Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.31 no.4 Bogotá Oct./Dec. 2016
Occult Hepatitis B Virus Infection in Liver Transplant Patients
Alejandra Duque-Jaramillo Biol.(1), Julio C. Rendón Biol. MSc (1), Fabián Cortés-Mancera Bact. MSc (1,2), Gonzalo Correa MD (1), Juan Carlos Restrepo MD (1,3), Sergio Hoyos MD (1,3), María-Cristina Navas Bact. MSc PhD (1)
(1) Gastrohepatology Group of the Faculty of Medicine at the University of Antioquia in Medellín, Colombia
(2) Biomedical Research and Innovation Group GI2B in the Faculty of Exact Sciences of the Metropolitan Technological Institute (ITM) in Medellín, Colombia
(3) Hospital Pablo Tobón Uribe in Medellín, Colombia
Received:Â Â Â 07-03-16Â Â Accepted:Â Â Â 01-11-16
Abstract
Introduction: Occult hepatitis B virus infection is characterized by the presence of the viral genome in serum and/or liver tissue from individuals who test negative for the HBsAg surface antigen. Occult infection has been associated with the development of cirrhosis and hepatocellular carcinoma.
Objective: The objective of this study was to identify cases of occult hepatitis B virus infection in patients with cirrhosis and/or hepatocellular carcinoma undergoing liver transplantation.
Materials and methods: Between February 2013 and March 2014 hepatic explant samples were obtained from patients with cirrhosis and/or hepatocellular carcinoma. The hepatitis B virus genome was detected by amplification of three regions of the viral genome (S, Core and X). Positive samples were confirmed by real-time PCR for the S region.
Results: Fifteen hepatic tissue samples were analyzed. The genome of the hepatitis B virus was detected in two (13.3%) samples by nested PCR for the S region and by semi-nested PCR for region X. The results were confirmed by real-time PCR. These samples came from patients who had tested negative for anti-HBc and anti-HBs serological markers for hepatitis B virus infection.
Conclusion: The frequency of occult infection reported in this study is similar to that reported in Brazil in biopsy specimens obtained from patients with chronic hepatitis. Additional studies are needed to estimate the frequency of occult hepatitis B in patients with end-stage liver disease in Colombia.
Keywords
Hepatitis b virus, hepatitis b occult, surface antigen, liver diseases, liver transplantation.
INTRODUCCIÓN
Hepatitis B viral (HBV) infections are a global public health problem. The World Health Organization (WHO) estimates that there are more than 240 million cases of chronic HBV infection worldwide and more than 780,000 deaths each year due to this infection and due especially to the development of terminal liver diseases such as cirrhosis and hepatocellular carcinoma (HCC). (1)
The HBV genome is 3,200 bp partial double stranded circular DNA (rcDNA). It contains four open reading frames (ORFs) encoding seven proteins: ORF S for the three forms of the surface antigen (HBsAg), the ORF Core for the structural subunit of the capsid and the e antigen, ORF P for viral polymerase, and ORF X for the HBx protein. (2, 3)
In 1979 occult HBV infection (OBI) was described for the first time. (4) It is characterized by the presence of the viral genome in hepatic tissue of individuals but has only undetectable levels of the surface antigen (HBsAg) serological marker, and HBV DNA is not present in the serum. (5, 6) In these cases, the viral load is <200 IU/mL or <1000 IU/mL. (5, 6) About 80% of individuals with occult infections have antibodies against the core protein (anti-HBc+) and/or against the surface antigen (anti-HBs+). (7) Affected individuals are identified as negative for HBV infection by ELISA for HBsAg even though they are infected with the virus.
As various studies have shown, the clinical importance of OBI lies in the risk of developing cirrhosis and HCC. (8, 9) In addition, the infection can be reactivated in cases of immunosuppression and can even cause hepatic insufficiency and death. (10, 11) Importantly, individuals with OBI can transmit the virus through blood transfusions and organ transplants, especially liver transplants. (7, 12).
