Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.31 no.3 Bogotá jul./set. 2016
MRI Staging of Colorectal Cancer
Felipe Aluja Jaramillo MD (1), Daniel Upegui Jiménez MD (2)
(1) Resident in radiology and diagnostic imaging at Fundación Universitaria Sanitas in Bogotá, Colombia.
(2) Medical radiologist specializing in the abdomen and pelvis at Clínica Universitaria Colombia and Hospital Universitario Mayor - Méderi. Professor of Radiology at Fundación Universitaria Sanitas and Universidad del Rosario in Bogotá, Colombia. Mail: d_upegui@hotmail.com
Received: 17-02-16 Accepted: 25-07-16
Abstract
Colorectal cancer is the fourth leading cause of death worldwide and the fifth leading cause of cancer death in Colombia. MRIs are ideal for evaluating colorectal cancer, especially for screening, because they allow staging by determining the extent of any invasion beyond the muscularis and into adjacent organs. This is key for identifying candidates for chemotherapy and/or preoperative radiation therapy and for planning surgical procedures. The key is differentiation between stages T2 and T3 by using T2 sequences with high resolution information. In addition, MRIs allow assessment of the size and morphology of the lymph nodes and greatly increases the specificity of detection of nodal involvement. Magnetic resonance imaging has high specificity and reproducibility.
Keywords
Magnetic resonance imaging, neoplasms of the colon, rectal neoplasms, staging of neoplasms.
INTRODUCTION
Colorectal cancer is the fourth leading cause of cancer‑related deaths worldwide. (1,2) The frequency of colorectal cancer has been increasing in Colombia. Currently, colorectal cancer is the fourth most prevalent and fifth leading cause of death in Colombia among different kinds of neoplasia. (2-4)
Magnetic resonance imaging, first used for evaluation of rectal cancer in 1986, is now considered the ideal method for evaluating the pelvises of patients with this disease. (5-7, 8-12) This imaging technique relies on a display in multiple planes combined with high-contrast images of soft tissues which allow local staging by identifying invasion beyond the muscularis propria. (13, 14) Also, MRIs can be used to classify patients for treatment with either pre‑surgical chemotherapy or radiation therapy, and can help planning of surgical procedures. (15,16) It is a reliable technique, with good reproducibility and specificity as high as 92%. It is especially useful for predicting negative resection margins by determining the relation of the tumor with the resection margin or the compromised region beyond the muscularis propria. (13, 15,16) The presence of a tumor or nodule 1mm from the resection margin increases the risk of recurrence. (15)
The advantage of MRIs lies in the use of sequences that allow adequate contrast between the tumor and the surrounding soft tissue which is not possible with other imaging techniques such as computed axial tomography (CAT) scans. (8, 17) It has been shown that the distance from the tumor to the resection margin is the most important predictor of local recurrence. (13, 18 to 21)
Magnetic resonance imaging is also the method of choice for patients receiving radiation therapy. (22)
High-resolution sequences with T2 information are the keys to evaluation of rectal cancer. (8, 15) These sequences consist of fine axial images that are less than 3 mm long with a resolution of 0.5 to 0.8 mm and which are orthogonal to the plane of the tumor. (15) Such sequences make it possible to differentiate between a stage T2 tumor (confined to the rectal wall) and a T3 tumor (with extension beyond the muscularis propria). (10, 15)
The assessment of compromise of, and extension to, lymph nodes remains a controversial determining factor. (15) MRI allows not only the assessment of the size of lymph nodes, but also the identification of the nodal morphology which considerably increases the specificity of detecting nodal compromise. (15)
