Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.31 no.4 Bogotá Oct./Dec. 2016
A Case and Control Study of the OLGA System's Impact on Detection of Chronic Atrophic Gastritis in Colombia
Diana Martínez MD (1), William Otero MD (2), Orlando Ricaurte MD (3)
(1) Pathologist in the Pathology Unit at the National University of Colombia in Bogotá, Colombia
(2) Professor of Medicine in the Gastroenterology Unit at the National University of Colombia and the National University Hospital of Colombia in Bogotá, Colombia
(3) Professor of Medicine in the Unit of Pathology of the National University of Colombia and the National University Hospital of Colombia in Bogotá, Colombia
Received:Â Â Â 16-08-16Â Accepted:Â Â Â 01-11-16
Abstract
Introduction: Chronic atrophic gastritis (GCA) is a clinicopathological entity related to intestinal type gastric cancer (GC) whose main cause is helicobacter pylori. Currently, in addition to the diagnosis, it is recommended that the extent of atrophy or intestinal metaplasia be evaluated in order to stage the GC risk. The most accurate method for atrophy is OLGA which requires five biopsies: two from the corpus, two from the antrum and one from the angular incisure. Each biopsy is marked placed in a separate tube and marked. In Colombia, the use of OLGA to study gastric atrophy had not been evaluated previously.
Materials and methods: This is a case and control study whose cases are patients who had biopsies taken to be studied with OLGA. Control patients had less than five gastric biopsies, without OLGA sampling.
Results: This study includes 1,599 cases and 4,191 controls. The average age of cases was 49 +/- 12 years, and the average age of controls was 54 +/- 10 years (p: NS). H. pylori infections were found in 60% of the cases and in 57% of the controls while 42% of the cases were found to have gastric cancer and 26% of the cases were found to have GC. 12.3% had OLGA III or IV and 88% had OLGA 0, I or II and did not merit endoscopic monitoring.
Conclusion: The OLGA system detects 61.8% more atrophy than is detected with less sampling of gastric biopsies. Most of the cases (88%) had low risk of GC (stages 0-II) and did not require endoscopic monitoring.
Keywords
Atrophic gastritis, intestinal metaplasia, helicobacter pylori, Sidney system, OLGA system.
Introduction
Chronic gastritis (CG) is a very common pathological entity in countries such as Colombia that have high prevalence of gastric cancer and high mortality rates due to gastric cancer. (1-3). It is a very important step in the natural history of gastric carcinogenesis which has a well-documented relationship with intestinal gastric carcinoma. (4) Atrophy of the gastric mucosa and intestinal metaplasia are considered to be precursors of gastric cancer (GC). (5-10) A study in the Netherlands has found that the annual incidence of GC in patients with atrophy is 0.1% while in patients with intestinal metaplasia its 0.25%. (11) The study found that the risk of GC is 2% to 3% at the ten-year follow-up examination. (11) Appropriate diagnosis through endoscopic biopsies is fundamental for correct follow-up of these patients. (12) The histopathological features of chronic atrophic gastritis (CAG) are chronic inflammation and loss of glands in the gastric mucosa through atrophy or replacement by intestinal type epithelium (atrophy with intestinal metaplasia). (13-16) The presence of anthropological glands in the body mucosa (pseudopyloric metaplasia) is also part of this process in its initial phase. (1, 11-15)
CAG develops late in the evolution of chronic gastritis whose main cause is Helicobacter pylori which is also the main cause of GC. (1-4, 7, 12). Another less common type of CAG is autoimmune gastritis which predominates in other latitudes but is considered to be very rare in Colombia. (7, 17, 18) CAG, associated with H. pylori is multifocal and compromises the mucosa of both the antrum and corpus, whereas autoimmune atrophic gastritis is restricted to the mucosa of the corpus. (1, 13-16) Its distribution in patches motivated Sidney (19) to recommended that two biopsies be taken from the corpus of the body and two more from the antrum to adequately identify CAG. However, when this system was updated, the last Maastricht consensus considered it necessary to add a biopsy of the incisura angularis. (12, 20) Other authors have considered that this biopsy is unnecessary, and the recent European guideline on management of precancerous gastric lesions supports taking biopsies only from the corpus and antrum without any biopsy from the incisura angularis. (21) Nevertheless, the incisura angularis is the site atrophy and metaplasia occur earliest in multifocal CAG. (22, 23) This was taken into account by a group of gastroenterologists and expert pathologists who proposed using the OLGA (Operative Link for Gastritis Assessment) staging system as the instrument for evaluating and determining the extent of atrophy and intestinal metaplasia. (24) OLGA scores range from 0 to IV: 0 is no atrophy, I is minimum atrophy, and IV is the most severe degree of atrophy. (24) The ultimate purpose of the OLGA system is to stratify GC risk into the future. (12, 24) It is recommended that patients with OLGA III or IV be monitored with endoscopy to detect early GC. (4, 12, 24) A study of 93 patients with dyspepsia who were followed for 12 years found that the only two patients who had GC at the end of follow-up had been classified as OLGA III/IV at the initial endoscopy. (25)
