Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.31 no.4 Bogotá oct./dic. 2016
Useful Algorithms for Histopathological Diagnosis of Liver Disease Based on Patterns of Liver Damage
Rocío del Pilar López Panqueva MD (1)
(1) Pathologist at Hospital Universitario Fundación Santa Fe de Bogotá and Universidad de Los Andes in Bogotá, Colombia
Received:Â Â Â 01-11-16Â Accepted:Â Â 15-11-16
Abstract
Previous articles have reviewed the most frequent liver pathologies from the morphological point of view and looked at the importance of adequate correlation for obtaining better understanding between clinicians and pathologists. The next exercise is directed toward histopathological diagnosis of some of the liver diseases for which biopsies are performed most frequently. It takes into account algorithms based on patterns of liver damage.
Keywords
Liver biopsy, algorithms, patterns, portal infiltrates, granulomas, duct reaction, cholangiolar proliferation, lobular injury, pigments, fibrosis, cirrhosis, steatosis, hepatic mass.
PATTERNS OF LIVER DAMAGE
A simple description of what is observed on a microscope slide with a slice of a liver biopsy mounted on it can have very limited diagnostic value. The pathologist must ensure optimal management of the biopsy, proper fixation, and proper processing and must understand additional histochemistry, immunohistochemistry and molecular studies that can add value to the study to finally identify and analyze the type of liver injury present in the tissue sample and to finally make a diagnosis. Fitting the morphological findings of a lesion within one of the patterns of liver damage, always correlated with clinical information, is key to diagnosis.
The liver's response to injury is limited, no matter what cause triggers the damage. It should be remembered that morphological changes are summarized into cellular edema (ballooning), steatosis, cholestasis, necrosis and/or apoptosis, inflammation, regenerative changes and architecture alteration with or without fibrosis. These characteristics, presented in isolation or several simultaneously, give rise to patterns of liver damage which are distinctly morphological. Each encompasses several differential diagnoses. Analyzed in an algorithmic way, they guide diagnosis. Used together with the clinical history, they can achieve very accurate diagnoses.
Most Important Patterns:
- Pattern I, cellular infiltrates
- Pattern II, ductal reaction
- Pattern III, minimal or almost normal changes
- Pattern IV, injury or lobular injury
- Pattern V, steatosis
- Pattern VI, fibrosis
- Pattern VII, presence of masses
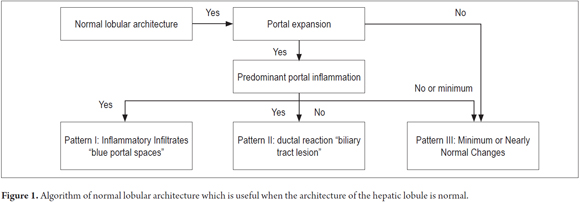
The first steps are to identify whether or not there are any alterations of normal lobular architecture and to determine the existence of portal spaces with presence of the normal structures of the portal triad, central vein and uniformly distributed hepatocellular trabeculae (Figure 1).
PATTERN I: INFLAMMATORY INFILTRATES
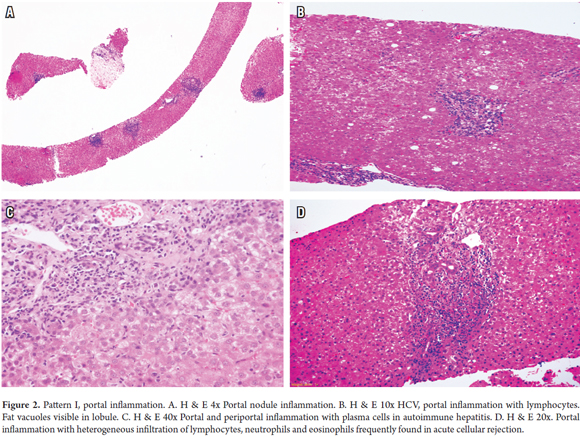
One of the most frequent findings in hepatic pathology is the presence of portal inflammatory infiltrates which are observed in a wide variety of liver diseases that mainly include necroinflammatory diseases, such as viral hepatitis, autoimmune and drug induced hepatitis. They are also found in chronic cholestatic diseases such as primary biliary cholangitis, primary sclerosing cholangitis, metabolic diseases such as Wilson's disease, α1 antitrypsin deficiency and neoplastic conditions, such as lymphoma infiltration, leukemia and post-transplant lymphoproliferative syndrome (Figure 2A).
After identifying the inflammatory infiltrates pattern of portal expansion, it is important to consider other keys for histopathological diagnosis such as the type of infiltrate, the pattern of inflammation of the limiting plaque or lobule, association with necrosis and presence of fibrosis. Some of these characteristics were discussed above and will be summarized below.