In Colombia, OBI studies have been performed among patients with human immunodeficiency virus (HIV) infection (13-15), blood donors (16-19), patients undergoing hemodialysis (20), and university students. (21). Study of liver tissue samples is of great importance because OBI depends on the persistence of the viral genome in the hepatocytes. (5) Added to this, the low replication rate characteristic of occult infections make it difficult to detect the viral genome in serum. (22)
Based on this, and considering that only one single study has been carried out in this country, with only preliminary results, (23) our objective was to identify cases of occult infection in patients who had received liver transplant and who had tested negative for the HBsAg serological marker.
Materials and Methods
Study Population
This is a descriptive study of liver tissue samples from Colombian patients diagnosed with cirrhosis and/or HCC who underwent liver transplantations between February 2013 and March 2014 at the Pablo Tobón Uribe Hospital in Medellín, Colombia. All patients had tested negative for the HBsAg serologic marker.
The diagnosis of liver cirrhosis was established by clinical criteria such as parotid hypertrophy, jaundice, spider nevus, gynecomastia, palmar erythema and/or associated complications such as hepatic encephalopathy, ascites, esophageal variceal bleeding, coagulopathy, hepatorenal syndrome and spontaneous bacterial peritonitis. Diagnoses were all confirmed by imaging, and some were also confirmed by histopathology. The diagnosis of HCC was established following the criteria of the European Association for the Study of the Liver (ESAL) and the American Association for the Study of Liver Diseases (AASLD).
Samples
Hepatic explant samples were obtained, stored at 4° C for one to twelve days in sterile tubes, and then stored at -70° C until analysis. Diagnostic information, serological markers and viral loads of HBV, HCV and HIV were obtained from medical histories.
DNA Extraction
DNA was extracted with TRIzol® (Invitrogen) according to the manufacturer's instructions. DNA was quantified by spectrophotometry (Nanodrop 2000, Thermo Scientific), and 260/280 ≥1.80 ratio was considered indicative of good quality DNA.
Detection of Hepatitis B Virus
Prior to amplification of the viral genome, PCR was performed for the GADPH gene as quality control of the total DNA extracted, adapting the protocol described by Pitzurra et al. (24).
To detect HBV, PCR was performed for ORF S, ORF X and ORF Cores following the laboratory's protocols and methods described previously. (19, 25-29) The ORF S region was amplified by a nested PCR with YS1-YS2 and S3s-S3as primers to obtain amplifications of 584 and 336 bp (19, 25, 26). Semi-randomized PCR with X4F and X3R or X1R primers was performed for ORF X. The amplification of the first round was 425 bp and that of the second round was 139 bp. (27, 30) Finally, semi-random PCR with 1101P and 2440n or P2 primers was performed for the ORF Core. The sizes of the amplifiers were 1333 bp and 747 bp, respectively. (28, 29)
The PCR detection limits for ORF S (50 IU/mL) and for ORF X and ORF Core (500 IU/mL) were determined in a previous study. (19) All amplifications used 60-80 ng of DNA. A 1:10 dilution of the extracted DNA was also included in order to exclude possible inhibitors that could affect amplification. All samples were analyzed in duplicate.
In all experiments, DNA extracted from a sample with no evidence of HBV was included as a negative control. DNA extracted from a sample from an individual diagnosed with HBV cirrhosis who had tested positive for the HBsAg serologic marker was used as a positive control. PCR products were evaluated by 1.5% agarose gel electrophoresis and ethidium bromide staining.
Real-time PCR
Real-time PCR was performed on samples that were positive for at least one region of the HBV genome by nested or semi-nested PCR. The QuantiTect SYBR Green PCR kit (QIAgen) and HBV359F and HBV425R primers used for real time PCR were donated by Dr. Tonya Mixson-Hayden of the Center for Disease Control and Prevention (CDC, Atlanta, USA). (19, 31) Serial dilutions of known concentrations of plasmid pJetTH24_1.5 which had been previously constructed in the laboratory, and which contained the complete genome of HBV, were used as the standard.
Ethical Issues
Individuals voluntarily participated in the study after signing informed consent forms. This project was approved by the ethics committees of the University of Antioquia and the Hospital Pablo Tobón Uribe.
