with liver cirrhosis slowly deteriorates to a point at which decompensation occurs and is manifested by complications associated with portal hypertension. At this point, the only treatment option is liver transplantation. In contrast, patients with ACLF have more or less preserved liver functions but experience a precipitating hepatic or extrahepatic event such as an infection. Because of the exaggerated immune response, this quickly develops into multisystem organ failure with a high risk of death. Consequently, treatment and management of ACLF must be different from those appropriate for decompensated cirrhosis.
The Asian Pacific Association for the Study of the Liver (APASL) issued on of the first definitions of ACLF as a condition that develops in the context of a patient who may or may not have been diagnosed with chronic liver disease and who suffers an acute insult manifested by jaundice and coagulopathy and which can become complicated within the first four weeks by ascites and/or encephalopathy. (2) The European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) define this entity as an acute deterioration of a chronic underlying liver disease triggered by a precipitating event that leads to increased 3 month rates and multisystem organ failure. (3)
Recently the European Association for the Study of the Liver Chronic Liver Failure (EASL CLIF) Consortium has defined acute on chronic liver failure based on the CANONIC study. The study analyzed development of organ dysfunction according to the criteria of CLIF-SOFA (defined later in this text) in cirrhotic patients with acute decompensation which was defined as encephalopathy, ascites, gastrointestinal tract bleeding and bacterial infection. (4). This study demonstrated that ACLF patients exist and have prognoses different from those of patients with decompensated cirrhosis.
Due to the absence of a consensus definition, it has been difficult to estimate the incidence and prevalence of ACLF, Nevertheless, the CANONIC study, which had a sample of 1,343 patients hospitalized for acute decompensated cirrhosis in 29 specialized centers in 8 European countries, had made it possible to calculate a prevalence of 31% for ACLF. The study also found that the mortality rate among ACLF patients was 34% in the first 28 days while it was only 1.9% for patients without ACLF. (4)
Other studies have estimated that 40% of patients with advanced cirrhosis over a period of 5 years could develop ACLF. (5) This same study identified that patients who had no prior episodes of acute decompensation had a higher mortality rate than patients who had had prior acute decompensation. (6)
DIAGNOSTIC CRITERIA
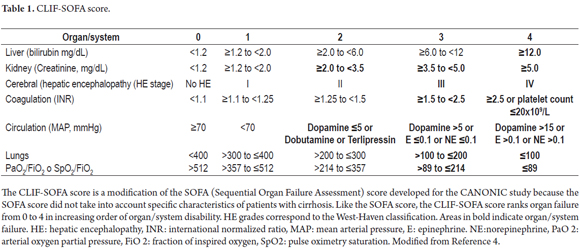
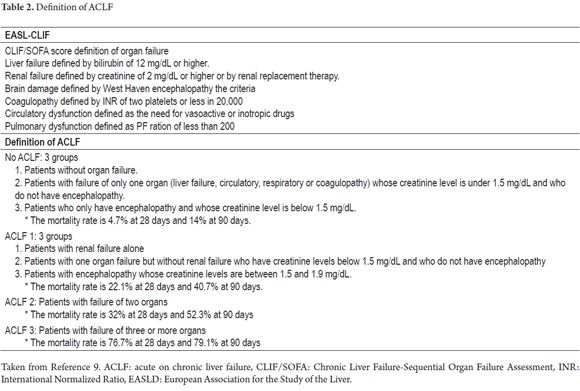
The lack of diagnostic criteria is currently one of the biggest problems with ACLF. In 2013, the CANONIC study set a goal of establishing diagnostic criteria for ACLF based on analysis of patients with organ failure as defined by the CLIF-SOFA score (Table 1) and the at 28-day mortality rate as shown in Table 2. The study shows that most patients with ACLF were young alcoholics who had associated bacterial infections and who had higher white blood cell counts and levels of C-reactive protein (CRP) than those found in patients without ACLF. The CLIF-SOFA score, which was higher in patients with ACLF, and leukocyte counts were independent predictors of mortality in these patients. Accordingly, in addition to organ failure and high mortality rates, diagnosis of ACLF should also consider age at presentation, the precipitator of the event precipitant, and any systemic inflammation which may develop. (4)
PHYSIOPATHOLOGY
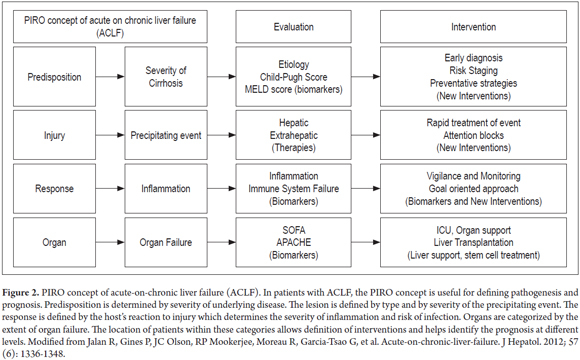
The pathophysiology of ACLF has not yet been fully described, and much remains to be understood. However, what is known is that the immune response to injury, infection and uncontrolled inflammatory responses changes in ways that lead to damage characteristic of ACLF. Something similar to the predisposition, infection, response and organ failure (Piro) sepsis classification system concept can be applied to description of the clinical manifestations and pathophysiology of these patients (Figure 2). (3, 7)