Inflammation of Portal Lymphocytes
This is observed mainly in chronic hepatitis B and C, acute hepatitis caused by hepatotropic viruses, non-hepatotropic viruses and drug toxicity (Figure 2B) (1, 2).
Chronic hepatitis C
- Inflammation of portal lymphocytes with lymphoid aggregates or follicles with germinal centers
- Interface hepatitis
- Lobular hepatitis with rows of lymphocytes
- Hepatocellular necrosis and/or apoptosis
- Macrovesicular steatosis
- Fibrosis depending on the stage
Hepatitis crónica C
- Inflamación portal linfocitaria
- Hepatitis de interfase
- Hepatitis lobulillar
- Necrosis y/o apoptosis hepatocelular
- Células en vidrio esmerilado
Chronic hepatitis B
- Inflammation of portal lymphocytes
- Interface hepatitis
- Lobular hepatitis
- Hepatocellular necrosis and/or apoptosis
- Ground glass cell appearance
Portal inflammation with plasma cells
Portal inflammation with a predominance of plasmocytes occurs in autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, IgG4 disease, transplant pathologies, recurrent diseases such as autoimmune hepatitis and biliary cholangitis, and late cellular rejection rich in plasma cells (Figure 2C). (3-6)
Autoimmune hepatitis
- Portal infiltrate with predominance of very dense plasma cells
- Interface hepatitis
- Formations of hepatic liver cell rosettes
- Lobular hepatitis
- Hyaline globules in Kupffer cells
Portal inflammation with eosinophils
There are numerous liver diseases in which eosinophils are present, but when they are predominant they do not necessarily imply toxicity or drug hypersensitivity. Also, systemic conditions with eosinophilia, eosinophilic gastroenteritis or infrequent parasitic diseases such as schistosomiasis of the portal system can produce eosinophilic abscesses. The differential diagnosis also includes some tumors and pseudotumors that compromise the liver such as Langerhans cell histiocytosis, inflammatory pseudo-tumors, myofibroblasts tumors, and post-transplant acute cellular rejection (Figure 2D). (7, 8)
Mixed portal inflammation with lymphocytes, plasma cells and neutrophils or polymorphonuclear eosinophils
This condition is characteristic of acute cellular rejection, Hodgkin's disease, viral hepatitis and drug-induced hepatitis.
Inflammation with granulomas
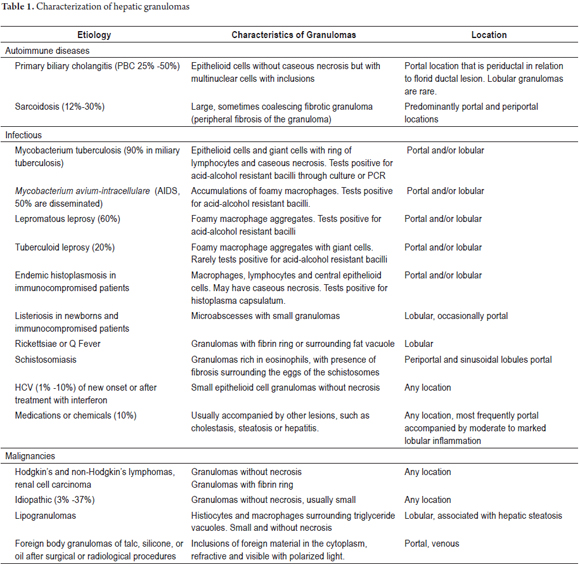
The prevalence of granulomas ranges from 2.4% to 15%, of which more than 60% is secondary to systemic processes, one third to primary hepatic conditions while 6% to 10% are idiopathic. (7, 9, 10)
Granulomas can be located in the portal region, in the parenchyma, or in both. The location and characteristics of granulomas can guide diagnosis. Table 1 summarizes some of these characteristics.