Results
Sample Characteristics
In total, 15 samples of liver tissue from patients with cirrhosis and/or HCC who underwent liver transplantation were obtained. Of these, nine came from men, and six came from women. Their average age was 49 years old with a range of 20 to 64 years old. All patients except for one tested negative for HBsAg and anti-HBc serological markers. The exception tested positive for anti-HBc IgG. Five patients tested positive for anti-HCV antibodies, and the viral genome was detected in one of them. Anti-HCV antibodies were detected by chemiluminescent microparticle immunoassay (CMIA; ARCHITECT anti-HCV, Abbott), and viral load was determined by real-time PCR. The diagnosis recorded in the clinical history of patient HT127 indicated HCV cirrhosis genotype 1B, although there was no information on viral load or anti-HCV antibodies. None of the individuals tested positive for HIV infections.
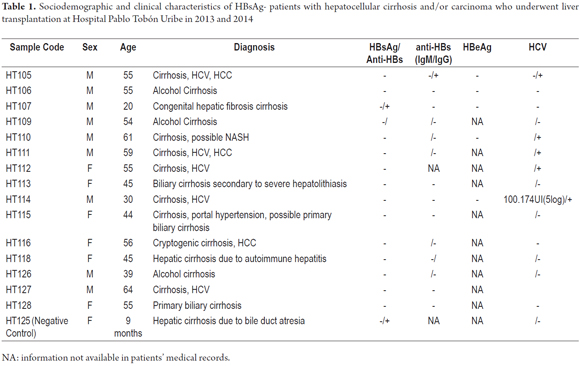
All patients had been diagnosed with cirrhosis, and three (20%) had HCC. Five patients (33.3%) had HCV infections, two of which also had HCC while the other three (20%) cases were due to chronic alcohol consumption. Only one case was diagnosed as cryptogenic cirrhosis. The characteristics of the patients are summarized in Table 1.
HBV Detection
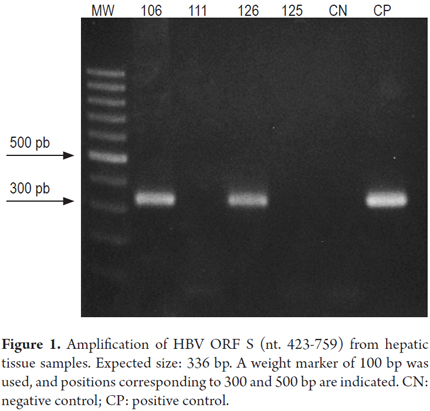
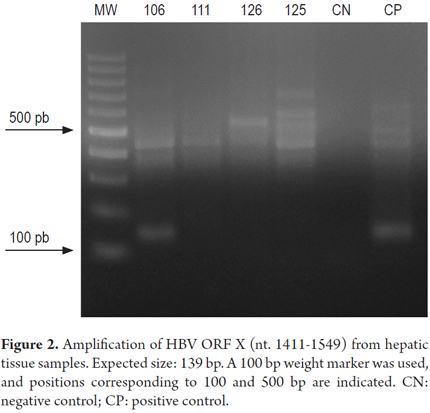
Fragments of the HBV genome were amplified from samples HT106 and HT126 by PCR for ORF S (Figure 1). The ORF X strategy amplified the viral genome from the HT106 sample (Figure 2), but the ORF Core strategy could not achieve amplification in any sample.
Real-time PCR
Taking into account the recommendation that diagnosis of OBI should be made on the basis of amplification of at least two regions of the viral genome, and the fact the amplification of the HBV genome in the HT126 sample had been achieved in only one sample through nested PCR, real-time PCR was used to corroborate the presence of the viral genome in this sample as well as in sample HT106. The result of the amplifications were both positive. This confirmed the presence of the HBV genome in samples HT106 and HT126 which were taken from patients who had tested negative for HBsAg thus indicating that these are cases of occult infection.
Discussion
This study describes a 13.3% (2/15) frequency of occult hepatitis B virus infection in samples from individuals diagnosed with cirrhosis and/or HCC who underwent liver transplantation. The samples in which the viral genome was detected had tested negative for anti-HBc and anti-HBs markers, hence they are cases of seronegative OBI which are estimated to account for 20% of total cases of OBI. (7) This underlines the importance of including individuals without serological markers of HBV infection in OBI studies, since their exclusion may lead to an underestimation of the frequency of this type of infection.
