Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.32 no.1 Bogotá Jan./Mar. 2017
https://doi.org/https://doi.org/10.22516/25007440.124
Identification of Blood Biomarkers for Detecting Premalignant Lesions and Gastric Cancer
Martin A. Gómez Zuleta, MD,1 Karen E. Torres, MSc,2 Michael T. Falduto, PhD,2 Scott R. Magnuson, PhD.2
(1) Associate Professor of Gastroenerology at the National University of Colombia and Gastroenterologist at Hospital Nacional Universitario UGEC in Bogota, Colombia
(2) Paradise Genomics Inc. in Northbrook, Illinois USA
Received:Â Â Â 05-11-15 Â Â Accepted:Â Â 16-12-16
Abstract
Introduction: Gastric cancer is very common in Colombia where it is the leading cause of death due to cancer. According to the Pelayo Corre, there is a cascade of stages from gastritis through atrophy, metaplasia and dysplasia to cancer. In the intermediate stages, it might be possible to detect and prevent the development of cancer, but there are no known markers in the blood other than pepsinogen to help to detect premalignant stages and diagnose cancer. Research is the key to discovery of new biomarkers.
Objective: The aim of this work is to identify molecular markers (mRNA expression profiles) that distinguish patients who have premalignant conditions (atrophy, metaplasia) and gastric cancer from patients who only have gastritis.
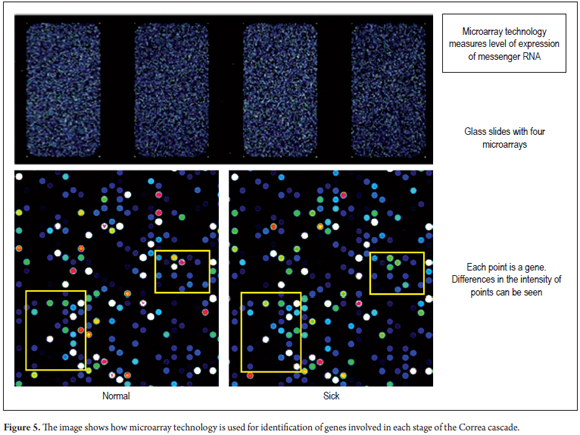
Methodology: Following an initial endoscopy, patients in each stage of the Pelayo cascade fasted and then provided a 2.5 ml blood sample which was analyzed for gene expression. All participating patients signed consent forms prior to tests. The blood was placed in a PAXgene RNA Blood tube, RNA was extracted from the blood and then analyzed. A microarray platform which identified changes in messenger RNA expression was used to differentiate each of the stages described.
Results: Endoscopic findings for the eighty-nine patients included showed that 25 (28%) had advanced gastric cancer, 7 (7.8%) had early cancer, 27 (30.3%) had chronic antral gastritis, 15 (16.8%) had chronic pangastritis, three (3.3%) were suspected of having atrophic gastritis, six (6.6%) were suspected of having intestinal metaplasia, and two (2.2%) had peptic ulcers. Pathological reports showed 20 cases of intestinal adenocarcinoma (4 women), 11 cases diffuse cancer (7 women), 34 cases of chronic gastritis (22 women), one case of atrophy alone, 18 cases of intestinal metaplasia (13 women), four cases of low-grade dysplasia, and one case of high-grade dysplasia. The analysis of genetic expression found 48 genes which could be used for differentiation of patients with chronic gastritis from patients with gastric cancer. We also found 14 genes that could be used to differentiate patients with diffuse cancer from patients with intestinal type gastric cancer, and a group of 48 genes that could be used to differentiate patients with chronic gastritis from those with intestinal metaplasia.
Conclusions: This is the first work anywhere in the world that has identified new biomarkers through the genetic expression of messenger RNA which differentiates the stages of the Pelayo Correa cascade and permits diagnosis of gastric cancer. It is likely that in the future they may be used as diagnostic and/or follow-up tests.
Keywords
Gastric cancer, biomarkers, prevention.