The sensitivity and specificity of MRI for the detection and staging of rectal cancer depends on the experience of the radiologist. (8, 10, 15, 23, 24) Rafaelsen et al. demonstrate that, for tumor staging, an experienced radiologist's sensitivity is 96% and her or his specificity is 74% while for a general radiologist sensitivity is only 77%, and specificity is only 40%. (25)
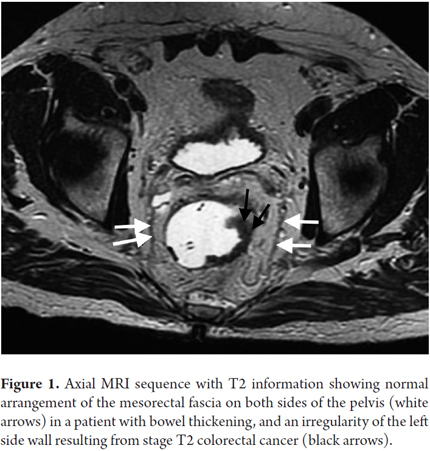
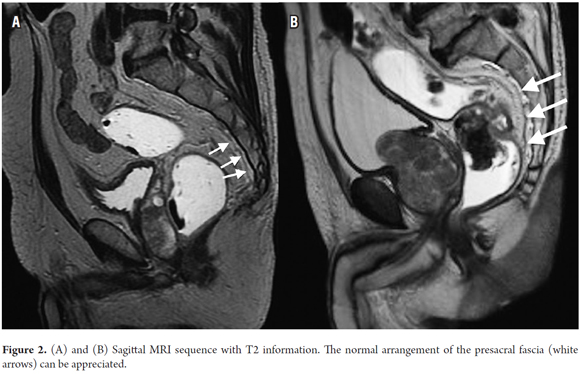
The elements that should be considered in an MRI for primary rectal cancer are the stage, the depth of invasion beyond the muscularis propria, and the relationships of the tumor to the mesorectal fascia (Figure 1), the presacral fascia (Figure 2), the anal sphincter, and the pelvic walls. (15)
STRUCTURES TO EVALUATE WITH MAGNETIC RESONANCE IMAGING
Tumor Stage (T)
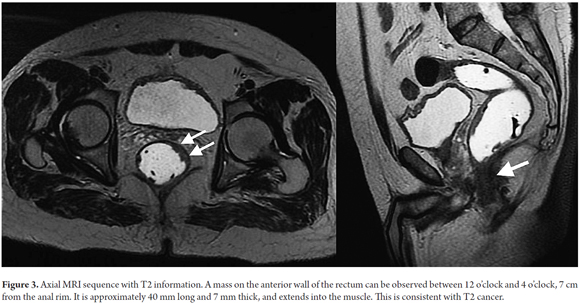
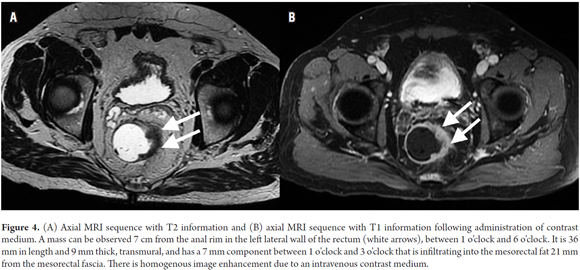
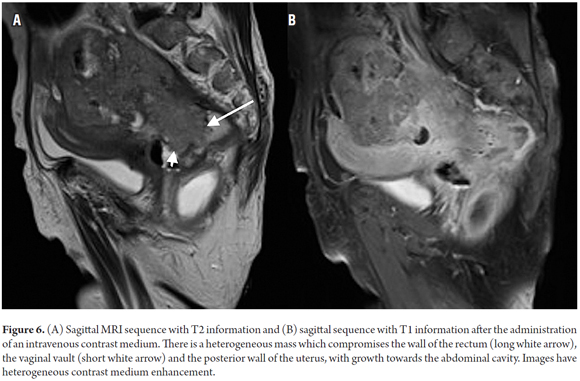
Tumor staging is directly dependent on the relation of the tumor with the muscularis propria and the invasion of adjacent organs. (8) A T1 tumor is one that is confined to the mucosa. A T2 tumor extends to the muscularis propria (Figure 3). A T3 tumor invades and extends beyond the muscularis propria (an early T3 tumor spans less than 5 mm, whereas an advanced T3 tumor spans 5 mm or more) (Figures 4 and 5). A T4 tumor is one that invades the pelvic organs (Figure 6). (15) The pelvic vessels, the pelvic parietal fascia, and the mesorectal fascia are not considered organs. (8)
Definitions of TNM staging for rectal cancer were taken from the Radiological Society of North America (standardized MR reporting criteria incorporated into the Radiological Society of North America's radiology reporting template for primary rectal cancer). (26) Physicians and radiologists should take into account the type of TNM classification being used to simplify interpretation and unify terms in imaging reports. (8)
80% of rectal tumors are in stage T3, a heterogeneous group of lesions that have variable survival rates depending on the extent of invasion beyond the muscularis propria. (15) Stage T3 have invasions of less than 5 mm and similar survival rates to stage T2 tumors. For this reason, both can be grouped together for prognostic and therapeutic purposes. (15)
Sixty to 100 mL of rectal contrast medium, composed of ultrasound gel at body temperature, improves visualization of polypoid growths or tumors of less than 3 cm across. (15, 27) Nevertheless, the use of intra-rectal contrast is controversial, and there is still no consensus on the use of intravenous contrast medium. (16, 27, 28)
To avoid errors due to partial volume, images should be obtained in a plane perpendicular to the muscle layer, (8) since this layer is a thin hypointense (black) structure which surrounds the rectum. (8) Among the signs that suggest an extension beyond the muscularis propria (T3 Advanced) (Figure 5) are tumor ulceration, tumors that occupy more than half the circumference of the rectum, tumors with great longitudinal compromise, and metastasis to a lymph node or at a distance. (8)
The presence of fat between the tumor and adjacent structures indicates absence of invasion. (8) The most reliable signs of invasion are nodular growth to adjacent organs and ureteral obstruction that generates hydronephrosis. (8, 29)
Differentiating between stage T2 and T3 injuries is not always straightforward, since desmoplastic reactions and fibrosis can generate spiculation of perirectal fat, and since it is not always possible to ascertain whether or not there are tumor components in these lesions. This can lead to overestimation in staging. (14, 18, 23, 30-32) This is particularly important in patients who have been treated with radiation therapy. In these cases, unlike in cases of spiculation, nodular lesions point to recurrence of tumors or to residue rather than to changes due to radiation therapy.