It has been proposed that the frequency of OBI is related to the endemicity of HBV infections in the study population and HBV risk factors in that population. However, because there is no clarity regarding the mechanisms of pathogenesis of occult infections, factors such as hosts' immune systems, viral load and virus genotype may be involved. (32) In addition, frequencies of OBI that have been reported are also conditioned by the methodological approach used to detect the HBV genome including the nature of the sample (serum or hepatic tissue), pool analysis, and the sensitivity and specificity of the technique all of which may affect the detection of the viral genome. (33, 34)
Despite the fact that the most recent analysis indicates that Colombia has low to moderate endemicity for HBV infections, (35, 36) the country's pattern of prevalence is very heterogeneous: there are regions with low, medium and high prevalences. According to National Institute of Health statistics for 2015, there were 2,227 cases of hepatitis B which computes to a countrywide incidence of 4.67 cases/100,000 inhabitants (range 0.29-25.20). Incidence in the department of Antioquia during this period was 6.60 which is the eighth highest in the country. (37)
In Colombia, the beginnings of research into OBI study were in studies of patients with HIV infections and of blood donors. Ramírez Sánchez and Cataño Correa evaluated 50 HIV-positive patients, twelve of whom tested negative for HBsAg but positive for anti-HBc IgG. In one sample, viral genome was detected by PCR and accounted for 2% of the total sample and 8.3% of the anti-HBc + samples. (13) Another study of 103 HIV positive patients by Polo et al. found six patients who tested negative for HBsAg but positive for anti-HBc, but viral load could only be determined for one of them (1/103, equivalent to 0.97%). (14) In this population, the most recent study was performed by Bautista et al. on 275 serum samples: all samples were analyzed by nested PCR for ORF S, and OBI was diagnosed in 8.7% of the samples. (15)
Beltrán et al. analyzed 129 serum samples from blood donors to blood banks in Bogotá, Cali, Barranquilla and Valledupar for total reactive anti-HBc serological profiles and anti-HBs titer ≤30 mIU/mL. The analysis was performed by amplification of the pooled viral genome of six samples. The authors reported 0% incidence of OBI. (16) In Antioquia, Arroyave Ospina et al. analyzed 207 serum samples from HBsAg-/anti-HBc + blood donors which had tested negative for other infection markers. They used two nested PCR strategies for ORF S and reported an OBI frequency of 3.4% (7/207). (17) Ríos-Ocampo et al. evaluated 302 HBsAg-/anti-HBc + donor serum samples by nested PCR for ORF Ss, ORF Cores and ORF Xs. They detected the HBV genome in six samples (2%). In addition, they found mutations in the S and P ORFs was found in the study by Ríos-Ocampo et al., And four mutations were found to be synonymous and three non-synonymous in S, and six non-synonymous mutations in P (19). Finally, Castellanos and collaborators analyzed by nested PCR for the ORF S 160 sera from blood donors with a HBsAg-/total anti-HBc + serological profile, from Santander, and found an OBI frequency of 8.75% (18).
Other populations in which OBI has been studied are patients undergoing hemodialysis in Bogotá (20) and university students in Bucaramanga, with a frequency of 0% OBI in the two studies (21). In addition, samples of indigenous children from the department of Amazonas detected the viral genome in 2/24 HBsAg-/anti-HBc + samples (8.3%) by nested PCR for different regions of the ORF S (Jaramillo et al., Manuscript in preparation). These studies have reported OBI frequencies ranging between 0% and 8.75% in several different populations in Colombia.