In the PIRO sepsis classification system, predisposition is based on the severity and etiology of the underlying chronic liver disease. Any disease that leads to chronic liver damage is classified as an underlying cause. These include cholestasis, metabolic disease and steatohepatitis not classified as steatosis. (2) In these cases the severity of liver disease is classified according to Child-Pugh and MELD (Model for End Stage Liver Disease) scores.
The appearance of ACLF in a patient who had compensated cirrhosis is the result of a precipitating factor which corresponds to infection/inflammation in the PIRO system. Infection and inflammation can directly increase liver damage, as in the cases of alcoholic or drug-induced hepatitis, viral hepatitis, ischemic hepatitis and thrombosis of the portal vein. It can also be extrahepatic, highlighting the trauma, surgery, variceal bleeding and infections (8). It is important to note that, despite the variety of these precipitating events, in a substantial proportion of patients (up to 40%) no cause can be found that explains the appearance of ACLF. (3)
Some authors have proposed, a classification of ACLF into Type I and Type II based on precipitating factors. In Type I, the precipitant is a extrahepatic event, most commonly infections. In Type II, the precipitating event is intrahepatic such as alcoholic hepatitis, a flare-up of autoimmune hepatitis and reactivation of chronic hepatitis B. (6)
The PIRO system also takes into account immune response. In recent years there has been a breakthrough in the understanding of the pathophysiology of the transition from stabilized cirrhosis to the appearance of ACLF. Systemic inflammatory response syndrome (SIRS) in which cytokines play an important role in inflammation is considered to be the starting point in this transition. (9) This syndrome results in liver damage and is characterized by inflammation, neutrophil dysfunction, necrosis, apoptosis of liver cells, cholestasis and eventually fibrosis. (2, 8)
Nitric oxide (NO) production induced by inflammation and oxidative stress seems to explain the appearance of circulatory and renal disorders in ACLF. (10) In addition to ACLF's relation to proinflammatory, it has been linked to the onset of encephalopathy through the modulating effect it can have on concentrations of ammonium. (2)
Immune system dysfunction in ACLF resulting from the inflammatory response of patients makes them more prone to infections which in turn induce inflammation, resulting in a vicious circle. (11) Infections have been associated with increased morbidity and mortality of patients with ACLF. The mortality rate of patients with cirrhosis who develop severe bacterial infections accompanied by septic shock is somewhere between 60% and 100%. (12-14) In cirrhotic patients, sepsis is an important precipitating factor for significant comorbidities including hepatic encephalopathy and bleeding varices which have significant impacts on mortality. (3)
Various authors have shown that immune deficiencies in ACLF are comparable to those of patients with sepsis. The clinical pictures are very similar: shock is progressively evidenced by vasodilation and multiple organ failure. (15) The most important immune deficiency is functional failure of neutrophils which decreases phagocytic capacity and causes oxidative stress which has been linked to increased risk of infection and the subsequent development of organ failure and death. (16) Other cells of innate immunity, such as monocytes and natural killer (NK) cells are also affected. Cells of acquired immunity area also affected as they fail to proliferate and apoptosis increases. (3)
Role of Cytokines
