Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.31 no.4 Bogotá Oct./Dec. 2016
For How Long Should Adjuvant Therapy for Gastrointestinal Stromal Tumors (GIST) Be Administered?
Ricardo Oliveros Wilches MD (1), Ana D. Bonilla Castañeda MD (1), Helena Facundo Navia MD (1), Â Ricardo Sánchez Pedraza MD (2)
(1) Gastrointestinal Surgery and Digestive Endoscopy Group at the National Institute of Cancer in Bogotá, Colombia
(2) Clinical Research Group at the National Institute of Cancer and the Faculty of Medicine of the National University of Colombia in Bogotá, Colombia
Received:Â Â Â 29-02-16Â Accepted:Â Â Â 01-11-16
Abstract
Surgery is the established treatment for patients with primary gastrointestinal stromal tumors (GIST) that are completely resectable. After surgery, up to 50% of patients suffer recurrences in the first two years. This is especially true for high risk patients. This is the justification for adjuvant therapy with imatinib (IMB). Based on clinical evidence, three years is the established treatment time for patients at high risk of recurrence. However, the same clinical studies also show that recurrences begin to be detectable six to nine months after discontinuation of the drug. We present the cases of three patients who underwent complete resection of GIST who then received imatinib for three years. Months after the medication was discontinued, recurrences required administration of the drug to be restarted. The optimal duration of adjuvant therapy with IMB has not been established, and it is unclear whether IMB actually cures the disease. For this reason, a review of how long adjuvant therapy with IMB should be administered and of what the effect of IMB on the disease really is was needed. In conclusion, on the basis of currently available evidence, we know that the recommendation is three years of treatment with IMB as adjuvant therapy, but, based on daily experience and expert recommendations, there are patients who probably need to continue treatment with imatinib for much longer while we wait for reports of the results of clinical studies of five year IMB treatment.
Keywords
Gastrointestinal stromal tumor, adjuvant therapy, imatinib, treatment, metastatic and/or advanced GIST, risk stratification, molecular profile, mutational state.
Introduction
Gastrointestinal stromal tumors (GIST) are the most frequent mesenchymal tumors of the gastrointestinal tract, with an estimated incidence of 10 to 20 cases per 1 million people. (1, 2) The site where it occurs most frequently is the stomach, followed by the small intestine, colon, rectum, and esophagus. They have also been described in the mesentery and retroperitoneum. GISTs originate from interstitial cells of Cajal which also regulate the rhythm of intestinal muscles. GISTs and interstitial cells of Cajal both express the KIT protein and have similar ultrastructural features. (2, 3)
Several studies have shown that the constitutive activation of the c-KIT receptor plays a central role in the pathogenesis of most GISTs. This occurs as a consequence of point mutations that can occur in both the extracellular and intracytoplasmic regions of receptor tyrosine kinases. (3, 4)
Management of GISTs is primarily surgical through complete resection of the lesion, but 30% to 50% of patients undergoing complete resection suffer recurrences, especially in the first 2 years. The 5-year survival rate of patients with completely resected GISTs is 50%. (5) These data indicate the need for adjuvant therapy.