PATTERN II: DUCTAL REACTIONS
Previously, the term ductal or cholangiolar proliferation was used to refer to the proliferation of small bile ducts in the periphery of the portal tracts, but this pattern not only includes the proliferation of cholangioles, but also changes in the portal stroma, at the interface and in the parenchyma. The combination of these elements helps define diseases that are characterized by prominent ductal reactions. It should be remembered that hepatic progenitor cells react to damage to bile ducts and hepatocytes and that interface biliary metaplasia of hepatocytes represents a regenerative process. When there is an important ductal reaction, it is related, in the first instance, to acute or chronic biliary tract diseases. (11, 12)
Prominent Ductal Reaction
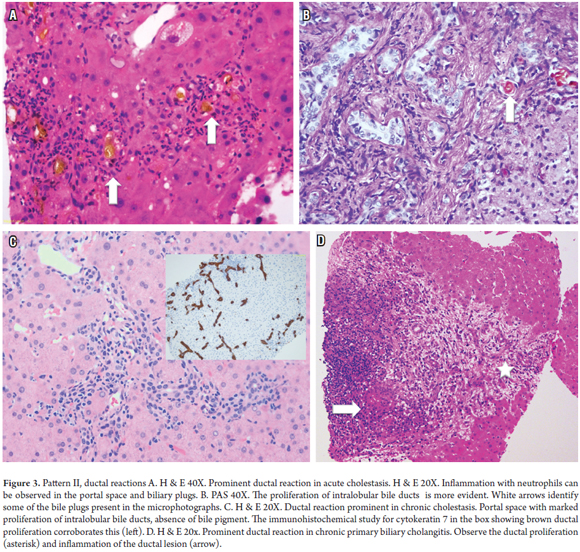
Acute or chronic biliary tract blockage due to stones, parasites, fibrosis, or masses does not usually require a biopsy because the clinical and/or imaging diagnosis is usually clear. Blockages are consistently found in post-transplantation biopsies and during autopsies. Other causes of ductal reactions are ascending cholangitis, slow cholangitis, acute hepatitis with submassive or massive necrosis, and chronic cholestatic diseases such as primary biliary cholangitis and primary sclerosing cholangitis. Biliary ductal reaction is defined as the proliferation of bile ducts accompanied by neutrophils and is divided into three types: Type I is associated with biliary obstructions, Type II is related to active hepatitis, and Type III is associated with massive hepatic necrosis. This terminology is only useful for guiding diagnosis of possible etiologies (Figure 3). (13-15)
Acute biliary obstruction
- Proliferation of ducts that appear to anastomose with small lights
- Marked edema of the surrounding stroma
- Variable neutrophil infiltrate
- Variable hepatocellular and ductal cholestasis
- Biliary infarction in severe obstructions
Ascending cholangitis
- Proliferation of cholangiocytes with presence of intraluminal neutrophils
- Acute inflammation of the ductal epithelium
- Dilation of the duct with attenuation of the epithelium
- Variable hepatocanalicular cholestasis
- Periportal biliary infarctions are common
Slow cholangitis
Slow cholangitis occurs in patients with impending sepsis or sepsis, obstruction of the biliary tract or as the result of endotoxins. It is considered a medical emergency and is characterized by ductal proliferation with dilated ducts filled with bile (ductal cholestasis), bile concretions in the portal space or interface zone and minimal inflammation.
Acute Hepatitis with Massive or Submassive Necrosis
- Prominent ductal reaction due to activation of the stem cells for regeneration of parenchyma and restoration of its function
- Minimal edema
- Mild inflammation, usually due to neutrophils
- Macrophages with PAS material which tests positive for diastase
Chronic Obstruction and Chronic Cholestasis
- Cholangiolar proliferation
- Minimal edema
- Slight inflammation due to lymphocytes
- Effect of clear halo in periportal or septal region
- Hepatobiliary xanthomatosis (cholestasis)
- Periportal copper deposit
- Periductal fibrosis
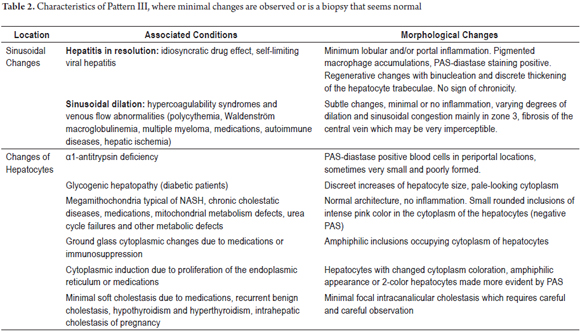
PATTERN III: NEARLY NORMAL WITH MINIMAL CHANGES
About 5% of biopsies have no specific changes but may have minimal portal or lobular inflammatory infiltrates. There is no evidence of fibrosis or structural changes. In 80% of these patients, there are persistent anomalies in liver function tests and 20% have unexplained ascites which requires a biopsy.
It is possible to define an etiology for approximately half of the patients who have adequate clinical histories and paraclinical studies. These include hepatic compromises due to systemic autoimmune conditions such as systemic lupus erythematosus and rheumatoid arthritis (15%), autoimmune hepatitis, primary biliary cholangitis (10%), metabolic syndrome (10%), chronic inflammatory conditions of the gastrointestinal tract (5%), hepatic ischemia (1% -5%) and non-cirrhotic portal hypertension (1%). Many of these pathologies are heterogeneous and focal including chronic cholestatic diseases and fatty liver disease. Others have periods of latency and inactivity during their clinical courses.