High levels of TNF-α, sTNF-αR1, sTNF-αR2, IL-2, IL-2R, IL-4, IL-6, IL-8, IL-10 and IFN-γ have been described in ACLF. (2, 17) These high levels may result from endotoxemia which stimulates production or release of necrotic hepatocytes and which are not removed due to liver failure. Although TNF-α induces apoptosis of hepatocytes, it has been found that this cytokine, along with IL-6, can promote regeneration and proliferation of hepatocytes through the production of acute phase reactants which have the effect of countering apoptosis. (18) Because SIRS, the transition from compensated cirrhosis to liver failure, is mediated by these cytokines, inhibition of inflammatory responses that these cytokines induce has been presented as an alternative for reducing morbidity and mortality in patients with ACLF. (2)
Multiple organ dysfunction in the PIRO system, regardless of the cause of the underlying liver disease, is what determines the prognoses of patients with ACLF. As inflammatory disorders progress they ultimately lead to alteration of macrocirculation, microcirculation, endothelial dysfunction and organ failure. The multiple hit and critical mass hypothesis proposes that multiple injuries are what lead to organ damage. Studies have shown that patients who had been hospitalized in the six months prior to an episode have higher mortality than patients who had not been hospitalized with mortality rates of 78% versus 34%. (10)
CLINICAL MANIFESTATIONS
Liver Dysfunction
Coagulopathy and hyperbilirubinemia, which manifests as jaundice, are fundamental criteria for diagnosis of acute on chronic liver failure. The problem is that tests are difficult to interpret in patients with cirrhosis since they may be affected by the underlying liver disease which makes diagnosis and determination of prognosis based on the scores difficult. (19)
Renal Dysfunction
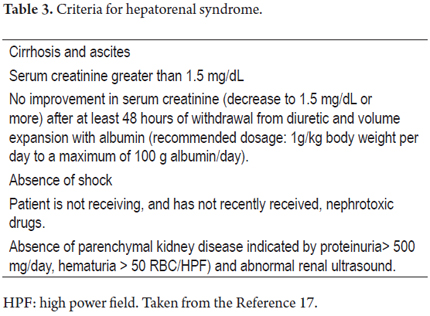
Following the liver, the organ that fails most frequently is the kidney. Kidney damage can be classified into four types: pre-renal, parenchymal kidney disease, renal damage secondary to medication, and hepatorenal syndrome. The prerenal component is the cause of 68% of cases, followed by parenchymal kidney disease which causes 32% of cases. (20) Renal failure associated with liver cirrhosis is defined as creatinine levels over 1.5 mg/dL. International Ascites Club criteria guide diagnosis of hepatorenal syndrome and differentiate it from other causes of renal failure (Table 3). (21)
There are two types of hepatorenal syndrome. Type I evolves more rapidly and is characterized by baseline serum creatinine increases of more than 100% to values over 2.5 mg/dL in less than two weeks. Its appearance is usually related to a precipitating factor, the most common of which is spontaneous bacterial peritonitis (SBP). (22) It is associated with a rapid deterioration of liver function and encephalopathy, and its prognosis is poor. Type II is more stable and evolves more slowly. It is characterized by creatinine levels over 1.5 mg/dL. It occurs spontaneously in most cases and has a better prognosis, but it does indicate progression of liver disease. (1)
Portal hypertension in cirrhotic patients produces splanchnic vasodilation which leads to low blood pressure. This in turn activates regulatory mechanisms such as antidiuretic hormone (ADH) from the renin-angiotensin system and the sympathetic nervous system leading to the occurrence of ascites. (23) Finally, hepatorenal syndrome develops as the result of vasoconstriction of the renal vessels. (1)
The development of renal failure depends largely on rapid and effective diagnosis. Monitoring of blood pressure and urine output should be continuous when patients admitted with complications of cirrhosis are admitted to emergency services. A complete physical examination and laboratory tests including serum creatinine are vital. (1) When there are signs of hemodynamic instability and renal failure, it is necessary to control urine output and central venous pressure through placement of a urinary catheter and a central catheter. Since ascites, edema and hyponatremia are results of the expansion of extracellular volume in hepatic impairment, fluid intake and sodium levels must be reduced. (1)
Given the marked hypotension in these cases, the first treatment option has been considered terlipressin and albumin which has managed to reverse this syndrome in 40% to 70% of patients thus improving prognoses in these cases. The treatment scheme should be adjusted according to the patient's progress.