Another more recently proposed staging system that is similar to OLGA is called the Operative Link on Gastric Intestinal Metaplasia Assessment (OLGIM), but it only evaluates changes in intestinal metaplasia. (26) Although both scoring systems are useful for stratifying GC risk and identifying patients requiring follow-up or monitoring, OLGIM has the advantage of greater interobserver agreement. Nevertheless, it has recently been found that it may classify cases in lower stages that would be severe (III and IV) in the OLGA system, and this warrants additional vigilance. (27)
The correlation between the appearance of the mucosa and the degree of histological atrophy is very poor with white light endoscopes, it is very high with magnification and high resolution endoscopes and with enhanced image endoscopes combined with magnification and electronic chromoendoscopy (NBI). This is especially true in the hands of endoscopists who are well trained in their use. (28-33) Using this technology, biopsies can be targeted to morphologically altered mucosal sites and not simply taken at random sites. (30-32) These advanced methods are routinely used in Japan, but are not yet available in the rest of the world, including Colombia.
In our country, there are high prevalences of H. pylori and CAG and a high incidence of GC, the leading cause of cancer death among men and the third leading cause of death among women. (34). For this reason, and because of the scarcity of research in Colombia, we decided to carry out this study in order to determine whether appropriate sampling of the gastric mucosa and the use of the OLGA system can increase the number of diagnoses of CAG beyond those found with biopsies without the protocol required to apply such a system.
Materials and Methods
This is a case-control study based on the database of histopathological studies carried out during between 2010 and 2013 in the Department of Pathology of the Faculty of Medicine of the National University of Colombia (UN) in Bogotá. The Department of Pathology of the UN receives samples from patients sent from all over the city of Bogotá which is probably the population that best most represents the entire country. Official statistics show that between 400,000 and 600,000 people come to this city every year. Thus, many regions of Colombia are represented in Bogotá.
Cases were defined as patients whose gastric biopsies were taken following the updated Sydney protocol which allowed the use of the OLGA system. This protocol calls for two samples from the antrum, one from the minor curvature and one from the major curvature taken at 3-4 cm from the pylorus; two biopsies from the corpus, one from the greater curvature more than eight cm from the cardia, and one from the lesser curvature less than four cm proximal of the angular incisure; and one from the angular incisure. Each sample suitably marked and sent separately to the laboratory. (20, 24) These biopsies were taken during digestive endoscopies performed by gastroenterologists or endoscopic surgeons. Once taken, they were fixed in 10% formaldehyde and sent to the Department of Pathology. In the laboratory, the standard paraffin embedment process was followed. From each block, multiple histological sections of 5 microns were obtained. Sections were then stained with hematoxylin and eosin (HE) When H. pylori was not detected following HE staining, sections were also stained with Giemsa. (18) All biopsies were evaluated by expert pathologists who generally had the report of the patient's endoscopy. When there were doubts, the final report was decided by consensus between two pathologists. Each biopsy was evaluated according to the updated system of Sydney, and the atrophy and metaplasia of fragments were staged according to the recommendations of the OLGA scoring system. (24) The analysis of each biopsy was done using a database checklist of diagnostic criteria of the Sydney system. (20)
Controls were patients whose gastric biopsies were not taken following the updated Sydney sampling system. The processing of gastric biopsies was the same for case biopsies.