In some cases, especially in entities that cause non-cirrhotic portal hypertension, vascular changes are subtle and focal. Sinusoidal dilation, dilated portal vessels or those with fibrosis can easily pass unnoticed. In 25% of cases, functional abnormalities will normalize and in another 25% they will persist for many years. Table 2 summarizes probable diagnoses and describes morphological changes found when specific lesions are not identified in the biopsy and when a liver biopsy appears to be normal or almost normal (Figure 4). (7)
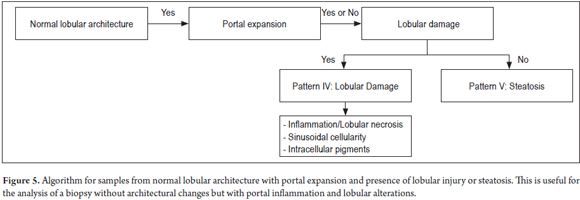
PATTERN IV: LOBULAR DAMAGE
Lobular Inflammation and Damage
Figure 5 shows an algorithm for normal lobular architecture and portal expansion requiring search for lobular injury or steatosis.
When a lobule is viewed under low magnification, the lobule looks restless. This is due either to occupation by inflammatory cells or because of necrosis which would indicate an injury or lobular damage. There are multiple possibilities which require detailed study with greater magnification to find additional keys to guide later diagnosis together with the medical history.
Some examples of this pattern are acute hepatitis; subacute hepatitis; non-hepatotropic hepatitis such as cytomegalovirus, herpes virus, adenovirus, Epstein-Barr and hepatitis E; compromises due to systemic diseases such as Celiac disease, lymphomas, leukemia, hypervitaminosis A and when pigments of lipofuscin, copper or iron are found. (16-20)
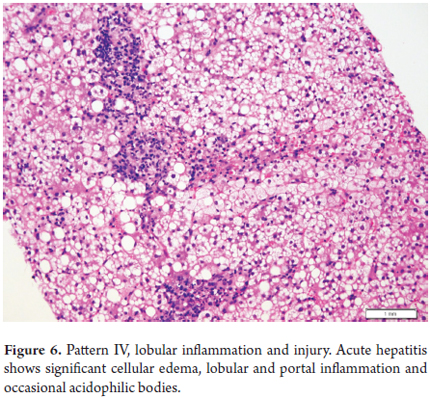
Acute Hepatitis
A biopsy is normally not indicated for acute hepatitis except in rare cases such as when there is doubt or error in the clinical diagnosis and when, despite a well-established diagnosis, the clinician needs more accurate information on the stage or severity of the disease, and following a transplant. Moderate and severe cases of acute hepatitis show prominent lobular inflammation, mild lymphocytic portal inflammation, hepatocellular edema (ballooning), isolated necrosis or apoptosis dispersed in the lobule, acidophilic bodies, accumulations of macrophages with material that is PAS-diastase positive and hepatocanalicular cholestasis (Figure 6).
Non-Hepatotropic Viral Hepatitis
Cytomegalovirus (CMV)
CMV occurs in immunosuppressed patients and presents with portal and lobular inflammation with lymphocytes, neutrophils, microabscesses of polymorphonuclear neutrophils located in the lobule, and viral inclusions of CMV without zonal distribution.
Herpes Simplex
Hepatitis secondary to infection with herpes simplex virus is similar to CMV hepatitis, is rare, and is seen in immunosuppressed patients. It produces inflammation and scattered patchy necrosis in the lobule and viral or nuclear amphiphilic or eosinophilic inclusions with a peripheral ring due to chromatin margination.
Adenovirus
Adenovirus infections are rare and usually fatal and in immunosuppressed patients. The virus is similar to herpes virus, and infections present large areas of parenchymal involvement with necrosis but without zonal distribution, lymphocytic inflammation at the extremities of the necrosis zones and presence of adenovirus inclusions.
Sinusoidal Cells
An increase of cells in the sinusoidal space can be a subtle finding that requires analysis with high magnification. When sinusoidal cellularity is very noticeable, one must think of a serious infection or neoplasia.
Celiac disease, Epstein-Barr hepatitis, lymphoma, leukemia, extramedullary hematopoiesis, hypervitaminosis A and lipidosis of stellate cells are the most frequent reasons for increased sinusoidal cellularity.
Celiac Disease
Up to 30% of patients at the time of diagnosis of Celiac disease have compromised livers. Findings range from normal appearance to lobular hepatitis and changes suggestive of autoimmune diseases such as autoimmune hepatitis, biliary cirrhosis and primary sclerosing cholangitis associated , with or without fibrosis. (19) Sinusoidal lymphoid inflammatory cells, scattered apoptotic cells, mild portal inflammation of lymphocytes, changes in fat and nodular regenerative hyperplasia are likely.
Epstein-Barr Hepatitis
Epstein-Barr hepatitis is most frequent among immunosuppressed patients, but it can also occur in apparently healthy young individuals. (20, 21) It is characterized by numerous sinusoidal lymphoid inflammatory cells simulating a predominantly lobular mononucleosis-like syndrome, small epithelioid cell granulomas with fibrin rings, absence of apoptosis or very occasional and dispersed apoptosis, mild lymphocyte portal inflammation and is positive for localized hybridization.