- Initially, low doses of intravenous terlipressin (0.5-1 mg every 4 hours) should be administered. Dosage should remain low during the first 48 hours. Simultaneously, 1 g/kg of albumin should be slowly administered over 24 hours.
- According to the patient's blood volume, doses of albumin between 20 and 40 g/day should then be administered. If there is fluid overload (central venous pressure greater than 15 mm Hg), albumin should be discontinued.
- Serum creatinine levels must be constantly monitored to determine response to treatment. If the decline in creatinine in the first 72 hours is less than the expected decrease of 25% or more compared to baseline, the dose of terlipressin should be increased to the maximum allowable of 2 mg every 4 hours.
It should be noted that the treatment has no definite time limit for discontinuation. The range reported in various studies is s from seven to 14 days or the treatment is discontinued when creatinine levels fall below 1.5 mg/day. Terlipressin is contraindicated in patients with heart conditions. It is important to monitor vital signs and constantly look for signs of peripheral ischemia. (24)
Other vasoconstrictors including midodrine have been tested for treatment of hepatorenal syndrome, but they not are yet recommended for therapeutic management because there has been insufficient study of their usefulness for clinical treatment of disease. (25) Another option is norepinephrine. Studies have shown that its effectiveness is equivalent to terlipressin for management of both Type 1 and Type 2 hepatorenal syndrome, but that it is less expensive.
The aim of reversing hepatorenal syndrome is to enable the patient to undergo transplantation which is the final therapeutic approach in these cases. If patients undergo transplantation without prior resolution of hepatorenal syndrome, their prognoses are poorer. (26)
Hepatic Encephalopathy
Acute or chronic liver disease can lead to different neurological manifestations which together are called hepatic encephalopathy. These manifestations are mediated by two events: the portosystemic shunting and liver failure. (27) The most common pathophysiological process is based on passage of unaltered nitrogenous substances such as ammonia from the liver through the systemic. There may also be alterations in systems that inhibit neurotransmission (GABAergic, glutamatergic, and/or serotoninergic agents), changes in blood flow, and changes in brain permeability to different products which cause changes in the form and function of astrocytes and affect cerebral ammonium metabolism. (29)
The classification of HE is based on the West Haven criteria which takes into account the neurological manifestations of patients (Table 4). (30)
The diagnosis is eminently clinical and is based on exclusion of other entities that affect functioning of the central nervous system (CNS) such as head injuries, poisoning, drugs overdoses, epilepsy, strokes, CNS infections, uremia, hypercapnia, and psychiatric disorders. (31) After establishing the diagnosis, it is necessary to determine the precipitating factors such as gastrointestinal bleeding, CNS inhibitory drugs, constipation, SBP, impaired renal function and electrolyte balance in order to begin treatment rapidly and in the best way.
These factors can be found in up to 80% of patients with HE. (1, 32)
Therapeutic management of HE depends on its grade. If a patient has HE Grade II to IV, protein intake should not be restricted as this may complicate the metabolism of ammonium ion to muscles. At all levels of encephalopathy adequate nutrition must be ensured. (32, 33)
Given that the consciousness of these patients is impaired, and hence their eating habits are poor, nasogastric tubes are usually used for both feeding and administration of medicines. Intubation of HE patients is only indicated for those who are in a coma (HE grade IV) in order to prevent aspiration. (1) Identification of the precipitating factor is key because may allow reversal and avoid more complex states of altered consciousness. When the precipitating factor is not known with certainty, general measures for suspected clinical events are taken. For example, if an infection is suspected, antibiotics should be started empirically. Similarly, when there are electrolyte imbalances or impaired renal function, administration of diuretics should be discontinued. (32)
Nonabsorbable disaccharides (lactulose) should be administered to lower levels of ammonia, one of the most toxic molecules that triggers HE. These disaccharides cut fatty acid chains with the help of the colonic bacterial flora, thus achieving an acidification of the medium and increasing hydrogen which favors the conversion of ammonium into the nonabsorbable ammonia molecule. Administration may be oral or rectal (enemas). (34, 35)