Relation of a Mass to Adjacent Structures
The relationship of the tumor to adjacent structures such as the mesorectal fascia, the peritoneal reflection, the pelvic organs, the anal sphincter, and the pelvic side wall should be taken into account. (8,15)
Mesorectal Fascia
The mesorectal fascia is an anatomical reference point for diagnostic evaluation of tumor extension. (33, 34) This is the most important variable in terms of prognosis, (8) since compromise of the mesorectal fascia increases the risk of local and distant recurrence. (8) The mesorectal fascia is the visceral layer of the pelvic fascia, (15) but it is a distinct anatomical unit insofar as it surrounds the rectum and contains the mesorectal fat, lymph nodes, and vessels. (15) In high-definition images with T2 information, it is displayed as a straight hypointense image lateral to the rectum (Figure 1). (15)
The relationship between a tumor and the mesorectal fascia is essential for surgical planning. (15) Histologically, a distance greater than 1 mm between the tumor and the resection margin implies a lower probability of local recurrence. (15, 35) As the distance decreases, the possibility of compromise increases. (8, 36) For this reason, in sequences with high resolution T2 information, a distance of less than 1 mm between the tumor and the mesorectal fascia indicates compromise. However, the margin used may vary in different medical centers. (8, 15, 37-39) This measurement can be taken from the margin of the tumor at its extension beyond the muscularis propria, from tumor deposits in the mesorectum, from a thrombus tumor within a vascular structure, or from a lymph node that appears to be tumorous. (8, 15)
It should be noted that the administration of a rectal enema at the time of examination may affect the distance between the tumor and the mesorectal fascia as a result of distention of the rectum. However, there are no studies showing that this factor is relevant. On the contrary, the use of rectal enema improves the visualization of lesions. (8, 40)
Peritoneal Reflection
The peritoneal reflection is located between the superior bladder wall and the anterior rectal wall, forming the rectovesical pouch. (15) In axial high-definition images with T2 information it is displayed as a hypointense V‑shaped image. (15) Compromise of this fascia by a tumor implies stage T4a cancer. (15)
Pelvic Organs
The pelvic organs most often compromised by rectal cancer are the uterus, the vagina, the prostate, and the seminal vesicles. (15) The assessment of tumor involvement of these structures, as well as of compromise of the presacral fascia and of the sacral nerves, have implications in terms of surgical planning, especially because compromise of any of either the presacral fascia or the sacral nerves make the tumor unresectable. (15)
Anal Sphincter
The anal sphincter consists of an internal sphincter of smooth muscle, a circular layer of the rectum, and an external sphincter of striated muscle which comprises the elevator muscle of the anus and an extension of the puborectal muscle. (15) The location of the tumor in relation to the anal sphincter should be assessed in coronal images that allow identification of the relation between the upper margin of the puborectal muscle and the tumor to determine whether surgical resection is possible. (15) Describing the relation between the tumor and the anal sphincter is particularly important in cases in which tumors involve the distal rectum.