Lymphoma and Leukemia
Most case of hepatitis due to lymphoma and leukemia are secondary. They present atypical sinusoidal lymphoid and/or portal cells. Immunohistochemical studies and/or flow cytometry are essential for establishing the phenotype.
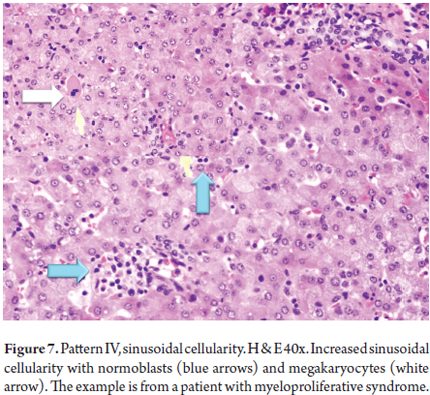
Extramedullary Hematopoiesis
Extramedullary hematopoiesis is a compensatory mechanism through which blood cells are produced outside the bone marrow when production in the bone marrow is unable to maintain the needs of the organism. Extramedullary hematopoiesis can be observed in a variety of hematological disorders such as myeloproliferative diseases, chronic anemia in neonates and children with giant hemocytes and other pathologies (22, 23). Normoblasts, megakaryocytes, evidence of myelopoiesis, and erythropoiesis in the sinusoidal space are commonly found (Figure 7).
Hypervitaminosis A or Lipidosis of Stellate Cells
In normal livers, vitamin A is stored in stellate liver cells which activate and acquire a phenotype similar to myofibroblasts which produce the extracellular matrix. Toxicity or hypervitaminosis can be serious and can even lead to cirrhosis. (24, 25) Hypervitaminosis A is characterized by prominent stellate cells in sinusoids in Dissé's space, crescent-shaped cells with dark nuclei which appear like an image of a starry sky due to expansion of lipid vacuoles, proliferation of myofibroblasts, and subsinusoidal fibrosis.
Intracellular Pigments
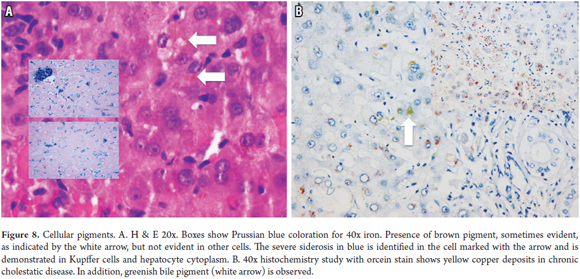
Intracellular pigments are most frequent in the cytoplasm of hepatocytes. They are fine or coarse granular aggregates that acquire various colorations and that may require special histochemistry studies to determine the etiology. The most frequent are deposits of lipofuscin, iron, copper or bile. (Figure 8)
Lipofuscin
Lipofuscin is a normal constituent of adult livers, especially during the fifth and sixth decades of life. It is also deposited in chronic liver diseases such as HCV and primary biliary cirrhosis. (7) It is most prevalent in gold and brown colored granular pigment depositsand is distributed in perivenular zones 2 and 3 or in the area between the zones. It occurs near the bile or canalicular canaliculi in the cytoplasm of hepatocytes.
Siderosis (Iron Deposits)
Siderosis is observed in primary conditions such as hereditary and non-hereditary hemochromatosis but can also result from transfusions, hemodialysis, and diet due to increased iron intake. Liver diseases such as alcoholic and nonalcoholic steatohepatitis and hepatitis C can also cause siderosis. (7, 26) It is characterized by refractile gold or brown colored granular pigment deposits in periportal areas. In hereditary hemochromatosis it is also identified throughout the lobule. Located in the canalicular areas of hepatocytes in hereditary forms, excess iron is also deposited in the Kupffer cells, endothelium, stromal cells and ductal epithelia. In siderosis due to transfusions or diet, iron deposits are located in the Kupffer cells. Iron reacts with Prussian blue.
Copper and/or copper binding serum proteins
Copper and/or copper binding serum proteins are found due to interference with excretion in chronic cholestatic diseases and due to defects in excretion in hereditary conditions such as Wilson's disease. (7, 27) They appear as deposits of grayish pigment in the cytoplasm. Copper is shown with yellow brick granules in rhodamine, orcein and Victoria blue histochemical studies. It can be observed indirectly from deposits of its fixative protein shown with fuscin aldehyde staining. Frequently, PAS diastase is positive. In chronic cholestatic diseases, deposits are located in the periportal area or at the interface.