Before the imatinib (IMB) era, patients with resectable GIST-like lesions underwent surgery and then observation since neither chemotherapy nor radiation therapy plays a role in the management of this pathology. (5, 6) In 2002, IMB was approved for management of metastatic GISTs. Later, it was approved for 12 month use as adjuvant management. (7) However, once the drug is discontinued, a significant proportion of patients experience recurrences of the disease which require renewed administration IMB which fortunately has had good responses. (8) Subsequently, it has been shown that 36 months of treatment with IMB is better than 12 month treatment although it was again observed that upon discontinuance recurrence is very likely. (9, 10)
It is known that the standard management in the care of patients with GIST who have a high risk of recurrence is systemic adjuvant therapy with IMB, but the optimal duration of treatment is still the object of debate. (11)
Molecular Pathology
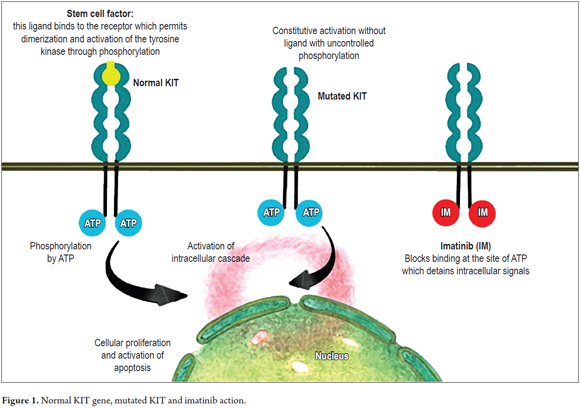
GISTs originate from the interstitial cells of Cajal. The KIT gene which is located on chromosome 4q11-21 encodes a type III tyrosine kinase receptor (KIT) to which stem cell factor binds. The binding of this ligand to the membrane receptor determines the dimerization that follows with the activation of the tyrosine kinase in the intracellular region. This in turn determines the activation of an intracellular cascade leading to the control of cell differentiation, proliferation and growth (Figure 1). (3, 12)
Eighty-five percent of GISTs have mutations that are activated at c-KIT, 5% of mutations are activated through platelet-derived growth factor (PDGF). The rest are known as wild type mutations because they do not contain mutations that have been identified. Mutations in KIT exon 11 are the most common and have b initial responses to treatment with imatinib. Mutations of KIT exon 9 are the second most common in this transmembrane receptor, and are associated with a different sensitivity or response to IMB and other tyrosine kinase inhibitors (TKIs). Mutations in KIT exons 13 and 17 have been less common described, and in some cases are related to primary or secondary resistance to IMB. (11, 13)
Mutations in PDGF exons 12, 14 and 18 have been found and are considered to be the cause of several GISTs that do not have mutations activated in KIT. They each have different prognoses based on responses to currently available medications. Wild-type GISTs without mutations in KIT or PDGF have increased expressions of insulin-like growth factor 1. Potentially, they have worse prognoses and are associated with a higher mitotic index, larger sizes and greater numbers of recurrences and metastases. (13, 14)
The development of molecularly targeted therapy has revolutionized the treatment of GIST. However, individual tumor analysis (molecular profiling) is the first step in maximizing treatment success since it allows the identification of patients who can benefit from such therapies. (13, 15)
Analysis of mutations can provide information that indicates IMB should not be used for treatment. PDGF D842V mutations, which are insensitive to IMB, are examples. On the other hand, analysis of mutations may suggest increases in the dosage of IMB, as is the case of exon 9 GIST mutations. Nevertheless, despite the benefits provided by mutational status information, there is no established therapeutic approach for each molecular subtype of GIST. The guidelines of most European countries recommend mutational analysis. In contrast, less than 10% of GIST patients in the USA have their molecular profile's analyzed. Mutational analysis should be as common as in other types of tumors such as those in the breast. (13) Ascertaining mutational status is recommended for all patients with GIST for whom tyrosine kinase inhibitor (TKI) therapy is proposed. (16)
Stratification of Risk
Because several risk factors are associated with tumor recurrence, risk stratification is essential for identifying patients with GIST who might benefit from adjuvant therapy. These risk factors are the mitotic index, tumor location, tumor size and whether a tumor has ruptured during surgical manipulation. (11, 17)
Complete surgical resection is possible in approximately 85% of these cases and is important for the average survival time of patients with GIST. The average survival time after complete resection is 66 months, but the average survival time after incomplete resection is only 22 months. (11, 17)