Lateral Wall of the Pelvis
The structures of the lateral wall of the pelvis are in close contact with the rectum. (15) The tumor may compromise the common arteries and external and internal iliac veins, as well as the ureters, the piriformis and obturator muscles, and the sacral nerve. (15) Compromise of the mesorectal fascia in the upper or lower rectum (sites which cannot be differentiated by their layers) implies compromise of the lateral wall of the pelvis. (15) High‑resolution coronal and sagittal images are recommended for this assessment. (15) Using extended fields of vision leads to underestimation of the proximity of the tumor to the sidewall and underestimation of the degree of compromise of structures of the side wall. (15)
Vascular and Lymphatic Involvement
The assessment of rectal cancer should include not only tumor staging and estimation of compromise of adjacent structures, but also the evaluation of key structures such as lymph nodes and nearby vascular structures.
Vascular Invasion
Invasion of vascular structures is irrelevant to treatment but plays an important role in prognosis and therefore should be analyzed with images. (15) Perivascular growth is not synonymous with intravascular growth. (8)
Identifying invasion in small vascular structures is not simple. (15) The presence of a tumor in the opening of larger vascular structures such as the upper or middle arteries and veins is indicative of tumor invasion. (10, 15) Other findings that indicate invasion include nodular growth at the site of extramural growth, tumor proximity to the veins or arteries of the pelvis, heterogeneity of signal strength inside vessels, and increasing size of the vessels. (8, 12, 41) Smith et al. conclude that when two of these signs are present, the prognosis is similar to that when vascular invasion is evident in histology. (12, 41)
Lymph Nodes (N)
Lymph nodes play a decisive role in the evaluation of rectal cancer and therefore affect prognosis. (8) Groups that should be evaluated are the mesorectal, upper rectal, inferior mesenteric, common iliac, and the internal, external, and surface retroperitoneal and inguinal groups. (15) Compromise of lymph nodes is a prognostic factor for recurrence and distant metastasis. (18, 42) Whether or not a lymph node tumor is benign or malignant should be ascertained. (15) If any lymph nodes are located less than 1 mm from the mesorectal fascia, they should be included in the resection margins to obtain clean margins. (15)
The size of lymph nodes is of limited value for determination of whether metastasis is present. (8, 15) As is well known, there can be microscopic metastasis in normal sized lymph nodes. (5, 43, 44) A diameter of 5 mm implies a sensitivity of 68% and specificity of 78% for differentiation of malignant from benign lymph nodes. (8, 10, 15, 45) Size is not relevant in rectal cancer, since between 30% and 50% of lymph nodes with metastasis are less than 5 mm across. (15, 46, 47) Lymph node morphology, including its margins, contours and internal characteristics (homogeneity), is the key for determination of the existence of metastasis. (8, 10, 48-51) A node with metastatic appearance has spiculated margins and heterogeneous content. (15) Calcifications within a node suggest malignancy. (8) Despite this, the problems of relying on morphology are that it is impossible to differentiate between neoplastic and reactive lymph nodes when they are large, and that it is impossible to identify microscopic metastasis when they are small (Figure 7A). (18) It should be noted that the sensitivity of magnetic resonance imaging reported in the literature for detection of lymph nodes ranges from 39% to 95%. (5-7,22,52-55)
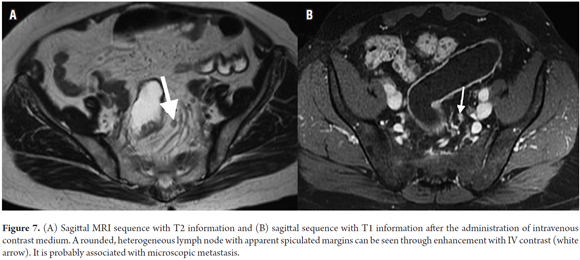
Gadolinium, a contrast agent, has been used for evaluation of lymph nodes with reports of up to 70% accuracy (Figure 7B). (5, 8) Heriot et al. report sensitivity and specificity higher than 80% for detection of nodal compromise using rectal antennas. (22, 56)
THE PRESENT AND FUTURE OF PREOPERATIVE MRI ASSESSMENTS