In both groups the proportion of CAG cases was determined in relation to cases of other types of gastritis and to diagnoses broken down by age group, gender and H. pylori infection status. The frequency of CAG was compared in both groups.
Samples obtained through procedures other than gastric biopsies were excluded. These included specimens from gastrectomies and polypectomies, those obtained as part of gastroesophageal junction biopsies, histological follow-up studies and gastric biopsies with tumor diagnosis. All follow-up studies were also excluded.
Statistical Analysis
All data collected were processed in Microsoft Office 2007 and analyzed with GraphPad StatMate 2. The Chi square test was used for comparison between proportions and dichotomies. Student's t test was used between averages. Statistical significance was defined as a difference less than 0.05.
Results
Cases
During the study period, 1,599 cases were staged and graded using the OLGA system. In this group, 1,028 (64%) patients were women and 571 (36%) were men. The mean age was 49 ± 10 years (range: 6-88).
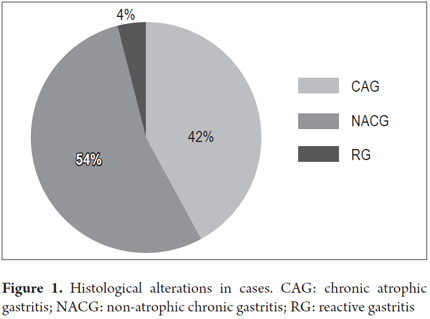
CAG was found in 42% (n = 665) of patients, and absence of atrophy was found in 54% (n = 869). Reactive gastropathy was found in 4% (65 patients). These histological alterations are shown in Figure 1.
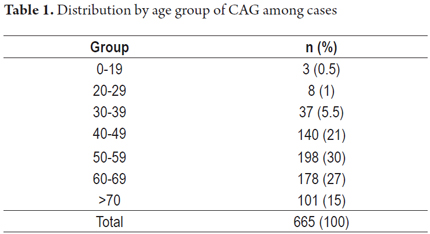
The distribution of chronic multifocal atrophic gastritis (CMFAG) by age group is shown in Table 1.
Other concomitant alterations found included reactive gastropathy in three patients with CMFAG. Four cases with autoimmune atrophic gastritis in the corpus were also reported. The mean age of these patients was 46 years, while the age of those with CMFAG was 56 years (p <0.05).
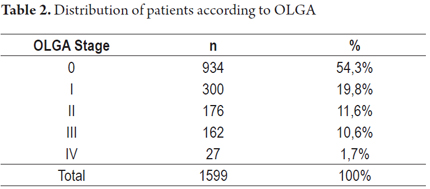
The distribution of cases according to OLGA stage is shown in Table 2.
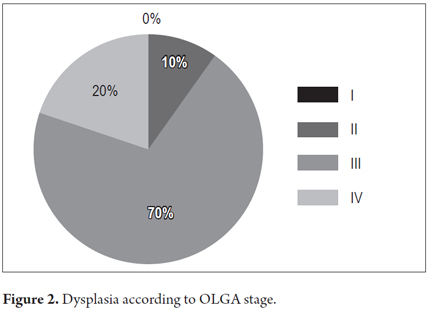
Dysplasia was found in 3% of patients with CAG (n = 20). The frequency of dysplasia according to OLGA category was as follows: 70% occurred in patients with OLGA III (n =Â 14); 20%, with OLGA IV (n = 4); and 10% with OLGA II (n = 2) (Figure 2).
Patients with OLGA 0 have significantly younger age (44 years) than OLGA III and IV (59 and 65 years, respectively). The distribution of OLGA stages by age group is shown in Table 3. In 98.5% of the patients with NACG (OLGA 0) there were no other alterations; 1.5% also had reactive gastritis.
H. pylori infections were found in 400/665 cases (60%) with CAG alone or associated with another diagnosis, in 633/869 cases (73%) of NACG (OLGA 0) alone or associated with another diagnosis (p < 0.05) and were absent in the 65 cases of reactive gastropathy.