The first scheme for the determination of biological risk was the product of the 2001 National Institute of Health (NIH) consensus. It only considered tumor size and mitotic index to be risk factors. Later, this scheme was modified to take the work of Miettinen et al. at the Department of Soft Tissue Pathology, Armed Forces Institute of Pathology into account. This classification system included the location of the tumor within the prognosis scheme. Tumor rupture and non-gastric location were later considered to be independent prognostic variables in risk classification systems. (18)
In the modified classification system, patients with non-gastric GISTs smaller than or equal to 5 cm and with mitotic indices greater than 5x50 high power fields (HPF), those with non-gastric GISTs with sizes 5 to 10 cm and less than 5 mitosis per 50 HPF, and those with ruptured tumors had a risk of recurrence of over 50%. (11)
Miettinen et al. observed that patients with gastric GISTs larger than 10 cm and with mitotic indices less than 5 x 50 HPF had a recurrence rate of only 10%. Patients with similar tumors in the small intestine or rectum had a recurrence rate of more than 50%. This classification allowed the identification of low, intermediate and high risk patients of whom the latter would probably benefit from adjuvant therapy. (18)
After surgery, the five year recurrence rate may be 50%, but for large tumors it can be as high as 85% to 90%. (19) In 2009, Gold developed a nomogram for stratification of recurrence risk. Recently, Rossi and colleagues have developed a nomogram to predict overall survival. These stratification systems are the beginning of clinical research to measure the efficacy and safety of adjuvant therapy in patients at high risk of recurrence. (20, 21) To improve treatment of GISTs, the consensus is that it is necessary to add molecular analysis to risk stratification. (22)
Adjuvant Therapy
Surgery continues to be potentially curative therapy for primary GIST. The National Comprehensive Cancer Network (NCCN) guidelines recommend surgical resection of all lesions larger than two cm. Nevertheless, results after surgery are still poor due to the risk of recurrence. (10)
Management for patients with primary GIST aims to prevent recurrences and metastases. Survival at five years for patients with resected GISTs is approximately 50%, and the median time of recurrence is close to two years. These statistics support rational use of adjuvant therapy following surgery to reduce the risk of recurrence and improve the clinical course and outcomes of these patients. (23)
The use of IMB adjuvant therapy is supported by two studies by the American College of Surgeons Oncology Group (ACOSOG). (23)
The Z9000 study, the first of the two, was a multicenter, single arm study of 107 patients who were at high risk of recurrence. Patients received 400 mg of IMB for 1 year with significant improvement in recurrence-free survival (RFS) and also improvement in overall survival compared to historical controls at a 4-year follow-up. First year RFS was 94%, second year RFS was 73%, and third year RFS was 61%. Overall survival rates were 99%, 97% and 97%, respectively. Adjuvant IMB was well tolerated and 83% of the patients completed the treatment. The majority of adverse events were severity 1 or 2. (24)
The second study, ACOSOG Z9001, was a phase II, randomized, double-blind study of 713 patients who had lesions 3 cm or larger resected. Patients were randomized to receive 400 mg of IMB per day or placebos for 1 year. DeMatteo et al. demonstrated that 400 mg IMB per day orally for 1 year improved recurrence-free survival from 83% with placebos to 98% ( HR 0.35, 95% CI: 0.22- 0.53, p = 0.001). There was no statistically significant improvement in overall survival with IMB (HR 0.66, 95% CI: 0.22-2.03, p = 47). The study was discontinued as the result of interim efficacy analysis. and all patients receiving placebos were treated with IMB. (25)
Based on these results, in 2008 the FDA approved the use of IMB in the USA for adjuvant treatment of completely resected GISTs greater than 3 cm but did not provide definitive guidance about optimal duration of treatment or about which patients are most likely to benefit. (23)
These studies provide support for the recommendation to prolong adjuvant therapy. During the first year of treatment, there was only one recurrence in the arm with IMB, compared with 41 in the placebo arm. Moreover, six to twelve months after administration of IMB was suspended, recurrence numbers increased rapidly in the IMB arm. This raises the question of whether therapy with IMB beyond 1 year could extend recurrence-free survival (23).