INTRODUCTION
Gastric cancer is a highly prevalent multifactorial entity: in 2012 more than 1 million new cases were reported. About two-thirds of these occurred in developing countries with high-risk areas in East Asia (China and Japan), Eastern Europe and parts of Central and South America. Globally, gastric cancer (GC) is the fifth most common type of cancer, and the second leading cause of cancer death, but in Japan it is the leading cause of death from cancer. In Colombia the incidence is 10 times higher than in the USA. (1-8)
According to the Lauren classification, gastric cancer (GC) is histologically divided into two types: intestinal and diffuse. These types of tumors have clear differences from the epidemiological, histopathological, endoscopic, clinical and pathogenetic points of view. (9-12)
Intestinal GC develops through a multi-step process that can last 20 years or more. According to the gastric carcinogenesis model proposed by Correa, GC starts with chronic gastritis produced by H Pylori, progresses first to gastric atrophy, then to intestinal metaplasia followed by dysplasia and finally to cancer. In this model, gastric atrophy and intestinal metaplasia are considered to be precursors of GC. The final appearance of the tumor involves host genetic characteristics and environmental factors such as lack of consumption of fruit and vegetables and excessive consumption of salt. Among the genetic factors are immune response polymorphisms as well as their protein products. These include genes for cytokines involved in the adaptive immune system as well as pattern recognition factors that initiate the innate immune system response. (13-15)
The main cause of high mortality in GC is late diagnosis. When GC is detected early, 5-year survival rates are 90%, but when it is detected in advanced stages, five-year survival rates are only 15% to 20%. (16) Unfortunately, most GC patients in developing countries are diagnosed in advanced stages when the prognosis is very poor. Therefore, it is very important to identify the premalignant states of atrophy, intestinal metaplasia and dysplasia, and to monitor them.
Generally, when GC is at an early stage most cases are completely asymptomatic. Unfortunately, many premalignant lesions and even early cancer can be asymptomatic. By the time they present alarming symptoms such as bleeding and weight loss which lead patients to see a physician, GC is already well advanced.
Japan is a pioneer in GC screening programs whose goal is to identify GC in early stages in order to provide timely treatment. For this purpose, annual barium x-rays are used for people over 40 years of age. (15, 16) In spite of this, only 10% of the candidate population for screening is subjected to this method. (17) Another option is to perform endoscopies (UGIE) on a massive basis, but symptomatic people's acceptance of this is not ideal. The result is that the vast majority do not undergo studies. This strategy is also expensive and, in certain areas, it may not be cost effective.
Consequently, an ideal preventive measure would be to have reliable serological markers to detect premalignant conditions or GC before they reach the stage of advanced cancer. A serological method aimed at noninvasive determination of atrophy was described more than 20 years ago. It uses measurement of biochemically and immunologically distinct types of pepsinogens (PG) in blood serum without the need for endoscopy. PGs are classified into two types: PG-I, or PG-A, and PG-II, or PG-C. (18-20)
PG-I is produced by the principal and mucosal cells of the glandular necks of the fundic glands. PG-II is produced by these same cells as well as by the cells of the pyloric glands and Brunner's glands. When atrophy occurs in the proximal gastric body, PG-I levels progressively decrease while PG-II levels remain constant. This results in progressively decreasing levels of PG-I and therefore in the ratio of PG- I/PG-II thus reflecting the progression of normal gastric mucosa to chronic atrophic gastritis. When PG-I is less than 70 ng/mL and PG-I/PG-II is less than three, atrophy is indicated. However, this method is not widely accepted since it only seeks to detect atrophy, does not identify intestinal metaplasia or cancer, is costly and is not available in the medium. (18-22).