Controls
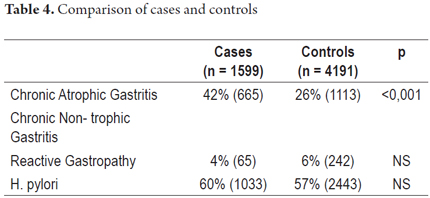
During the study period there were 4,191 controls. Of these, 2,553 (61%) were women. The mean age of the whole group was 54 years (range 10-98). CMFAG was found in 26% (1,113) of the patients, NACG in 68% and reactive gastropathy in 6%. These histological characteristics are shown in Figure 3. H. pylori infections were present in 58% (2,443) of the patients.
Of the patients with CAG, 42% were men (471). The average age was 61 years (range 50-80 years). CAG was diagnosed alone in 946/1,113 cases (85%) and associated with GR in 167/1,113 cases (15%). NACG was diagnosed in 68% of the total cases (n = 2,836). It was diagnosed alone in 2,585 cases (91%) and associated with RG in 251 cases (9%). RG was diagnosed alone in 6% of cases (242/4,191). Table 4 shows the comparison of main characteristics of cases and controls.
Discussion
This case and control study's population included more than 5,700 patients. The frequency of atrophy was compared between cases defined as samples taken in accordance with the updated Sydney system and controls defined as samples taken without following this protocol. The OLGA system was used to classify and stage atrophy of cases. Multifocal atrophic chronic gastritis was found in 42% of the patients versus 26% of the control group (p <0.001). This means a 61.8% higher yield when five biopsy samples were taken according to updated Sydney system. In addition to a greater number of biopsies from the corpus and antrum, the key difference between these two groups was the biopsy from the incisura angularis, whose impact has been demonstrated. (35, 36) Recently, a study from Lithuania found that when a biopsy from the incisura angularis is not included, the OLGA degree of severity of atrophy decreases by 18% and the OLGIM degree of severity of intestinal metaplasia decreases by 4%. When high-risk (grade III and IV) conditions are evaluated, 30% and 35% of cases respectively are no longer detected. (That study and another study have shown that biopsies from the incisura angularis are more reliable than those from the corpus and the antrum for detecting atrophy and intestinal metaplasia. (35, 36) By definition it was not possible to use OLGA staging for controls in this study, but CAG was found more frequently among cases. That the difference was greater than the one found in the Lithuanian study is probably due to the fact only one or two biopsies from each control was included. This also limited identification of atrophy. The importance of complete gastric sampling including five biopsies and additional biopsies for specific lesions was taken into account by the last Maastricht consensus (12) and more recently by the Global Kyoto consensus on chronic gastritis and H. pylori. (37)
To determine that the OLGA system detects atrophy better than the practice of taking a limited number of biopsies, it is necessary to rule out other variables that might explain the great difference in the results. These other variables include patients' ages and prevalence of H. pylori. With regard to age, the cases were younger and theoretically should have less atrophy, so the difference most probably was due to the way biopsies were taken. With regard to H. pylori, the prevalence of infection was similar in both groups, 60% and 57%, respectively (p: NS), and both were practically the same as that found in a recent study in Bogotá. (38)
Although gastric biopsy is the gold standard for evaluation and follow-up of chronic gastritis, sampling usually used in our environment does not follow the Sydney protocol and is therefore inadequate for diagnosis and staging of atrophy, the most important alteration in the chain of carcinogenesis. (1-3, 12, 38) Colombia has a high incidence of GC, (34) so it is noteworthy that this study found that with the OLGA protocol only 12.3% of the patients had a high GC risk (OLGA III And IV) which would merit endoscopic surveillance after eradicating H. pylori. (12, 37) The vast majority (88.7%) had minimal risk (OLGA 0, I and II) and theoretically would not require endoscopic surveillance after eradicating H. pylori. (4, 12). We consider that these findings could impact the routine of digestive endoscopies in our setting, since with adequate biopsy sampling, information should be available to decide whether or not to monitor patients with endoscopies in the future. If only 12.3% need control endoscopy, costs incurred in repetition of unnecessary gastritis endoscopies could be avoided for most patients.