A study by Joensuu et al. of the Scandinavian Sarcoma Group (SSG) has compared 12-month IMB therapy with 36 month IMB therapy (400 mg/day orally in 400 patients with high-risk GISTs). The median follow-up time was 54 months. It was found that three years of IMB treatment resulted in higher rates of RFS and overall survival than did one year of treatment. Five year RFS was 66% compared to 48% with an HR of 0.46 (95% CI: 0.32-0.65), and overall survival was 92% compared to 82%. Adverse events were more frequent in the long-term treatment group, but most of the effects were grade 1 or 2. These data established a minimum of 36 months of adjuvant IMB as a new standard for patients with high-risk GISTs. Despite this, doubts persist as to whether treatment should continue for more than 3 years. Recurrence rates increased in both groups six to twelve months after discontinuation of adjuvant IMB. This finding raises the question of whether recurrences are truly prevented or simply delayed. (9)
As previously noted, the optimal duration of IMB therapy still needs to be defined. At present, for patients at high risk of relapse, it is unknown whether the duration of therapy should be longer than three years and whether such patients should be maintained with adjuvant IMB continuously in a manner similar to patients with chronic myeloid leukemia. (CML). (23)
In patients with marginal or borderline lesions for which surgery may involve resection of neighboring organs, neoadjuvant therapy is recommended because it reduces surgical morbidity by reducing the size and vascularity of the lesion. Adjuvant therapy is indicated for at least three years after resection of the primary tumor in patients receiving neoadjuvant therapy. Therefore, the association between neoadjuvant and adjuvant therapy is not surprising. (16)
National Comprehensive Cancer Network (NCCN) clinical practice guidelines suggest adjuvant IMB for 36 months in patients with high-risk GISTs (tumors larger than five cm with mitotic index over 5 mitosis x 50 HPF) or a risk of recurrence over 50%. (19)
The risk of recurrence of GISTs is greater during the first and second years after discontinuation of adjuvant therapy. Restoring therapy during this period mitigates this risk. While the optimal duration of adjuvant therapy remains unknown, three years is better than one year in terms of recurrence-free survival and overall survival. Therefore, several authors recommend adjuvant therapy with IMB for three years as standard management for patients with high-risk resected GIST. (19)
Three drugs with clinical benefits for GISTS have been identified: imatinib, sunitinib and regorafenid. (26)
IMB has been effective for up to 8 years without evidence of recurrences. (9, 26) The efficacy of IMB varies among cases and depends on the sensitivity of the type of mutation. Risk estimation is of paramount importance when selecting patients who might benefit from IMB therapy. (11)
Le Cesne et al. have reported the results of a randomized study of patients with GISTs who maintained control of their metastatic disease during three years of treatment with IMB. Patients were randomized into groups that continued or discontinued treatment. The mean time to progression was nine months after allocation to the untreated group. After a 35-month follow-up after randomization, the RFS at 2 years was 80% in the group that continued with the drug compared to 16% in the group who discontinued it. Fortunately, reintroduction of the IMB was associated with 100% tumor control in the group for whom the drug was discontinued. This study can be assimilated into adjuvant management since they were patients with metastatic disease. (27)
PERSIST-5, a non-randomized, single-arm, phase II study is currently underway to evaluate RFS after complete resection and five years of adjuvant IMB treatment (400 mg orally per day). The study includes patients with completely resected high risk GISTs. The main objective of the study is to evaluate RFS. Preliminary reports from this ongoing study suggest a benefit from extended IMB therapy. After 44-month follow-up, a significant improvement in RFS was observed in patients taking IMB. The RFS numbers for those receiving IMB (35 patients) compared to follow-up (55 patients) were 100% compared to 70.9% for the first year; 88% compared to 37.8% for the second year and 88% compared to 27.5% for the third year (HR: 0.122; 95% CI: 0.041-0.363; p = 0.000). Data are not yet reported for 5 years or more; However, this information supports extending therapy with IMB for at least three years to prolong RFS of patients at high risk of recurrence. (28)
One of the objectives of the BFR14 phase III study of the French Sarcoma Group was to compare the effects of continuing adjuvant therapy with IMB with the effects of discontinuing it. This study was performed on patients with advanced GISTs by discontinuing therapy after one, three, or five 5 years of daily treatment with 400 mg oral IMB. The risk of progression was found to increase with IMB discontinuation. Moreover, patients whose disease progressed after discontinuation of the drug did not always achieve the same degree of tumor control as they had had prior to discontinuation of therapy. (29) The authors of the BRF14 study conclude that 5 years of IMB is not sufficient in patients with advanced GISTs to achieve complete remission, and wonder if these findings could be similar for adjuvant therapy use. (30, 31)
Below we presents the cases of three patients with high-risk GISTs treated with surgery and adjuvant therapy IMB that was discontinued after three years of administration. These patients relapsed in the months after discontinuation and improved after medication was restarted. This raises the questions of whether IMB therapy in patients with GISTs with high risks of recurrence and responders should continue beyond 36 months and, if so, for how much longer.