Diffusion of imaging sequences can improve detection of colorectal tumors. (15, 57) These sequences can also assist identification and location of tumors and metastasis in lymph nodes. (15) Moreover, there are reports of their usefulness for predicting responses to chemotherapy through the use of ADC values as presented by Dzik-Jurasz et al. (8, 15, 58) These authors find that low ADC values predict a favorable response to treatment. (58) Some authors have reported that a rapid increase in ADC values can precede changes in tumor size. (59) However, it should be noted that some authors have not found differences in precision, sensitivity, or specificity for tumor staging between T2 sequences and diffusion imaging sequences. (27, 60) The major limitation of diffusion sequences is the presence of artifacts originating in the intestinal gas that can hinder application the imaging procedure itself. (15)
Diffusion imaging sequences also can be used to evaluate lymph nodes, but their results in rectal cancer have not been fully tested. (8) Hyperintense lymph nodes in diffusion sequences are considered indications of metastasis. (61) Mizukami et al. report a sensitivity of 97% and a specificity of 81%. In their study, they use magnetic resonance imaging together with diffusion imaging sequences to detect metastatic lymph nodes. (61)
MRI Assessment of Recurrence
Tumors recur at a rate of 70% in the first two years, and at a rate of up to 85% by the third year. (62, 63) In cases of recurrence, MRI allows assessment of the extent of disease and compromise of adjacent organs with a sensitivity of 80% to 90% and a specificity of up to 100% (Figure 8). (62, 64-66) Recurrence can be classified according to location and involvement of structures in the axial plane, including recurrence in the anastomosis and local recurrence. Anterior recurrence involves organs in the pelvis anterior to the rectum, such as the bladder, the uterus, the vagina, the prostate, and/or the seminal vesicles. Posterior recurrence involves structures posterior to the rectum, such as the sacrum, the coccyx, or the presacral fascia. Lateral recurrence involves structures lateral to the rectum, such as the pelvic wall, the iliac vessels, and the ureters. (62, 67)
Postsurgical changes, granulation tissue, residual hematomas, inflammatory changes induced by radiation and neoadjuvant radiation therapy, all produce an increase in signal intensity in T2 sequences with that can be indistinguishable from tumor recurrence. (62, 68) This increase in signal strength may persist for up to 2 years after the surgical procedure. (62, 65) Because of this, morphological analysis and image enhancement with contrast media are especially important to differentiate postsurgical changes of residual or recurrent tumors. Residual tumors usually have rounded margins, while fibrosis causes angular or spiculated margins. (62) In many diagnostic centers it is deemed appropriate to begin image monitoring by no more than 6 months after surgery or the last session of radiation therapy. As mentioned above, the use of diffusion imaging sequences is useful for colorectal cancer screening. (62, 64)
CONCLUSIONS
MRI is the method of choice to assess the pelvis in patients with colorectal cancer because it allows the prediction of negative resection margins, the detection of metastasis to the lymph nodes, the compromise to adjacent organs. Also, newer sequences can even serve as a predictors of response to chemotherapy. In addition to these aspects, MRI has a high specificity and high reproducibility, so it should be used as the first-line imaging procedure of rectal cancer, especially when the interpretation of the study is in the hands of an expert radiologist.
Conflict of Interest
The authors declare that they have no conflicts related to his work.
Financing
Funding was assumed entirely by the authors.
REFERENCES
1. Jemal A, Bray F, Center M, Ferlay J, Ward E, Forman D. Global cancer statistics. Ca Cancer J Clin. 2011;61:69-90. [ Links ]
2. Ferreira EJ, Meléndez HJ. Características clínicas, demográficas e histopatológicas de los pacientes con cáncer colorrectal del Hospital Universitario de Santander. Revista Colombiana de Cirugía 2012;27(3), 213-220. [ Links ]