Of the 20 patients with dysplasia, eighteen (90%) were in high risk categories of the OLGA system (III and IV) and two (10%) were in OLGA II stage, representing 1.13% of these patients. The finding that the majority of cases with dysplasia (80%) were found in patients with OLGA III/IV coincides with the results of a Japanese study by Satoh et al. (39) In this study, 84 %% of GC patients had OLGA III/IV which contrasts with patients with duodenal ulcer, most of whom had OLGA 0/I although a minority had OLGA III/IV. The findings of Rugge et al. are similar. (40) In that study, seven patients with GC were among the 21/439 patients with OLGA III/IV. (40)
The finding of dysplasia in 2/176 patients with OLGA II (1.36%) should merit additional studies since this group of patients has traditionally not been considered require prospective surveillance with upper gastrointestinal endoscopy. (4) If these results are corroborated, whether these patients should also be monitored should be discussed.
Older case patients were more likely to have OLGA stages III and IV than Olga stages I and II which may indicate that they have had a chronic inflammation processes that have lasted longer than the milder stages. (4, 12, 23) In addition to allowing selection of patients at greater risk of developing gastric cancer based on the extent of changes in atrophy and intestinal metaplasia (stages III and IV), the OLGA system allows identification of changes in young individuals in early stages. In these patients the process may evolve in later decades to advanced stages if there is no intervention to halt its progression. (4, 12) The risk of GC in patients with OLGA IV is 10-90 times (41) and depends on the extent of atrophy rather than age since the latter is not an independent risk factor for GC. (41) In our country there is no exact data on the probability of GC in patients with mild or extensive atrophy, but we believe that this merits follow-up studies in these cohorts of patients.
Given the importance of surveillance for early detection of GC, we consider that patients with OLGA III/IV should be followed up, that endoscopy should be detailed and should follow the early GC detection protocol. (42)Â Performance of routine endoscopy and repeated suboptimal sampling of biopsies is not justified.
In this study, 99% of CAG cases were multifocal and only 1% had isolated foci, the pattern of autoimmune CAG. This was found only among cases that by definition had 5 biopsy samples taken. (24) Taking the patient population as a whole, these four patients account for only 0.25% of the total sample which corroborates the concept that this gastritis is very rare in our environment. (1, 2)
This study is the first study in Colombia to analyze the impact of the OLGA system on the risk staging of patients with chronic gastritis. We are aware of the limitations of this work. It is a retrospective study, there was no information on patients' clinical histories, socioeconomic statuses, family histories of GC, or past H. pylori eradication therapies. However, with respect to this latter limitation, the prevalence of H. pylori was equal to that found in a national study that had determination of the prevalence of infection in adults without a history of eradication therapies among its objectives.
Conclusions
- This study, with a sample of more than 5,000 patients, shows that 61.8% more cases of atrophy were identified with the OLGA protocol than with protocols using fewer biopsies (42% versus 26%).
- This is the first study in our country that demonstrates the association of dysplasia with advanced stages of OLGA (III/IV) which is similar to findings from studies in other countries such as Japan that have high incidences of gastric cancer (GC).
- A surprising finding that should be taken into account in programs for early identification of GC is that the majority of GC cases (88%) had no GC risk (OLGA 0-II). This finding implies that most people do not need to undergo surveillance endoscopies. However, it would be important to carry out studies in each region of Colombia, since health policies may differ regionally because the epidemiology of GC can vary, even within the same country. (34)
- Autoimmune gastritis is practically non-existent in our country (4/1,599) (0.4%).
- In countries like Colombia which have high incidences of GC, it is urgent that the community of gastroenterologists and endoscopists incorporate into daily practice the appropriate protocol of five gastric biopsies sent separately (two from the corpus, two from the antrum, and from the incisura angularis) because when atrophy is underdiagnosed when this protocol is not used. The biopsy from the incisura angularis is definitive. Although this study evaluated OLGA, OLGIM can also be used according to the most recent international consensus.
- Our results may have a national impact on the routine of gastric biopsies during endoscopies. We consider that public health care policy should require that the state and healthcare companies guarantee the cost involved in this method of taking biopsies of the stomach.
- With the OLGA protocol, the number or control endoscopies for gastritis in which insufficient biopsies are taken would decrease. In this study it has been observed that the majority of patients (> 85%), theoretically do not merit surveillance endoscopies. That saving could free up the resources needed for appropriate sampling with five biopsies during the initial endoscopy in accordance with the current standard.