The goal of treatment is for the patient to have two to three soft stools daily. The oral dose is 20 mL lactitol every 8 hours. If administration is rectal (rectal probe balloon), the dose should be 300 mL of lactulose in 700 mL of water, or 200g of lactitol diluted in one 1 liter of water. If the number of stools continues to increase, administration should be discontinued or the dosage should be decreased. (1, 32). Another option for reducing ammonium levels is to inhibit proteolytic flora of the colon with drugs such as neomycin, paromomycin, metronidazole, rifaximin and amoxicillin clavulanate. (36) Nevertheless, rifaximin is the only drug that has been shown to control acute encephalopathy and chronic symptoms. Rifaximin is administered in doses of 400 mg every 8 hours either orally or through a nasogastric tube. (37)
Drugs such as L-ornithine, L-aspartate, sodium benzoate and zinc that affect the urea cycle have also been tried. Recent treatment guidelines for encephalopathy have shown their effectiveness for controlling acute encephalopathy, but there is little evidence about the long-term use of these drugs. (32)
Cardiac Dysfunction
While patients with decompensated cirrhosis maintain high cardiac output, patients with ACLF do not: cardiac output falls, and there may be systolic and/or diastolic heart failure. This presentation is associated with increased mortality, especially when there is also renal dysfunction. (3)
Multiple Organ Failure
As defined by the EASL and the AASLD, organ failure plays a central role in ACLF, and the hypothesis that organ failure behaves differently in patients with ACLF than in patients with decompensated chronic liver cirrhosis arises. The development of multiple organ failure is characterized by significant alterations in systemic and hepatic hemodynamics and worsening liver function. Bacterial translocation plays an important role in the transition from compensated cirrhosis to decompensated cirrhosis, and together with the bacterial infection is the most common precipitant of deterioration through the systemic inflammatory response. (38)
Currently, prognostic factors that determine the outcome of patients with cirrhosis and multi-organ failure are under review, (39) but it seems that Child-Pugh and MELD scoring systems are less effective and accurate than the SOFA system. (40)
It has been shown that early intervention can prevent the occurrence of multisystem organ failure if the precipitating factors are attacked aggressively.
MANAGEMENT AND TREATMENT
Aside from the aforementioned support measures for the management of major complications, there is no specific treatment to improve the probability that these patients will survive. Artificial liver support systems appear to be an attractive strategy for management of ACLF. (41) They have the basic action mechanism of performing dialysis with albumin because the primary toxic substances that accumulate in the presence of hepatocellular insufficiency are not water-soluble but remain bound to proteins. Among these substances are bile acids, bilirubin, prostacyclin, nitric oxide, fatty acids, tryptophan, ammonium lactate and proinflammatory cytokines. (42)
In addition to reducing levels of proinflammatory cytokines TNF-α, IL-10, and IL-6 that can perpetuate liver damage and extend inflammatory cascade to other organs, the aim of this process is to detoxify the body of components of liver and kidney metabolism. (43). It has been shown that these toxic components trigger cell damage mediated by apoptosis and necrosis which leads to the release of more toxins which ultimately affect the renal, circulatory and central nervous systems with consequent multisystem organ failure. (2) Artificial liver support systems seek to reduce the time for patient recovery and thus serve as a bridge to liver transplantation which is the only effective treatment to date. (44)
The most common system is the Molecular Adsorbent Recirculation System (MARS) which uses exogenous albumin at 10% to 20% and a perforated polysulfone membrane with a 2.2 m2 absorption surface and pores that do not allow passage of substances with molecular weights larger than 50 kD. The technique involves conventional low-flow dialysis in which bicarbonate is incorporated to maintain fluid and electrolyte balance. (45-47)