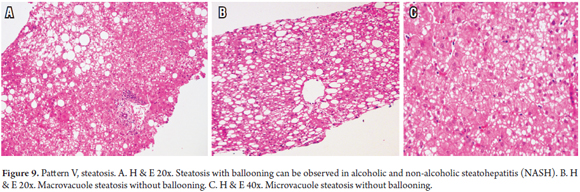
PATTERN V: STEATOSIS
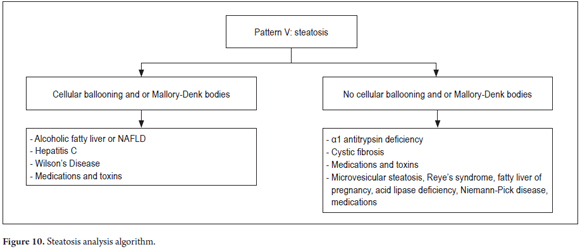
Steatosis is fat accumulation and is commonly observed in biopsies. A useful algorithm to guide diagnosis is to observe whether or not balloon cells and Mallory-Denk bodies are present (Figure 9). This subject was extensively discussed in an earlier article. (28)
Figure 10 shows the algorithm used for this pattern.
PATTERN VI: FIBROSIS
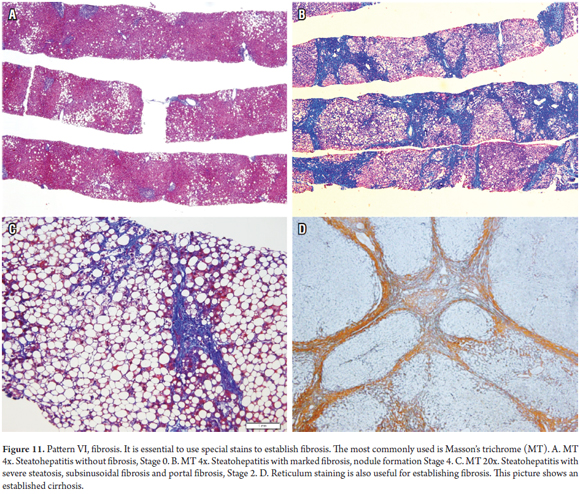
Fibrosis is the end result of the vast majority of liver diseases and is not a pattern of early damage. Determining the stage, amount and type of fibrosis is an indispensable component of the analysis of a biopsy. It should always be evaluated using histochemistry studies such as Masson's trichrome, the most commonly used, and Sirius red, less frequently used. The pattern of fibrosis provides the keys for diagnosis and subclassification, helps to establish the severity of the disease, and correlates directly with the severity of portal hypertension (Figure 11). (7, 29)
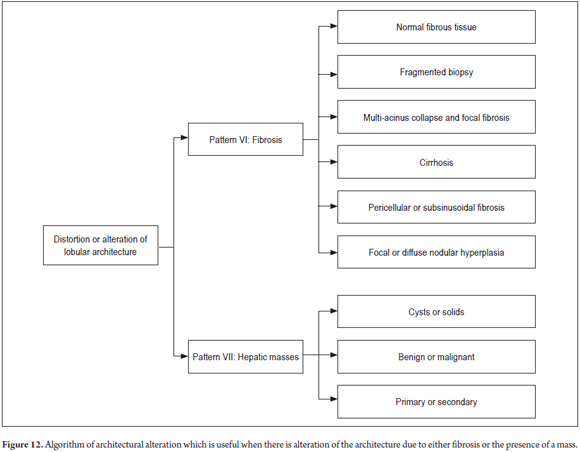
Figure 12 shows the algorithm used when to find alterations of the lobular architecture.
Frequently, fibrosis is overestimated in subcapsular biopsies. A thickness of up to 5 mm in the capsule is considered normal, with more fibrous tissue being observed in the portal spaces and around the central veins, including the presence of some fibrous partitions. In addition, the amount of fibrous tissue also depends on the size of the portal spaces and is directly proportional to the size of the structures of the portal triad. Also, longitudinal cuts that cross the length of the biopsy can suggest fibrous walls or bridges that do not really exist.
Fragmentation of percutaneous liver biopsies is common and increases with the progression of fibrosis from early to advanced stages. Specimens with significant fibrosis or cirrhosis are the most frequently and most extensively fragmented of all, but fragmentation is not always easy to identify when there are no fibrous edges. A rounded appearance with impaired lobular architecture (absence of central vein or portal triad structures) or fragmented reticular weave suggest that possible cirrhosis, despite the absence of true nodule formation. (30)
Deposits of young collagen (light blue with Masson's trichrome) are especially common in acute hepatitis with necrosis of the recent parenchyma but can be over-interpreted which leads to diagnostic errors. The collapse of the reticular weft helps establish the true nature of the lesion. To determine if fibrosis corresponds to the deposit of mature collagen, check for loss of the reticular weft and presence of cholangiolar proliferation in areas of necrosis and parenchyma with absence of its normal structures, because this will indicate if it is really fibrosis in bridges with incomplete or complete partitions (cirrhosis).