Only on study of OBI in liver tissue has been conducted in Colombia. Sixty three samples from patients with terminal liver pathologies who underwent liver transplantation whose blood tests were negative HBsAg and anti-HBc markers were included in the study. The preliminary results of amplification of at least two regions of the viral genome by nested and/or semi-nested PCR show that HBV genome was detected in 9.5% of the samples (6/63). (23)
A few studies of liver tissue samples from patients with terminal liver disease have been performed in other countries. In Brazil, an OBI frequency of 4.4% was found in a sample of 68 cirrhosis patients who underwent liver transplantation. (38). Another study included 17 liver biopsies from patients with chronic hepatitis who tested negative for HBsAg and positive for anti-HBc. OBI was found in 17.6% of the samples. (39)
In China, a study analyzed 43 samples from patients with alcoholic cirrhosis who underwent transplantation. Viral genome was detected in 41.9% of the samples. In Korea, a study of 33 samples from patients with cryptogenic HCC and 28 samples from patients with known etiologies were analyzed. The frequencies of OBI were 73% and 21.4%, respectively. (40) In Italy, 61.2% of 80 patients with cirrhosis and/or HCC who tested negative for HBsAg were found to have HBV DNA, and most of them were also infected with HCV. Other etiologies found included NASH, alcohol consumption, and cryptogenic disease. (41) This is consistent with a study of a similar population that detected HBV DNA in 64% of 14 patients, the majority of whom had HCV, who underwent transplantation. (42) The OBI frequency reported in this study is within the range of 4.4% to 64% that has been reported elsewhere among patients with terminal liver disease. Nevertheless, it is lower than that found in most studies. As mentioned above, this frequency is related to the prevalence of HBV in the study region and the sensitivity of the techniques used.
This is the second Colombia study of occult HBV infections in patients undergoing transplantation. Although the frequency reported in this study is greater than those described in other studies conducted in this country, the results are not comparable due to differences in the types of study populations, inclusion criteria and methodologies. In addition, three limitations of the present study should be taken into account: the small sample size which was due in part to the fact that liver transplantation is not a routine procedure; samples were obtained in a single hospital; and the study period was only one year.
Implementation and dissemination of these studies contributes to medical personnel's knowledge of OBI. Even though it was first described more than 30 years ago, it is still not widely recognized in the clinical setting. This is reflected in the lack of guidelines for diagnosis and management, although various studies have shown a relationship between OBI and the development of cirrhosis and HCC. The identification of OBI in patients with chronic liver disease allows diagnosis of HBV infections in individuals which would otherwise not be possible since the patients who tested negative for HBsAg could be identified through routine ELISA. In this way, clinical management can be adapted and, if necessary, antiviral treatment can be initiated. This would also reduce the risk of transmission.
Additional studies with representative samples from medical centers are needed to estimate the frequency of OBI in patients with terminal liver disease in Colombia.
Acknowledgements
This project was possible thanks to grant 2547 of the University of Antioquia, to the P10245 project of the Metropolitan Technological Institute and to the Sustainability Strategy 2013-2014 of the University of Antioquia.
Financing
This study was supported by the Medium-Term Project and Sustainability Strategy 2013-2014 of the Office of the Vice-Rector of Research at the University of Antioquia. It is registered as Project P10245 in the Directorate of Research of the Metropolitan Technological Institute.
Conflict of interests