Case 1
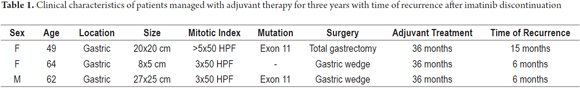
The patient was a 49 year-old woman with high risk of gastric GIST due to an exon 11 mutation. She was treated with total gastrectomy, splenectomy, distal pancreatectomy, and partial colectomy. She received IMB from September 2010 to November 2013. In January 2015, hepatic metastases were detected and IMB was restarted in April 2015, with partial response at the last check-up in December 2015 (Table 1).
Case 2
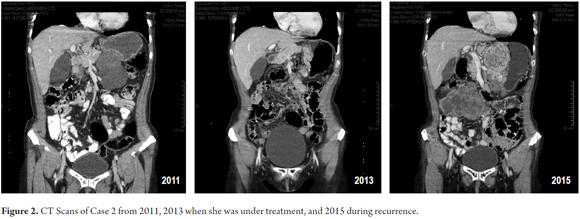
The patient was a 64-year-old woman with gastric GIST larger than ten cm who was treated with neoadjuvant IMB for 7 months. After good response reduced the size of the lesion, an 80% gastrectomy was performed. Subsequently, she received IMB from February 2012 until February 2015. After 6 months, recurrence was detected. Sunitinib was prescribed, but she did not tolerate it, so IMB was reinitiated with adequate tolerance and reduction in recurrence size at her last check-up in December 2015 (Figure 2).
Case 3
The patient was a 62-year-old man with gastric GIST and a mutation of exon 11 who was treated outside of our institution with a gastric wedge followed by imatinib for 3 years. He relapsed six months after suspension of the medication. He received nilotinib for eight months which resulted in a significant reduction in the size of the lesion. In November 2014 he underwent splenectomy, resection of 10 cm of small intestine, partial resection of the left diaphragm and gastric wedge. Since that time he has received imatinib with adequate clinical control and no recurrences as of his last check-up in December 2015 (Table 1).
Discussion
The optimal duration of adjuvant therapy with IMB is not yet known. Although surgery is the primary treatment for resectable GISTs, 30% to 50% of patients develop recurrences within the first five years, while those at high risk do so earlier. Survival of patients with metastatic GISTs has increased by up to three times since the introduction of tyrosine kinase inhibitors. (17, 32)
The selection of patients for adjuvant therapy should be based on one of the stratification schemes. Physicians should analyze the risks of recurrence for each patient on the basis of factors such as lesion size, location, mitotic index, and whether or not the tumor ruptured during surgery. Mutational analysis is important when deciding adjuvant treatment, but it is not taken into account by any of the systems of risk stratification. (17, 32)
The three patients presented here all had lesions larger than 10 cm (20x20 cm, 27x25 cm, larger than 10 cm). One of them received neoadjuvant IMB with a significant reduction (8x5 cm) of the lesion by the time of surgery.
The first patient would have benefited from this type of neoadjuvant treatment to reduce the size and vascularization of the lesion and avoid the need for organ resection due to contiguity. We propose that patients with lesions greater than 10 cm who have high mitotic indices first receive neoadjuvant treatment before surgery and continue with IMB for three years.
Similarly, two of the patients presented had mutations in exon 11 which respond well to IMB. In the first case, after recurrence was detected, the administration of the medication was resumed with partial response of the lesions. In the third case, after complete surgical resection was achieved, administrati0on of the drug resulted in excellent control with no new recurrence.