Conflicts of Interest
None. All costs of this study were assumed by the researchers.
References
1. Correa P, Cuello C, Duque E, et al. Gastric cancer in Colombia. III. Natural history of precursor lesions. J Natl Cancer Inst. 1976;57:1027-35. [ Links ]
2. Correa P, Piazuelo MB, Wilson KT. Pathology of gastric intestinal metaplasia: clinical implications. Am J Gastroenterol. 2010;105:493-8. [ Links ]
3. Correa P, Fontham ET, Bravo JC, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-Helicobacter pylori therapy. J Natl Cancer Inst. 2000;92:1881-8. [ Links ]
4. Graham DY. Helicobacter pylori update: gastric cancer, reliable therapy and possible benefits. Gastroenterology. 2015;148:719-31. [ Links ]
5. Kuipers EJ. Review article: Relationship between Helicobacter pylori, atrophic gastritis and gastric cancer. Aliment Pharmacol Ther. 1998;(12)Suppl 1:25-36. [ Links ]
6. Correa P. Human gastric carcinogenesis: a multistep and multifactorial process – a first American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52(24):6735-40. [ Links ]
7. Sipponen P, Maaroos HI. Chronic gastritis. Scand J Gastroenterol. 2015;50(6):657-67. [ Links ]
8. Correa P, Piazuelo MB. Natural history of Helicobacter pylori infection. Dig Liver Dis. 2008;40(7):490-6. [ Links ]
9. Atherton JC. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu Rev Pathol. 2006;(1):63-96. [ Links ]
10. Weck MN, Gao L, Brenner H. Helicobacter pylori infection and chronic atrophic gastritis: associations according to severity of disease. Epidemiology. 2009;20(4):569-74. [ Links ]
11. De Vries AC, van Grieken NC, Looman CW, et al. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945-52. [ Links ]
12. Malfertheiner P, Megraud F, O´Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646-64. [ Links ]
13. Rugge M, Pennelli G, Pilozzi E, et al. Gastritis: the histology report. Dig Liver Dis. 2011;(43)Suppl 4:S373-84. [ Links ]
14. Lewin D, Lewin K. Stomach. En: Weidner N, Cote RJ, Suster S, et al (editores). Modern surgical pathology. 2.a edición. Canada: Expert Consult; 2009. p. 703-10. [ Links ]
15. Fenoglio-Preiser C. Creating a framework for diagnosing the benign gastric biopsy. Curr Diagn Pathol. 1998;5:2-16. [ Links ]
16. El-Zimaity HM. Recent advances in the histopathology of gastritis. Current Diag Pathol. 2007;13:340-8. [ Links ]
17. Correa P. Chronic gastritis: a clinic-pathological classification. Am J Gastroenterol. 1998;83(5):504-9. [ Links ]
18. Ricaurte O, Gutiérrez O. Gastritis crónica. En: Alvarado J, Otero W, Archila PE, et al. Gastroenterología y hepatología. 2.a edición. Bogotá: Editorial Médica Celsus; Sociedad Colombiana de Endoscopia Digestiva; 2006. p. 549-64. [ Links ]
19. Price AB. The Sydney System: histological division. J Gastroenterol Hepatol. 1991;6:209-622. [ Links ]
20. Dixon MF, Genta RM, Yardley HJ, et al. Classification and grading of gastritis. The updated Sydney System. Am J Surg Pathol. 1996;20:1161-81. [ Links ]
21. Dinis-Ribeiro M, Arela M, de Vries AC, et al. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endodcopia Digestiva (SPED). Endoscopy. 2012;44:74-94. [ Links ]
22. Sugimura T, Sugano H, Terada M, et al. First International Workshop of the Princess Takamatsu Cancer Research Fund: intestinal metaplasia and gastric cancer. Mol Carcinog. 1994; 11(1):1-7. [ Links ]
23. Rugge M, Cassaro M, Pennelli G, et al. Atrophic gastritis: pathology and endoscopy in the reversibility assessment. Gut. 2003;52:1387-88. [ Links ]
24. Rugge M, Correa P, Di Mario F, et al. OLGA staging for gastritis: a tutorial. Dig Liver Dis. 2008;40(8):650-8. [ Links ]