The authors declare that they have no conflicts of interest. Preliminary results of this work were presented in a poster at the VI Colombian Virology Symposium held in Bogotá from May 27 to 29, 2015.
References
1. World Health Organization. Hepatitis B Fact Sheet [Internet]. World Health Organization. 2015 (citado el 1 de diciembre de 2015). Recuperado a partir de: http://www.who.int/mediacentre/factsheets/fs204/en/. [ Links ]
2. Baumert TF, Thimme R, von Weizsäcker F. Pathogenesis of hepatitis B virus infection. World J Gastroenterol WJG. 2007;13(1):82-90. [ Links ]
3. Samal J, Kandpal M, Vivekanandan P. Molecular mechanisms underlying occult hepatitis B virus infection. Clin Microbiol Rev. 2012;25(1):142-63. [ Links ]
4. Tabor E, Hoofnagle JH, Smallwood LA, et al. Studies of donors who transmit posttransfusion hepatitis. Transfusion (Paris). 1979;19(6):725-31. [ Links ]
5. Raimondo G, Allain J-P, Brunetto MR, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol. 2008;49(4):652-7. [ Links ]
6. Ocana S, Casas ML, Buhigas I, et al. Diagnostic strategy for occult hepatitis B virus infection. World J Gastroenterol WJG. 2011;17(12):1553-7. [ Links ]
7. Torbenson M, Thomas DL. Occult hepatitis B. Lancet Infect Dis. 2002;2(8):479-86. [ Links ]
8. Shi Y, Wu YH, Wu W, et al. Association between occult hepatitis B infection and the risk of hepatocellular carcinoma: a meta-analysis. Liver Int Off J Int Assoc Study Liver. 2012;32(2):231-40. [ Links ]
9. Covolo L, Pollicino T, Raimondo G, et al. Occult hepatitis B virus and the risk for chronic liver disease: A meta-analysis. Dig Liver Dis. 2013;45(3):238-44. [ Links ]
10. Hui C, Cheung WWW, Zhang H, et al. Kinetics and risk of de novo hepatitis B infection in HBsAg-negative patients undergoing cytotoxic chemotherapy. Gastroenterology. 2006;131(1):59-68. [ Links ]
11. Zachou K, Sarantopoulos A, Gatselis NK, et al. Hepatitis B virus reactivation in hepatitis B virus surface antigen negative patients receiving immunosuppression: A hidden threat. World J Hepatol. 2013;5(7):387-92. [ Links ]
12. Squadrito G, Spinella R, Raimondo G. The clinical significance of occult HBV infection. Ann Gastroenterol. 2014;27(1):15-9. [ Links ]
13. Ramírez Sánchez IC, Cataño Correa JC. Prevalencia de hepatitis B oculta en una cohorte prospectiva de pacientes con VIH. Iatreia. 2008;21(1):S10-1. [ Links ]
14. Polo P, Castañeda C, Sierra M, et al. Hepatitis B oculta en pacientes VIH positivos de una institución de salud en Barranquilla, Colombia. Infectio. 2010;14(1):39-46. [ Links ]
15. Bautista-Amorocho H, Castellanos-Domínguez YZ, Rodríguez-Villamizar LA, et al. Epidemiology, risk factors and genotypes of HBV in HIV-infected patients in the northeast region of Colombia: high prevalence of occult hepatitis B and F3 subgenotype dominance. PLoS ONE. 2014;9(12):e114272. [ Links ]
16. Beltrán M, Berrío-Pérez M, Bermúdez MI, et al. Detección de hepatitis B oculta en donantes de bancos sangre, Colombia 2008-2009. Biomédica. 2011;31(4):580-9. [ Links ]
17. Arroyave Ospina JC, Loureira CL, Pujol FH, et al. Caracterización molecular de la infección por el virus de la hepatitis B en donantes de sangre HBsAg negativo/anti-HBc positivo. Hechos Microbiológicos. 2011;1(2):22. [ Links ]
18. Castellanos Y, Portilla V, Chacón L, et al. Identificación de casos de hepatitis B oculta en donantes de bancos de sangre de Santander, Colombia. Hechos Microbiológicos. 2013;4(2 Supl 1):43. [ Links ]
19. Rios-Ocampo WA, Cortes-Mancera F, Olarte JC, et al. Occult hepatitis B virus infection among blood donors in Colombia. Virol J. 2014;11(1):206. [ Links ]
20. Delgado S, Triana A. Identificación de infección oculta por virus de hepatitis B en pacientes con insuficiencia renal crónica en hemodiálisis en Unidad Renal del Hospital Militar Central, Bogotá, Colombia, Noviembre 2008. GEN. 2009;63(1):29-31. [ Links ]
21. Bautista Amorocho H, Castellanos Domínguez YZ, Farfán García AE. Marcadores serológicos y moleculares de infección por el virus de la hepatitis B en estudiantes universitarios colombianos. Rev Colomb Gastroenterol. 2012;27(4):282-90. [ Links ]