Some studies have demonstrated the ability of MARS to decrease amounts of vasoactive substances such as angiotensin, aldosterone, renin, norepinephrine and vasopressin which results in improvement of hemodynamic patterns. Studies have also found improvement in portal pressure and increased liver, renal and cerebral blood flow. (48, 49) However, other studies indicate no significant improvement of clinical parameters and transplant-free survival compared with standard medical management. (50, 51) It is argued that improvements in hepatorenal syndrome and hepatic encephalopathy are not enough to make a significant difference in survival compared to standard medical management's achievements. Nevertheless, more study is needed to determine some characteristics of the use of MARS such as frequency of treatment. (41) The most widely accepted use is for earliest response to the complication of hepatic encephalopathy. (52)
Use of MARS is contraindicated in patients with severe coagulopathy because there is a slight decrease in platelets during the procedure and because anticoagulation is required during the dialysis process. 53) The most important adverse effect has been bleeding, but frequent infections have also been reported. To reduce the risk of infection, concomitant use of antibiotics is recommended. Other side effects include disseminated intravascular coagulation, anaphylaxis, fever, sepsis, hypotension and renal insufficiency. (42)
Artificial liver support systems such as MARS are only bridges to liver transplantation. At present there is little data regarding outcomes of patients with ACLF, so it is difficult to determine exactly at what point in the process is best for patients to undergo transplantation. This is especially true given the multiple complications that patients in this state can have. Potential complications include hemodynamic instability, need for high doses of inotropic agents, high intracranial pressure, and low cerebral perfusion pressure. Bin-Wei and colleagues have described 100 patients who underwent transplantation because of ACLF. Transplant patients had high MELD scores (32 on average), high levels of bilirubin, and high INRs, and SOFA scores of nine or higher. The one-year survival rate of these patients was 76.8%, their three-year survival rate was 75.6%, and their five-year survival rate was 74.1%. Overall mortality was 20%. The same study found no statistically significant difference in mortality between the group of patients whose donor organs came from living donors and the group of patients whose donor organ came from a deceased donor.
Another study of 238 patients by Finkenstedt and colleagues evaluated acute-on-chronic liver failure in 94 patients who were evaluated for liver transplantation. Seventy-one patients were placed on the transplant list, but only 33 patients were able to undergo transplantation. The mortality rate was 54%. The one-year survival rate was 84%, and the five-year survival rate was 82%, (55). Bahirwani and colleagues described a retrospective cohort of 332 patients who underwent liver transplantations because of ACLF. Their objective was to assess whether acute-on-chronic liver failure was associated with worse than usual outcomes after transplantation. There were no statistically significant differences in mortality, renal dysfunction, recurrence of cirrhosis or retransplantation between these patients and patients who underwent transplantation for other reasons which indicates that there are no differences in prognoses with patients with decompensated cirrhosis. (56)
These studies conclude that liver transplantation is the option for improving survival of ACLF patients; it is a safe option with high one-year and five-year survival rates a year.
To avoid futile procedures, what remains to be determined is the optimal moment for patients to undergo transplantation and which patients are most likely to benefit.
CONCLUSION
ACFL is a syndrome affecting a group of patients with chronic liver failure. They develop multiple organ dysfunction that impacts morbidity and mortality. ACFL is under investigation, but many questions remain, especially regarding definitions and its pathophysiology. Nevertheless, it clearly has prognoses that differ from those of acute liver failure and patients with decompensated cirrhosis. This, added to the heterogeneity of current definitions, makes ACFL an entity under construction and in which there are many areas of uncertainty. Currently, treatment aims at mitigating organ dysfunction. Liver transplantation is the option that confers improved survival in the short and long terms. The future is focused on defining the moment patients should undergo transplantation time, determination of which patients are candidates for this measure, and on understanding ACLF's pathophysiology to allow improved diagnosis and management strategies.