Pericellular or subsinusoidal fibrosis is characteristically observed in zone 3 in nonalcoholic and alcoholic steatohepatitis. In the latter it can progress to occlusion of the central vein. It also occurs in diseases that cause obstruction to chronic venous flow such as sinus veno-occlusive disease and Budd Chiari syndrome and metabolic diseases such as Wilson's disease, tyrosinemia, and galactosemia, and in infectious diseases such as leishmaniasis and congenital syphilis. Portal space-centered fibrosis occurs in chronic liver disease of any etiology but especially in viral hepatitis B and C and in autoimmune hepatitis. It begins irregularly in the portal space, extends into periportal spaces, and later develops portal to portal partitions and finally, develops portal to center partitions until regenerative liver nodules are formed. The term "early cirrhosis" is used when nodularity is not complete. (29)
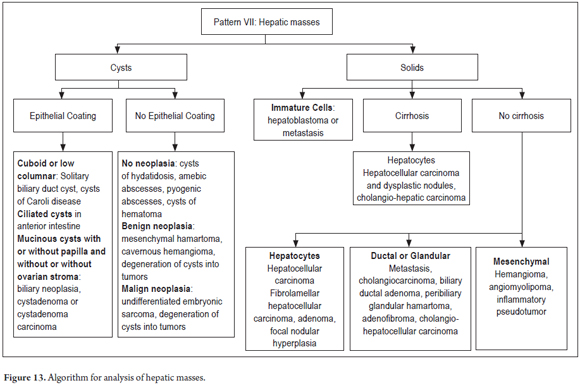
PATTERN VII: MASSES
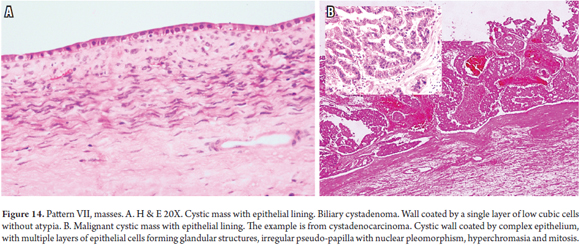
The algorithm (Figure 13) used to determine whether liver masses are cystic or solid mass involves observing the type of epithelial lining and/or the cells from which tumor proliferation originates (Figure 14). Additional histochemistry and immunohistochemistry may be useful. In addition, clinical and imaging correlation is fundamental for establishing whether it is a primary or secondary mass. (30, 31)
Conclusion
Liver pathology is extensive and complex. When the study of a liver biopsy is to be done and morphological findings are analyzed, it is very helpful to rely on algorithms that provide a simpler diagnostic path. Nevertheless, only proper correlation between the histopathology and the complete clinical history can achieve optimum results.
References
1. Villar LM, Medina Cruz H, Ribeiro Barbosa J, et al. Update on hepatitis B and C virus diagnosis. World J Virol. 12 de noviembre 2015;4(4):323-42. [ Links ]
2. Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG clinical guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. Julio 2014;109(7):950-66. [ Links ]
3. De Boer YS, van Nieuwkerk CMJ, Witte BI, et al. Assessment of the histopathological key features in autoinmune hepatitis. Histopathology. 2015;66:351-62. [ Links ]
4. Manns MP, Lohse AW, Vergani D. Autoimmune hepatitis-Update 2015. Journal of hepatology. 2015;62(1):S100-11. [ Links ]
5. Beuers U, Gershwin ME, Gish RG, et al. Changing nomenclature for PBC: from cirrhosis to cholangitis. Clin Gastroenterol Hepatol. Noviembre 2015;13(11):1867-9. [ Links ]
6. Putra J, Toor A, Suriawinata AA. The utility of repeat liver biopsy in autoimmune hepatitis: a series of 20 consecutive cases. Pathology. Agosto 2016;48(5):449-53. [ Links ]
7. Lefkowitch JH. Scheuer´s liver biopsy interpretation. 9a edición. Philadelphia: Elsevier. 2015. [ Links ]
8. Kleiner DE, Chalasani NP, Lee WM, et al. Drug-induced liver injury network (DILIN) Hepatic histological findings in suspected drug-induced liver injury: systematic evaluation and clinical associations. Hepatology. Febrero 2014;59(2):661-70. [ Links ]
9. Turhan N, Kurt M, Ozin Ozderin Y, et al. Hepatic granulomas: a clinicopathologic analysis of 86 cases. Pathology Research and Practice. 2011;207(6);59-365. [ Links ]
10. Coash M, Forouhar F, Wu CH, et al. Granulomatous liver diseases: a review. Journal of the Formosan Medical Association. Enero 2012;111(1):3-13. [ Links ]
11. Suzuki Y, Katagiri H, Wang T, et al. Ductular reactions in the liver regeneration process with local inflammation after physical partial hepatectomy. Lab Invest. Noviembre 2016;96(11):1211-22. [ Links ]