22. Marrero JA, Lok ASF. Occult hepatitis B virus infection in patients with hepatocellular carcinoma: Innocent bystander, cofactor, or culprit? Gastroenterology. 2004;126(1):347-50. [ Links ]
23. Rendón JC, Cortes-Mancera F, Duque A, et al. Identificación de casos de infección oculta por el virus de la hepatitis B en pacientes sometidos a trasplante hepático en la ciudad de Medellín. Hechos Microbiológicos. 2013;4(2 Supl 1):94. [ Links ]
24. Pitzurra L, Fringuelli R, Perito S, et al. A new azole derivative of 1,4-benzothiazine increases the antifungal mechanisms of natural effector cells. antimicrob agents chemother. 1999;43(9):2170-5. [ Links ]
25. Schaefer S, Glebe D, Wend UC, et al. Universal primers for real-time amplification of DNA from all known orthohepadnavirus species. J Clin Virol. 2003;27(1):30-7. [ Links ]
26. Zeng GB, Wen SJ, Wang ZH, et al. A novel hepatitis B virus genotyping system by using restriction fragment length polymorphism patterns of S gene amplicons. World J Gastroenterol WJG. 2004;10(21):3132-6. [ Links ]
27. Abedi-Ardekani B, Gouas D, Villar S, et al. TP53 mutations and HBX status analysis in hepatocellular carcinomas from Iran: evidence for lack of association between HBV genotype D and TP53 R249S mutations. Hepat Res Treat. 2011;2011:475965. [ Links ]
28. Günther S, Li BC, Miska S, et al. A novel method for efficient amplification of whole hepatitis B virus genomes permits rapid functional analysis and reveals deletion mutants in immunosuppressed patients. J Virol. 1995;69(9):5437-44. [ Links ]
29. Hu X, Margolis HS, Purcell RH, et al. Identification of hepatitis B virus indigenous to chimpanzees. Proc Natl Acad Sci. 2000;97(4):1661-4. [ Links ]
30. Navas MC, Suarez I, Carreño A, et al. Hepatitis B and hepatitis C infection biomarkers and TP53 mutations in hepatocellular carcinomas from Colombia. Hepat Res Treat. 2011;2011:1-10. [ Links ]
31. Mixson-Hayden T, Lee D, Ganova-Raeva L, et al. Hepatitis B virus and Hepatitis C virus infections in United States-Bound refugees from Asia and Africa. Am J Trop Med Hyg. 2014;90(6):1014-20. [ Links ]
32. Raimondo G, Caccamo G, Filomia R, et al. Occult HBV infection. Semin Immunopathol. 2013;35(1):39-52. [ Links ]
33. Alavian SM, Miri SM, Hollinger FB, et al. Occult hepatitis B (OBH) in clinical settings. Hepat Mon. 2012;12(8):e6126. [ Links ]
34. Negro F. Diagnostic pitfalls in chronic viral hepatitis: occult hepatitis B, hepatitis D and autoantibodies related to hepatitis C. En: EASL Postgraduate Course: Viral Hepatitis. Londres; 2014. [ Links ]
35. Ott JJ, Stevens GA, Groeger J, et al. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30(12):2212-9. [ Links ]
36. Schweitzer A, Horn J, Mikolajczyk RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. The Lancet. 2015;386(10003):1546-55. [ Links ]
37. Tolosa Pérez EN. Informe final hepatitis B y C, Colombia, 2014 (Internet). Bogota: Instituto Nacional de Salud; 2014. Recuperado a partir de: http://www.ins.gov.co/lineas-de-accion/Subdireccion-Vigilancia/Paginas/informes-de-evento.aspx. [ Links ]
38. Ferrari TCA, Xavier MP, Vidigal PVT, et al. Occult hepatitis B virus infection in liver transplant patients in a Brazilian referral center. Braz J Med Biol Res. 2014;47(11):990-4. [ Links ]
39. Júnior B, Maia G, Braga WSM, et al. Occult hepatitis B: prevalence and clinical characteristics in a population with high endemicity of hepatitis B infection in the western Brazilian Amazon region. Rev Soc Bras Med Trop. 2008;41(6):596-601. [ Links ]
40. Wong DKH, Huang FY, Lai CL, et al. Occult hepatitis B infection and HBV replicative activity in patients with cryptogenic cause of hepatocellular carcinoma. Hepatol Baltim Md. 2011;54(3):829-36. [ Links ]
41. Saitta C, Tripodi G, Barbera A, et al. Hepatitis B virus (HBV) DNA integration in patients with occult HBV infection and hepatocellular carcinoma. Liver Int. 2015;35(10):2311-7. [ Links ]
42. Ghisetti V, Marzano A, Zamboni F, et al. Occult hepatitis B virus infection in HBsAg negative patients undergoing liver transplantation: clinical significance. Liver Transpl. 2004;10(3):356-62. [ Links ]











 text in
text in