For these reasons, it is believed that there should be a method of identifying people who are at risk for GC, or who have early GC, that is not only simple but is also effective and inexpensive as is the case with testing for prostate antigen as an indication for prostate cancer. The most clinically useful test would reach the largest number of patients possible in an effective way. Such a test should be useful for detecting atrophy and intestinal metaplasia, which increase risk of GC, as well as for detecting gastric cancer itself. In this regard, some groups recommend using the combination of determination of H. pylori infection and serum biomarkers of atrophy, such as PG. Nevertheless, this strategy will not detect patients with metaplasia or early cancer since there are no serologic markers for these conditions. Consequently, research proposals are needed to identify new blood markers that will identify the stages of the Correa cascade of atrophy, metaplasia, dysplasia, early gastric cancer and advanced cancer. (23)
Recently, trefoil (TFF) factors and serum pepsinogens have been found to be new biomarkers of atrophy that have higher yields than those of PGs alone. Trefoil (TFF) factors are small and stable molecules (12-22 Kd) secreted by cells of the gastrointestinal tract. When TFF3 is combined with PG-I and the PG-I/PG-II ratio, the combination's sensitivity for detecting atrophy is 80% compared to 67% for PG alone. However, like PGs, this marker only evaluates atrophy. (24, 25)
Other biomarkers that have been considered are the microRNAs (miRNA). These single-stranded RNA vary in length between 21 and 25 nucleotides and have the ability to regulate the expression of other genes by various processes of RNA interference. It has been observed that the miRNA pattern correlates with the number of circulating tumor cells and that therefore the miRNA in peripheral blood may be a tool for follow-up rather than for diagnosis of cancer. A different miRNA pattern is observed in gastric cancer tissue than in non-malignant tissue of the same patient. Still, this cannot be used as a biomarker because the miRNA profile is not identical in several studies nor was it evaluated in the premalignant stages of the disease. (26)
As can be deduced, at present there are no blood markers that adequately allow identification of the stages of the Correa cascade through which a patient progresses to gastric cancer. This makes it imperative to develop research protocols in this area in order to identify these biomarkers so that they can be used in campaigns to prevent this terrible disease. GC kills more than 800,000 people around the world every year, and in Colombia it accounts for 50,000 deaths, more than those resulting from violence, which makes it the leading cause of cancer death. (1, 7)
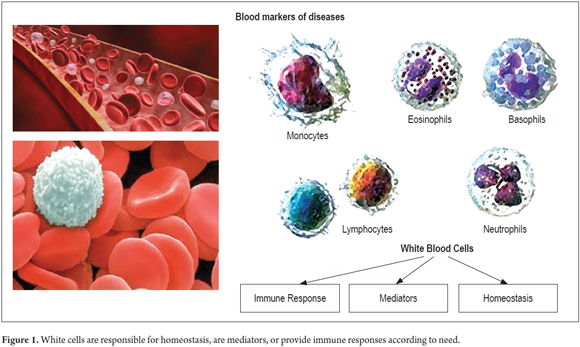
On the other hand, it is known that malignant tumors and metastatic cancer have a profound pathophysiological effect on multiple organs and systems of the human body. Because of this systemic effect, cancer is prone to mediate changes in white blood cells due to either a direct response to signaling by factors secreted by tumors, or to indirect reactions to physiological stress of white blood cells. (27) Since white blood cells mediate immune responses, they are known to produce antigens against tumor markers and to have a role in defense against primary tumors and metastases. (28)
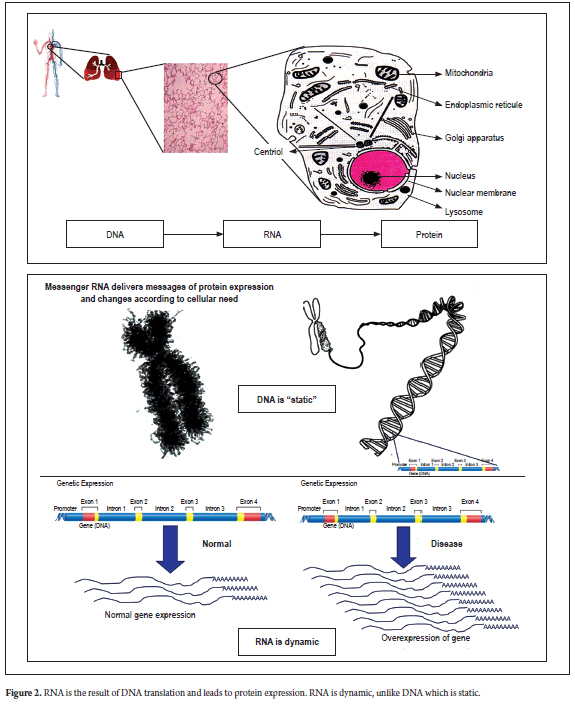
To a large extent, these responses are due to alterations in cellular proteins encoded by genes from these white cells. It is likely that white cells have specific programs for gene expression in the presence of a tumor (Figure 1). If these profiles can be correlated with the size, location or stage of the cancer, and to its progression, they could serve as biomarkers for diagnosis of gastric cancer, for determining a patient's prognosis, for therapeutic control and/or as adjuvants for anticancer drugs (Figure 2).