REFERENCES
1. Arvaniti V, DAmico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139(4):1246-56, 56.e1-5. [ Links ]
2. Rolando N, Wade J, Davalos M, Wendon J, Philpott-Howard J, Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32(4 Pt 1):734-9. [ Links ]
3. Mookerjee RP, Stadlbauer V, Lidder S, Wright GA, Hodges SJ, Davies NA, et al. Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome. Hepatology. 2007;46(3):831-40. [ Links ]
4. Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V. CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-Chronic Liver Failure Is a Distinct Syndrome That Develops in Patients With Acute Decompensation of Cirrhosis. Gastroenterology. 2013;144(7):1426-1437. [ Links ]
5. Ambrosino G, Naso A, Feltracco P, Carraro P, Basso SM, Varotto S, et al. Cytokines and liver failure: modification of TNF-and IL-6 in patients with acute on chronic liver decompensation treated with Molecular Adsorbent Recycling System (MARS). Acta Biomed. 2003;74(Suppl 2):7-9. [ Links ]
6. Olson JC, Wendon JA, Kramer DJ, Arroyo V, Jalan R, García-Tsao G, et al. Intensive care of the patient with cirrhosis. Hepatology. 2011;54(5):1864-1872. [ Links ]
7. García-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48(6):2064-2077. [ Links ]
8. Licata A, Maida M, Bonaccorso A, Macaluso FS, Cappello M, Craxi A, et al. Clinical course and prognostic factors of hepatorenal syndrome: A retrospective single-center cohort study. World J Hepatol. 2013;5(12):685-91. [ Links ]
9. Martin-Llahi M, Guevara M, Torre A, Fagundes C, Restuccia T, Gilabert R, et al. Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology. 2011;140(2):488-96.e4. [ Links ]
10. Mookerjee RP. Acute-on-chronic liver failure: the liver and portal haemodynamics. Curr Opin Crit Care. 2011;17(2):170-6. [ Links ]
11. Forcellini S, Fabbian F. The role of terlipressin in hepatorenal syndrome. G Ital Nefrol. 2010;27(5):469-76. [ Links ]
12. Restuccia T, Ortega R, Guevara M, Gines P, Alessandria C, Ozdogan O, et al. Effects of treatment of hepatorenal syndrome before transplantation on posttransplantation outcome. A case-control study. J Hepatol. 2004;40(1):140-6. [ Links ]
13. Salerno F, Gerbes A, Gines P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Postgrad Med J. 2008;84(998):662-70. [ Links ]
14. Prakash R, Mullen KD. Mechanisms, diagnosis and management of hepatic encephalopathy. Nat Rev Gastroenterol Hepatol. 2010;7(9):515-25. [ Links ]
15. Graziadei IW. The clinical challenges of acute on chronic liver failure. Liver Int. 2011;31 (Suppl 3):24-6. [ Links ]
16. Márquez-Aguirre AL, Canales-Aguirre AA, Gómez-Pinedo U, Galvez-Gastelum FJ. Molecular aspects of hepatic encephalopathy. Neurologia. 2010;25(4):239-47. [ Links ]
17. Gundling F, Zelihic E, Seidl H, Haller B, Umgelter A, Schepp W, et al. How to diagnose hepatic encephalopathy in the emergency department. Ann Hepatol. 2013;12(1):108-14. [ Links ]
18. Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35(3):716-21. [ Links ]
19. Atluri DK, Prakash R, Mullen KD. Pathogenesis, Diagnosis, and Treatment of Hepatic Encephalopathy. Journal of Clinical and Experimental Hepatology. 2011;1(2):77-86. [ Links ]
20. Córdoba J, López-Hellin J, Planas M, Sabin P, Sanpedro F, Castro F, et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41(1):38-43. [ Links ]
21. Chacko KR, Sigal SH. Update on management of patients with overt hepatic encephalopathy. Hosp Pract (1995). 2013;41(3):48-59. [ Links ]
22. Sharma P, Sharma BC. Disaccharides in the treatment of hepatic encephalopathy. Metab Brain Dis. 2013;28(2):313-20. [ Links ]
23. Mullen K, Prakash R. Rifaximin for the treatment of hepatic encephalopathy. Expert Rev Gastroenterol Hepatol. 2010;4(6):665-77. [ Links ]
24. Mas A, Rodes J, Sunyer L, Rodrigo L, Planas R, Vargas V, et al. Comparison of rifaximin and lactitol in the treatment of acute hepatic encephalopathy: results of a randomized, double-blind, double-dummy, controlled clinical trial. J Hepatol. 2003;38(1):51-8. [ Links ]
25. Olson JC, Kamath PS. Acute-on-chronic liver failure: concept, natural history, and prognosis. Curr Opin Crit Care. 2011;17(2):165-9. [ Links ]