3. Sanabria A, Martínez JI, Díaz JA. Cáncer de colon: análisis del costo de enfermedad para los estadios III y IV en Cansercoop IPS. Rev Colomb Cienc Quim Farm 2009;38(2):193-214. [ Links ]
4. Piñeros M, Hernández G, Bray F. Increasing mortality rates of common malignancies in Colombia. Cancer. 2004;101(10):2285-92. [ Links ]
5. Muthusamy VR, Chang KJ. Optimal methods for staging rectal cancer. Clin Cancer Res 2007;13(22):6877s-6884s. [ Links ]
6. Hodgman CG, MacCarty RL, Wolff BG, May GR, Berquist TH, Sheedy PF 2nd, et al. Preoperative staging of rectal carcinoma by computed tomography and 0.15T magnetic resonance imaging. Preliminary report. Dis Colon Rectum 1986;29(7):446-50. [ Links ]
7. Butch RJ, Stark DD, Wittenberg J, Tepper JE, Saini S, Simeone JF, et al. Staging rectal cancer by MR and CT. Am J Roentgenol 1986;146(6):1155-60. [ Links ]
8. Torkzad MR, Pahlman L, Glimelius B. Magnetic resonance imaging (MRI) in rectal cancer: a comprehensive review. Insights Imaging 2010;1(4):245-267. [ Links ]
9. Martling A, Holm T, Bremmer S, Lindholm J, Cedermark B, Blomqvist L. Prognostic value of preoperative magnetic resonance imaging of the pelvis in rectal cancer. Br J Surg. 2003;90(11):1422–1428. [ Links ]
10. Brown G, Richards CJ, Newcombe RG, Dallimore NS, Radcliffe AG, Carey DP, et al. Rectal carcinoma: thin-section MR imaging for staging in 28 patients. Radiology 1999; 211(1):215–222. [ Links ]
11. LeBlanc. Imaging and management of rectal cancer. Nat Clin Pract Gastroenterol Hepatol 2007;4(12):665-676. [ Links ]
12. Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg 2008;95(2):229–236. [ Links ]
13. Beets-Tan RGH, Beets GL. Rectal cancer: how accurate can imaging predict the T stage and the circumferential resection margin. Int J Colorectal Dis. 2003;18(5):385-391. [ Links ]
14. Beets-Tan RGH. MRI in rectal cancer: the T stage and circumferential resection margin. Colorectal Disease. 2003;5(5):392-395. [ Links ]
15. Kaur H, Choi H, You YN, Rauch GM, Jensen CT, Hou P, et al. MR imaging for preoperative evaluation of primary rectal cancer: practical considerations. Radiographics 2012;32(2):389-409. [ Links ]
16. MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ 2006;333(7572):779. [ Links ]
17. Matsuoka H, Masaki T, Sugiyama M, Nakamura A, Takahara T, Hachiya J, Atomi Y. Gadolinium enhanced endorectal coil and air enema magnetic resonance imaging as a useful tool in the preoperative examination of patients with rectal carcinoma. Hepatogastroenterology. 2004;51(55):131–135. [ Links ]
18. Beets-Tan RGH, Beets GL. Rectal cancer: review with emphasis on MR imaging. Radiology 2004;232(2):335-346. [ Links ]
19. Quirke P, Dixon MF. The prediction of local recurrence in rectal adenocarcinoma by histopathological examination. Int J Colorectal Dis. 1988;3(2):127–131. [ Links ]
20. Wibe A, Rendedal PR, Svensson E, Norstein J, Eide TJ, Myrvold HE, et al. Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer. Br J Surg 2002;89(8):327–34. [ Links ]
21. Nagtegaal ID, Marijnen CA, Kranenbarg EK, van De Velde CJ, van Krieken JH. Circumferential Margin Involvement Is Still an Important Predictor of Local Recurrence in Rectal Carcinoma: Not One Millimeter but Two Millimeters Is the Limit. Am J Surg Pathol. 2002; 26(3):350–7. [ Links ]
22. Kwok H, Bissett IP, Hill GL. Preoperative staging of rectal cancer. Int J Colorectal Dis. 2000;15:9-20. [ Links ]
23. Beets-Tan RG, Beets GL, Vliegen RF, Kessels AG, Van Boven H, De Bruine A, et al. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet. 2001;357(9255):497-504. [ Links ]
24. Blomquist L, Machado M, Rubio C, Gabrielsson N, Granqvist S, Holm T. Rectal tumour staging: MR imaging using pelvic phased-array and endorectal coils vs endoscopic ultrasonography. Eur Radiol. 2000;10(4):653-660. [ Links ]
25. Rafaelsen SR, Sorensen T, Jakobsen A, Bisgaard C, Lindebjerg J. Transrectal ultrasonography and magnetic resonance imaging in the staging of rectal cancer. Scand J Gastroenterol. 2008;43(4):440-446.