25. Rugge M, de Boni M, Pennelli G, et al. Gastritis OLGA-Staging and gastric cancer risk: a twelve-year clinico-pathological follow-up study. Aliment Pharmacol Ther. 2010;31:1104-11. [ Links ]
26. Capelle LG, de Vries AC, Haringsma J, et al. The staging of gastritis with the OLGA systems by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrintest Endosc. 2010;71(7):1150-8. [ Links ]
27. Marcos-Pinto R, Carneiro F, Dinis-Ribeiro M, et al. First-degree relatives of patients with early-onset gastric carcinoma show even at young ages a high prevalence of advanced OLGA/OLGIM stages and dysplasia. Aliment Pharmacol Ther. 2012;35:1451-9. [ Links ]
28. Anagnostopoulus GK, Yao K, Kaye P, et al. High-resolution magnification endoscopy can reliably identify normal gastric mucosa. Helicobacter pylori-associated gastritis and gastric atrophy. Endoscopy. 2007;39(3):202-7. [ Links ]
29. Pimentel-Nunes P, Dinis-Ribeiro M, Soares JB, et al. Multicenter validation of an endoscopic classification with narrow band imaging for gastric precancerous and cancerous lesions. Endoscopy. 2012;44(3):236-46. [ Links ]
30. Kikuste I, Stirna D, Liepniece-Karele, et al. The accrue of flexible spectral imaging color enhancement for the diagnosis of gastric intestinal metaplasia: do we still need histology to select individuals at risk of adenocarcinoma? Eur J Hastroenterol Hepatol. 2014;26:704-10. [ Links ]
31. Uedo N, Ishihara R, Iishi H, et al. A new method of diagnosing gastric intestinal metaplasia: narrow-band imaging with magnifying endoscopy. Endoscopy. 2006;38:819-24. [ Links ]
32. Tahara T, Shibata T, Nakamura M, et al. Gastric mucosal pattern by using magnifying narrow-band imaging endoscopy clearly distinguishes histological and serological severity of chronic gastritis. Gastrointest Endosc. 2009;70:246-53. [ Links ]
33. Kato M, Kaise M, Yonezawa J, et al. Magnifying endoscopy with narrow-band imaging achieves superior accuracy in the differential diagnosis of superficial gastric lesions identified with white-light endoscopy: a prospective study. Gastrointest Endosc. 2010;72:523-9. [ Links ]
34. World Health Organization (WHO). GLOBOCAN 2012: Estimated Incidence, Mortality and Prevalence Worldwide in 2012. Disponible en: http://globocan.iarc.fr/. [ Links ]
35. Isajevs S, Liepniece-Karele I, Janciauskas D, et al. The effect of incisura angularis biopsy sampling on the assessment of the gastritis stage. Eur J Gastroenterol Hepatol. 2014;26:510-3. [ Links ]
36. Zhang C, Yamada N, Wu YL, et al. Helicobacter pylori infection, glandular atrophy and intestinal metaplasia in superficial gastritis, gastric erosion, erosive gastritis, gastric ulcer and early gastric cancer. World J Gastroenterol. 2005;11:791-6. [ Links ]
37. Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64(9):1353-67. [ Links ]
38. Otero W, Trespalacios AA, Mercado M. Tasa de reinfección por Helicobacter pylori en una cohorte de pacientes colombianos tratados exitosamente con seguimiento superior a 2 años. Rev Col Gastroenterol. 2015;30:53-9. [ Links ]
39. Satoh K, Osawa H, Yoshizaewa M, et al. Assessment of atrophic gastritis using the OLGA system. Helicobacter. 2008;13(3):225-9. [ Links ]
40. Rugge M, Meggio A, Pennelli G, et al. Gastritis staging in clinical practice: the OLGA staging system. Gut. 2007;56:631-6. [ Links ]
41. Sipponen P, Graham DY. Importance of atrophic gastritis in diagnostics and prevention of gastric cancer: application of plasma biomarkers. Scand J Gastroenterol. 2007;42:2-10. [ Links ]
42. Yada T, Yokoi Y, Uemura N. The current state of diagnosis and treatment for early gastric cancer. Diagn Ther Endosc. 2013;2013:241320. [ Links ]











 text in
text in