26. Wlodzimirow KA, Eslami S, Abu-Hanna A, Nieuwoudt M, Chamuleau RA. A systematic review on prognostic indicators of acute on chronic liver failure and their predictive value for mortality. Liver Int. 2013;33(1):40-52. [ Links ]
27. Theocharidou E, Pieri G, Mohammad AO, Cheung M, Cholongitas E, Banwari A, et al. The Royal Free Hospital Score: A Calibrated Prognostic Model for Patients With Cirrhosis Admitted to Intensive Care Unit. Comparison With Current Models and CLIF-SOFA Score. Am J Gastroenterol. 2014;109(4):554-62. [ Links ]
28. Banares R, Nevens F, Larsen FS, Jalan R, Albillos A, Dollinger M, et al. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology. 2013;57(3):1153-62. [ Links ]
29. Kjaergard LL, Liu J, Als-Nielsen B, Gluud C. Artificial and bioartificial support systems for acute and acute-on-chronic liver failure: a systematic review. Jama. 2003;289(2):217-22. [ Links ]
30. Ichai P, Samuel D. Extracorporeal liver support with MARS in liver failure: has it a role in the treatment of severe alcoholic hepatitis? J Hepatol. England. 2003;38(1)104-6. [ Links ]
31. Koivusalo AM, Kantola T, Arola J, Hockerstedt K, Kairaluoma P, Isoniemi H. Is it possible to gain extra waiting time to liver transplantation in acute liver failure patients using albumin dialysis? Ther Apher Dial. 2009;13(5):413-8. [ Links ]
32. Cisneros Garza L. [Albumin dialysis using MARS. Principles and techniques. Initial experience in Mexico]. Gastroenterol Hepatol. 2005;28(2):85-94. [ Links ]
33. Pocze B, Fazakas J, Zadori G, Gorog D, Kobori L, Dabasi E, et al. MARS therapy, the bridging to liver retransplantation - Three cases from the Hungarian liver transplant program. Interv Med Appl Sci. 2013;5(2):70-5. [ Links ]
34. Kobashi-Margain RA, Gavilanes-Espinar JG, Gutierrez-Grobe Y, Gutiérrez-Jiménez AA, Chávez-Tapia N, Ponciano-Rodriguez G, et al. Albumin dialysis with molecular adsorbent recirculating system (MARS) for the treatment of hepatic encephalopathy in liver failure. Ann Hepatol. 2011;10(Suppl 2):S70-6. [ Links ]
35. Mitzner SR. Extracorporeal liver support-albumin dialysis with the Molecular Adsorbent Recirculating System (MARS). Ann Hepatol. 2011;10(Suppl 1):S21-8. [ Links ]
36. Laleman W, Wilmer A, Evenepoel P, Elst IV, Zeegers M, Zaman Z, et al. Effect of the molecular adsorbent recirculating system and Prometheus devices on systemic haemodynamics and vasoactive agents in patients with acute-on-chronic alcoholic liver failure. Crit Care. 2006;10(4):R108. [ Links ]
37. Atienza Merino G. Evaluation of extracorporeal liver support systems in the treatment of liver failure. A systematic review. Gastroenterol Hepatol. 2010;33(5):352-62. [ Links ]
38. Faenza S, Baraldi O, Bernardi M, Bolondi L, Coli L, Cucchetti A, et al. Mars and Prometheus: our clinical experience in acute chronic liver failure. Transplant Proc. 2008;40(4):1169-71. [ Links ]
39. Hassanein TI, Tofteng F, Brown RS, Jr., McGuire B, Lynch P, Mehta R, et al. Randomized controlled study of extracorporeal albumin dialysis for hepatic encephalopathy in advanced cirrhosis. Hepatology. 2007;46(6):1853-62. [ Links ]
40. Tan HK. Molecular adsorbent recirculating system (MARS). Ann Acad Med Singapore. 2004;33(3):329-35. [ Links ]
41. Bin-wei D, Chun Lu S, Wang M, Ning Liu J, Chi P, Lai W, et all. Liver transplantation in acute-on-chronic liver failure patients with high model for end-stage liver disease (MELD) scores: a single center experience of 100 consecutive cases. Journal of surgical research. 2013; 183(2):936-946. [ Links ]
42. Finkenstetedt A, Nachbaur K, Zoller H, Johannidis M, Pratschke J, Graciadei IW, et all. Acute-on-chronic liver failure: excellent outcomes after liver transplantation but high mortality on the wait list. Liver trasplantation. 2013;19(8):879-886. [ Links ]
43. Bahirwani R, Shaked O, Bewtra M, Forde K and Reddy R. Acute-on-Chronic Liver Failure Before Liver Transplantation: Impact on Posttransplant Outcomes. Trasplantation. 2011; 92(8):952-957. [ Links ]











 text in
text in