Some patients at high risk of recurrence, despite macroscopically complete surgeries, may benefit from adjuvant therapy with IMB for periods greater than three years. This hypothesis still has little supporting evidence which together with risks derived from the intake of the drug when it might not be needed mitigates against this practice. For these reasons, two ongoing European randomized trials are comparing adjuvant treatment with IMB for more than three years with standard three years treatment. (28)
Joensuu, who successfully treated the first patient with metastatic GIST in 2000, has said that at this time, "the standard duration of adjuvant IMB is three years". (33) Longer duration treatments have not proven to be beneficial, and there is no information from clinical studies of treatments of more than three years. For this reason, it is recommended that patients be checked through frequent imaging following suspension of medication in order to detect any recurrence as early as possible. Recurrences of GIST generally responds well to the resumption of IMB and risk of secondary resistance is very low. (30)
Currently, it is important to remember that one of the causes of recurrence is a second mutation, in which case the recommendation is to increase the dosage of IMB or move to a second line of treatment. Adjuvant therapy and the proper duration of that therapy are still being discussed, so after complete surgery with a high-risk of relapse, duration of adjuvant treatment must be decided upon. When the drug is discontinued, the patient suffers a recurrence six to nine months later which responds very well to the resumption of the medication. The question is: Should IMB simply be continued without ever being suspended?
There are records of patients at high risk of recurrence, progressing after IMB administration has been discontinued after three years. Whether or not to continue medication is a complicated decision, so individual variables that could measure benefit should be used to help patients and physicians make better informed decisions about continuing treatment. (30)
No clinical studies of patients treated with IMB for more than five years of treatment have been reported. Most GISTs recur after treatment is stopped. IMB should be administered continuously until the disease progresses, since the interruption of treatment results in progression within the first year. (34) For other authors, adjuvant therapy for a longer time is warranted if it is certain that the risk of recurrence is greater than 50%. (30)
The first two patients presented did not have residual disease after surgery but received three years of IMB management. When the drug was discontinued, they both consulted because of relapses of their disease. Currently, they receive IMB and show partial clinical responses. Should the drug be continued indefinitely? If the answer is yes, the third patient without current evidence of relapse should not discontinue the IMB.
According to findings, the pharmacological inhibition of c-KIT is insufficient to induce apoptosis and eradication of the mutant stem cells. Clinical and experimental findings support the need for continuous treatment with tyrosine kinase inhibitors. Discontinuation of treatment is associated with rapid repopulation of cancer cells differentiated from an intact population of stem and progenitor cells. (35)
It is thought that during administration of imatinib stem and progenitor cells are dormant, but that once the drug is discontinued the cells wake up. IMB binds to the activation sites of adenosine triphosphate (ATP), blocking phosphorylation and activation of the intracellular cascade. Once the drug is discontinued, its action disappears, so patients can respond for years, but this treatment usually does not cure the disease completely (Figure 1). (36)
If evidence is found that adjuvant IMB therapy cures patients with GIST, a finite period of therapy may become possible. Nevertheless, studies show that by discontinuing IMB, recurrences rapidly begin to appear. (30)
TKIs can improve the duration of disease control, but cannot cure the condition. Therapies are needed that can eradicate the initial stem cells in order to avoid the need for treatment with TKIs such as IMB. (35)
The advent of IMB therapy for metastatic GISTs has dramatically improved patients' prognoses. Therefore, if there is a clinical benefit, treatment is recommended indefinitely since interruption of treatment is related to relapse or disease progression. (37, 38)
Conclusions
The optimal duration of adjuvant therapy with IMB in patients with GIST is still unknown, but there are some groups of patients who can benefit from lifelong therapy and prolonged adjuvant therapy does not significantly increase treatment-related adverse effects.
Adjuvant treatment with IMB, the first-line adjuvant drug in the treatment of GIST, has shown a significant reduction in the risk of recurrence.
Recurrence-free survival correlates with the duration of adjuvant treatment. The risk stratification criteria should establish whether patients with a high risk of recurrence will benefit from adjuvant treatment and will probably also benefit from longer treatment.
Patients whose risk of recurrence is greater than 50% should be considered for adjuvant treatment with IMB longer than three years even though sufficient clinical evidence supporting this recommendation has not yet been established. The mutational state of KIT has a significant impact on the response to treatment. IMB may improve disease control but does not cure the disease.