26. Al‐Sukhni E, Milot L, Fruitman M, Brown G, Schmocker S and Kennedy E. RSNA Radiology Reporting Templates. MR rectum cancer. 2014. Disponible en: http://www.radreport.org/txt/0000068. [ Links ]
27. Kijima S, Sasaki T, Nagata K, Utano K, Lefor AT, Sugimoto H. Preoperative evaluation of colorectal cancer using CT colonography, MRI and PET/CT. World J Gastroenterol. 2014;20(45):16964-16975. [ Links ]
28. Vliegen RF, Beets GL, von Meyenfeldt MF, Kessels AG, Lemaire EE, van Engelshoven JM, Beets-Tan RG. Rectal cancer: MR imaging in local staging is gadolinium-based contrast material helpful? Radiology. 2005; 234(1):179-188. [ Links ]
29. Suzuki C, Torkzad MR, Tanaka S, Palmer G, Lindholm J, Holm T, Blomqvist L. The importance of rectal cancer MRI protocols on interpretation accuracy. World J Surg Oncol. 2008;20(6):6-89. [ Links ]
30. Vogl TJ, Pegios W, Mack MG, Hünerbein M, Adler A, Lobbeck H, et al. Accuracy of staging rectal tumors with contrast-enhanced transrectal MR imaging. AJR Am J Roentgenol. 1997;168(6):1427–1434. [ Links ]
31. Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg. 2003;90(3):355-364. [ Links ]
32. Meyenberger C, Huch Boni RA, Bertschinger P, Zala GF, Klotz HP, Krestin GP. Endoscopic ultrasound and endorectal magnetic resonance imaging: a prospective comparative study for preoperative staging and follow-up of rectal cancer. Endoscopy. 1995;27(7):469–479. [ Links ]
33. Klessen C, Rogalla P, Taupitz M. Local staging of rectal cancer: the current role of MRI. Eur Radiol. 2007;17(2):379-389. [ Links ]
34. Bisset IP, Chau KY, Hill GL. Extrafascial excision of the rectum: surgical anatomy of the fascia propria. Dis Colon Rectum. 2000;43(7):903–910. [ Links ]
35. Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection: histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;2(8514):996-999. [ Links ]
36. Mathur P, Smith JJ, Ramsey C, Owen M, Thorpe A, Karim S, Burke C, Ramesh S, Dawson PM. Comparison of CT and MRI in the pre-operative staging of rectal adenocarcinoma and prediction of circumferential resection margin involvement by MRI. Colorectal Dis. 2003;5(5):396-401. [ Links ]
37. Valentini V, Aristei C, Glimelius B, Minsky BD, Beets-Tan R, Borras JM, et al. Multidisciplinary rectal cancer management: 2nd European Rectal Cancer Consensus Conference (EURECA-CC2). Radiother Oncol. 2009;92(2):148-163. [ Links ]
38. Taylor A, Slater A, Mapstone N, Taylor S, Halligan S. Staging rectal cancer: MRI compared to MDCT. Abdom Imaging. 2007;32(3):323–327. [ Links ]
39. Kim NK, Min BS, Kim H, Pyo J, Kim MJ, Cha SH. A prospective comparison study for predicting circumferential resection margin between preoperative MRI and whole mount sections in mid-rectal cancer: significance of different scan planes. Eur J Surg Oncol. 2008;34(6):648-654. [ Links ]
40. Slater A, Halligan S, Taylor SA, Marshall M, Distance between the rectal wall and mesorectal fascia measured by MRI: effect of rectal distension and implications for preoperative prediction of a tumour-free circumferential resection margin. Clin Radiol. 2006;61(1):65–70. [ Links ]
41. Smith NJ, Shihab O, Arnaout A, Swift RI, Brown G. MRI for detection of extramural vascular invasion in rectal cancer. AJR Am J Roentgenol. 2008;191(5):1517–1522. [ Links ]
42. Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Rutten HJ, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345(9):638–646. [ Links ]
43. Andreola S, Leo E, Belli F, Bufalino R, Tomasic G, Lavarino C, et al. Manual dissection of adenocarcinoma of the lower third of the rectum specimens for detection of lymph node metastases smaller than 5 mm. Cancer. 1996;15(77):607-12. [ Links ]
44. Monig SP, Baldus SE, Zirbes TK, Schroder W, Lindermann DG, Dienes HP, et al. Lymph node size and metastatic infiltration in colon cancer. Ann Surg Oncol. 1999;6(6):579-81. [ Links ]
45. Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging a meta-analysis. Radiology. 2004;232(3):773-783. [ Links ]
46. Kotanagi H, Fukuoka T, Shibata Y, Yoshioka T, Aizawa O, Saito Y, et al. The size of regional lymph nodes does not correlate with the presence or absence of metastasis in lymph nodes in rectal cancer. J Surg Oncol. 1993;54(4):252-254. [ Links ]