12. Gouw AS, Clouston AD, Theise ND. Ductular reactions in human liver: diversity at the interface. Hepatology. Noviembre 2011;54(5):1853-63. [ Links ]
13. Li H, Xia Q, Zeng B, et al. Submassive hepatic necrosis distinguishes HBV-associated acute on chronic liver failure from cirrhotic patients with acute decompensation. J Hepatol. Julio 2015;63(1):50-9. [ Links ]
14. Lefkowitch JH. Bile ductular cholestasis: an ominous histopathologic sign related to sepsis and "cholangitis lenta". Hum Pathol. Enero 1982;13(1):19-24. [ Links ]
15. Kirsch R, Yap J, Roberts EA, et al. Clinicopathologic spectrum of massive and submassive hepatic necrosis in infants and children. Hum Pathol. Abril 2009;40(4):516-26. [ Links ]
16. Wang WH, Wang HL. Fulminant adenovirus hepatitis following bone marrow transplantation. A case report and brief review of the literature. Arch Pathol Lab Med. Mayo 2003;127(5):e246-8. [ Links ]
17. Adams DH, Hubscher SG. Systemic viral infections and collateral damage in the liver. Am J Pathol. Abril 2006;168(4):1057-9. [ Links ]
18. Buyse S, Roque-Afonso AM, Vaghefi P, et al. Acute hepatitis with periportal confluent necrosis associated with human herpes virus 6 infection in liver transplant patients. Am J Clin Pathol. Septiembre 2013;140(3):403-9. [ Links ]
19. Marciano F, Savoia M, Vajro P. Celiac disease-related hepatic injury: insights into associated conditions and underlying pathomechanisms. Dig Liver Dis. Febrero 2016;48(2):112-9. [ Links ]
20. Khoo A. Acute cholestatic hepatitis induced by Epstein-Barr virus infection in an adult: a case report. J Med Case Rep. Marzo 2016;10:75. [ Links ]
21. Kinch A, Baecklund E, Backlin C, et al. A population-based study of 135 lymphomas after solid organ transplantation: the role of Epstein-Barr virus, hepatitis C and diffuse large B-cell lymphoma subtype in clinical presentation and survival. Acta Oncol. Mayo 2014;53(5):669-79. [ Links ]
22. OMalley DP. Benign extramedullary myeloid proliferations. Modern Pathology. 2007;20:405-15. [ Links ]
23. Mohyuddin GR, Yacoub A. Primary myelofibrosis presenting as extramedullary hematopoiesis in a transplanted liver graft. Case report and review of the literature. Case Rep Hematol. 2016;2016:9515404. [ Links ]
24. Nollevaux MC, Guiot Y, Horsmans Y, et al. Hypervitaminosis A-induced liver fibrosis: stellate cell activation and daily dose consumption. Liver Int. Marzo 2006;26(2):182-6. [ Links ]
25. Levine PH, Delgado Y, Theise ND, et al. Stellate-cell lipidosis in liver biopsy specimens. Recognition and significance. Am J Clin Pathol. Febrero 2003;119(2):254-8. [ Links ]
26. Alwahaibi NY, Alkhatri AS, Kumar J. Hematoxylin and eosin stain shows a high sensitivity but sub-optimal specificity in demonstrating iron pigment in liver biopsies. Int J Appl Basic Med Res. 2015;5(3):169-71. [ Links ]
27. Karadag N. Tolan K, Samdanci E, et al. Effect of copper staining in Wilson disease: a liver explant study. Exp Clin Transplant. Octubre 2016. [ Links ]
28. López Panqueva RP. Enfermedad hepática grasa. Aspectos patológicos. Rev Col Gastroenterol. 2014;29(1):82-8. [ Links ]
29. Kim NY, Cho MY, Bail SK, et al. Histological subclassification of cirrhosis using the Laennec fibrosis scoring system correlates with clinical stage and grade of portal hypertension. J Hepatol. Noviembre 2011;55(5):1004-9. [ Links ]
30. Malik AH, Kumar KS, Malet PF, et al. Correlation of percutaneous liver biopsy fragmentation with the degree of fibrosis. Aliment Pharmacol Ther. Marzo 2004;19(5):545-9. [ Links ]
31. Bosman FT, Carneiro F, Hruban RH, et al. WHO classification of tumors of the digestive tract. 4a edición. Lyon: IARC. 2010. [ Links ]











 texto en
texto en