References
1. Corless C L. Gastrointestinal stromal tumors: what do we know now? Modern Pathology. 2014;27:S1-S16. [ Links ]
2. Ogata K, Mochiki E, Ojima H, et al. A Multicenter long term study of Imatinib treatment for japanese patients with unresectable or recurrent gastrointestinal stromal tumors. J Surg Oncol. 2014;110:942-6. [ Links ]
3. Hirota S, Isozaki K, Moriyama Y, et al. Gain of function mutation of c-KIT in human gastrointestinal stromal tumors. Science. 1998;279:577-80. [ Links ]
4. Cioffi A, Maki RG. GI stromal tumors: 15 years of lessons from a rare cancer. J Clin Oncol. 2015;33:1849-54. [ Links ]
5. Zhong JH, Ma L, Li LQ, et al. Adjuvant Imatinib for gastrointestinal stromal tumors: the current situation and problems. Scand J Of Gastroent. 2011;46:645-51. [ Links ]
6. Miettinen M, Makhlouf H, Sobin LH, et al. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, inmunohistochemical and molecular genetic study of 906 cases before imatinib with long term follow up. Am J Surg Pathol. Abril 2006;30(4):477-89. [ Links ]
7. Caram M, Schuetze S. Advanced or metastasic gastrointestinal stromal tumors: systemic treatment options. J Surg Oncol. 2011;104:888-95. [ Links ]
8. Blackstein ME, Corless CL, Ballman KV, et al. Risk assessment for tumor recurrence after surgical resection of localized primary gastrointestinal stromal tumor (GIST): North American Intergroup phase III trial ACOSOG Z9001. Presented at the 2010 Gastrointestinal Cancers Symposium: 22-24de enero de 2010; Orlando, FL, EE. UU. [ Links ]
9. Joensuu H, Eriksson M, Sundby HK, et al. One vs. three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. Marzo 2012;307(12):1265-72. [ Links ]
10. Maki R, Blay JY, Demetri G, et al. Key issues in the clinical management of gastrointestinal stromal tumors: an expert discussion. The Oncologist. 2015;20:823-30. [ Links ]
11. Reichardt P, Joensuu H, Blay JY. News fronts in the adjuvant treatment of GIST. Cancer Chemother Pharmacol. 2013;72:715-723. [ Links ]
12. Demetri G, Titton RL, Ryan DP, et al. Case 32-2004: a 68 year old man with a large retroperitoneal mass. N Engl J Med. 2004;351(17)21:1779-87. [ Links ]
13. Blay JY, Casali PG, Dei Tos AP, et al. Management of gastrointestinal stromal tumour: current practices and visions for the future. Oncology. 2015;89:1-13. [ Links ]
14. Demetri G, Mehren M, Antonescu C, et al. NCCN Task Force Report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8(2):S1-41. [ Links ]
15. ESMO. European Sarcoma Network Working Group: Gastrointestinal stromal tumors. ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(l7):vii49-55. [ Links ]
16. Bischof DA, Dodson R, Jimenez MC, et al. Adherence to guideliness for adjuvant imatinib therapy for GIST: a multi-institutional analysis. J Gastrointest Surg. 2015;19:1022-8. [ Links ]
17. Sicklick JK, Lopez N. Optimizing surgical and imatinib therapy for the treatment of gastrointestinal stromal tumors. J Gastroint Surg. 2013;17:1997-2006. [ Links ]
18. Miettinen M Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23(2):70-83. [ Links ]
19. Telzlaff E, Davey M. Optimizing adherence to adjuvant Imatinib in gastrointestinal stromal tumor. J Adv Pract Oncol. 2013;4(4):238-50. [ Links ]
20. Gold JS, Gonen M, Gutierrez A, et al. Development and validation of a prognostic nomogram for recurrence free survival after complete surgical resection of localized primary gastrointestinal stromal tumor: a retrospective analysis. Lancet Oncol. 2009;10(11):1045-52. [ Links ]