47. Dworák O. Number and size of lymph nodes and node metastases in rectal carcinomas. Surg Endosc. 1989;3(2):96-99. [ Links ]
48. Kim JH, Beets GL, Kim MJ, Kessels AG, Beets-Tan RG. High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol. 2004;52(1):78–83. [ Links ]
49. Koh DM, Brown G, Husband JE. Nodal staging in rectal cancer. Abdom Imaging. 2006;31(6):652–659. [ Links ]
50. Jager GJ, Barentsz JO, Oosterhof GO, Witjes JA, Ruijs SJ. Pelvic adenopathy in prostatic and urinary bladder carcinoma: MR imaging with a three-dimensional TI-weighted magnetization-prepared- rapid gradient-echo sequence. AJR Am J Roentgenol. 1996; 167(6):1503–1507. [ Links ]
51. Williams AD, Cousins C, Soutter WP, Mubashar M, Peter AM, Dina R, et al. Detection of pelvic lymph node metastases in gynecologic malignancy: a comparison of CT, MR imaging, and positron emission tomography. AJR Am J Roentgenol. 2001;177(2):343–348. [ Links ]
52. Okizuka H, Sugimura K, Ishida T. Preoperative local staging of rectal carcinoma with MR imaging and a rectal balloon. J Magn Reson Imaging. 1993;3(2):329-35. [ Links ]
53. Chan TW, Kressel HY, Milestone B, Tomochefski J, Schnall M, Rosato E, et al. Rectal carcinoma: staging at MR imaging with endorectal surface coil. Work in progress. Radiology. 1991;181(2):461-7. [ Links ]
54. Indinnimeo M, Grasso RF, Cicchini C, Pavone P, Stazi A, Catalano C, et al. Endorectal magnetic resonance imaging in the preoperative staging of rectal tumors. Int Surg 1996;81(4):419-22. [ Links ]
55. McNicholas MM, Joyce WP, Dolan J, Gibney RG, Mac Erlaine DP, Hyland J. Magnetic resonance imaging of rectal carcinoma: a prospective study. Br J Surg. 1994;81(6):911-4. [ Links ]
56. Heriot AG, Grundy A, Kumar D. Preoperative staging of rectal carcinoma. Br J Surg. 1999;86:17–28. [ Links ]
57. Ichikawa T, Erturk SM, Motosugi U, Sou H, Araki T, Fujli H, et al. High-b-value diffusion-weighted MRI in colorectal cancer. AJR Am J Roentgenol. 2006;187(1):181-184. [ Links ]
58. Dzik-Jurasz A, Domenig C, George M, Wolber J, Padhani A, Brown G, et al. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet 2002;360(9329):307-308. [ Links ]
59. Hein PA, Kremser C, Judmaier W, Griebel J, Kreczy A, Hub EB, et al. Diffusion-weighted magnetic resonance imaging for monitoring diffusion changes in rectal carcinoma during combined, preoperative chemoradiation: preliminary results of a prospective study. Eur J Radiol 2003;45(3):214-222. [ Links ]
60. Feng Q, Yan YQ, Zhu J, Xu JR. T staging of rectal cancer: accuracy of diffusion-weighted imaging compared with T2-weighted imaging on 3.0 tesla MRI. J Dig Dis. 2014;15(4):188-194. [ Links ]
61. Mizukami Y, Ueda S, Mizumoto A, Sasada T, Okumura R, Kohono S. Diffusion-weighted Magnetic Resonance Imaging for Detecting Lymph Node Metastasis of Rectal Cancer. World J Surg. 2011;35(4):895-899. [ Links ]
62. Sinaiei M, Swallow C, Milot L, Moghaddam PA, Smith A, Atri M, et al. Patterns and Signal Intensity Characteristics of Pelvic Recurrence of Rectal Cancer at MR Imaging. Radiographics 2003;33(5):E171-E187. [ Links ]
63. Palmer G, Martling A, Cedermark B, Holm T. A population-based study on the management and outcome in patients with locally recurrent rectal cancer. Ann Surg Oncol. 2007;14(2):447-454. [ Links ]
64. Lambregts DM, Cappendijk VC, Maas M, Beets GL, Beets-Tan RG. Value of MRI and diffusion-weighted MRI for the diagnosis of locally recurrent rectal cancer. Eur Radiol. 2011;21(6):1250-1258. [ Links ]
65. Tan PL, Chan CL, Moore NR. Radiological appearances in the pelvis following rectal cancer surgery. Clin Radiol. 2005;60(8):846-855. [ Links ]
66. Pema PJ, Bennett WF, Bova JG, Warman P. CT vs MRI in diagnosis of recurrent rectosigmoid carcinoma. J Comput Assist Tomogr. 1994;18(2):256-261. [ Links ]
67. Moore HG, Shoup M, Riedel E, Minsky BD Alektiar KM, Ercolani M, et al. Colorectal cancer pelvic recurrences: determinants of resectability. Dis Colon Rectum. 2004;47(10):1599-1606. [ Links ]
68. Messiou C, Chalmers AG, Boyle K, Wilson D, Sagar P. Pre-operative MR assessment of recurrent rectal cancer. Br J Radiol. 2008;81(966):468-473. [ Links ]











 texto em
texto em