21. Rossi S, Miceli R, Messerini L, et al. Natural history of imatinib naive GISTs: a retrospective analysis of 929 cases with long term follow and development of a survival nomogram base don mitotic index and size as continuous variables. Am J Surg Pathol. 2011;35:1646-56. [ Links ]
22. Pracucho EM, Lopes LR, Zanatto RM, et al. Profile of patients with gastrointestinal stromal tumors. Arq Bras Cir Dig. 2015;28(2):124-7. [ Links ]
23. Trent JC, Subramanian MP. Managing GIST in the Imatinib era: optimization of adjuvant therapy. Expert Rev Anticancer Ther. 2014;14(12):1445-59. [ Links ]
24. Dematteo RP, Ballman KV, Antonescu CR, et al. Long term results of adjuvant imatinib mesylate in localized high-risk primary gastrointestinal stromal tumor. ACOSOG Z9000 Intergroup phase 2 Trial. Ann Surg. 2013;258(3):422-9. [ Links ]
25. Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant Imatinib mesylate after resection of localized primary gastrointestinal stromal tumours: a randomized, double-blind placebo controlled trial. Lancet. 2009:373(9669):1097-104. [ Links ]
26. Niazi AK, Kaley K, Saif W. Gastrointestinal stromal tumor of colon: a case report and review of literature. Anticancer Research. 2014;34:2547-50. [ Links ]
27. Le Cesne A, Coquard IR, Bui BN, et al. Discontinuation of Imatinib in patients with advanced gastrointestinal stromal tumors after 3 years of treatment: an open label multicenter randomized phase 3 trial. Lancet Oncol. 2010;11:942-9. [ Links ]
28. Novartis Pharmaceuticals. Five year adjuvant Imatinib mesylate (Gleevec) in gastrointestinal stromal tumor (GIST). 2012. Disponible en: http//clinicaltrials.gov/ct2/show/NCT00867113 [ Links ]
29. Ray Coquard IL, Bin Bui N, Adenis A, et al. Risk of relapse with Imatininb (IM) discontinuation at 5 years in advanced GIST patients: results of the prospective BFR14 randomized phase III study comparing interruption versus continuation of IM at 5 years of treatment: a French Sarcoma Group Study. J Clin Oncol (Meeting Abstracts). 2010;28:10032. [ Links ]
30. Knox P. Adjuvant Imatinib study updates results. 15 de diciembre de 2015. Disponible en: https://liferaftgroup.org/2015/12/adjuvant-imatinib-study-updates-results/ [ Links ]
31. Le Cesne A, Blay J Y, Reichardt P, et al. Optimizing tyrosine kinase inhibitor therapy in gastrointestinal stromal tumors: exploring the benefits of continuous kinase suppression. The Oncolgyst. 2013;18:1192-9. [ Links ]
32. Reichardt P, Blay JY, Boukovinas I, et al. Adjuvant therapy in primary GIST: state of the art. Ann of Oncol. 2012;23:2776-81. [ Links ]
33. Joensuu H, Roberts PJ, Sarlomo Rikala M, et al. Effect of the tyrosine kinase inhibitor ST1571 in a patient with a metastasic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052-6. [ Links ]
34. Joenssu H, Hohenberger P, Corless CH. Gastrointestinal stromal tumor. Lancet. 2013;382:973-83. [ Links ]
35. Heinrich MC. Imatinib treatment of metastasic GIST: dont stop (believing). Lancet Oncol. 2010;11:910-1. [ Links ]
36. Bauer S, Fletcher J. El descubrimiento de nuevas terapias es posible si comprendemos como se desarrolla un GIST. Disponible en: http://alianzagist.org/assets/nuevas-terapias.pdf [ Links ]
37. Balachandran VP, DeMatteo RP. Targeted therapy for cancer. The Gastrointestinal Stromal Tumor Model. Surg Oncol Clin N Am. 2013;22:805-21. [ Links ]
38. Balachandran VP, DeMatteo RP. Gastrointestinal stromal tumors. Who should get imatinib and for how long? Advances in Surgery. 2014;48:165-83. [ Links ]











 text in
text in