The main objective of this study is to determine genetic expression profiles from the whole blood of patients submitted to upper digestive endoscopy during the various stages of the Correa cascade from gastritis to advanced gastric cancer.
General Objectives
The overall objective of this study is to identify molecular markers (mRNA expression profiles) that distinguish cases with premalignant conditions such as atrophy, metaplasia and gastric cancer from control patients with dyspepsia who have gastritis alone. This information can then be used to develop a blood-based expression profile that has high sensitivity and specificity for differentiating healthy patients, or those with gastritis alone, from those with gastric cancer or premalignant conditions. It is believed that the identification of this expression profile will be of great help for diagnosis of this serious disease.
Specific Objectives
- Identify a gene expression profile for chronic atrophic gastritis in the Colombian population.
- Identify a gene expression profile for complete intestinal metaplasia.
- Identify genetic expression profiles for low and high grade dysplasia.
- Identify a gene expression profile for gastric cancer.
MATERIALS AND METHODS
This is a prospective study of patients of the gastroenterology department of the Hospital el Tunal-Universidad Nacional de Colombia in Bogotá, Colombia. Patients were included prospectively for upper gastrointestinal endoscopy (UGIE). All patients signed informed consent forms after a thorough and detailed explanation of this study. Both the research protocol and informed consent form were approved by the Research Committee and the Ethics Committee of Hospital el Tunal.
Before performing UGIEs, patients answered a previously designed form containing the most important epidemiological and demographic variables in gastric cancer. All patients fasted prior to the procedure and the consumption of any medication or food was registered.
All digestive endoscopy was performed in the morning following minimum fasting of 6 hours. Patients were placed in the left lateral decubitus position. All patients received two 20 mg applications of given lidocaine spray (Roxicaine, topical solution, Ropsohn Therapeutics) to anesthetize the pharynx. The following samples were taken for histopathological study: three from the antrum marked as tube A; two from the corpus marked as tube B; and two for Helicobacter pylori infection. Biopsies were then immersed in 10% neutral buffered formalin and sent to the hospital laboratory for conventional processing pending histological diagnosis.
After endoscopy and recovery, a 2.5 mL sample of whole blood was taken for study. Then, the gene expression profile results were compared with pathology results to correlate gene expression with the presence of atrophy, metaplasia, dysplasia or cancer. In total, a sample size of 100 patients was calculated by summing all groups.
Inclusion criteria
Cases
- Patients (n = 20) suspected of gastric cancer because of endoscopic evidence.
- Patients suspected of atrophy (n = 20), metaplasia (20 patients) or dysplasia (20 patients) because of endoscopic evidence.
Controls
- Patients (n = 20) with endoscopic diagnoses of chronic gastritis later confirmed by the pathology.
Exclusion criteria
Cases and Controls
- Current use of narcotics
- Active infections of any kind
- Current use of prescription antibiotics
- History of any other type of cancer (excluding skin, melanoma or gastric cancer)
- Patients with serious concomitant diseases and conditions such as congestive heart failure, strokes, decompensated diabetes, clotting disorders, cirrhosis, previous gastric surgery, pregnancy or lactation, drug addiction, alcoholism, psychiatric illnesses, HIV infections, use of anticoagulants, and cancer (including those receiving chemotherapy).
Study Procedures
After the initial endoscopy and with prior written consent, cases and controls each provided a 2.5 mL sample of fasting blood for gene expression analysis taken. The blood was placed in a PAXgene blood RNA tube, inverted 8-10 times, incubated at room temperature for 2-8 hours and stored at -20° C (freezer) until the batch was shipped to the laboratory for processing and analysis.
RNA was extracted from the blood and analyzed using a microarray platform that identified changes in messenger RNA expression which allowed for differentiation of cases from controls. (Figure 3).
Sample Processing
Sample processing involves isolation of total RNA using modifications of standard procedures involving extraction and organic purification. (29) The quality and quantity of the total RNA was evaluated by spectrophotometry and Agilent Bioanalyzer equipment. Total RNA samples were amplified and prepared for microarray hybridization using an in-vitro transcription reaction with labeled nucleotide.
The quality and quantity of the resulting complementary RNA (cRNA) was also evaluated by spectrophotometry and the Agilent Bioanalyzer kit prior to hybridization. The cRNA was hybridized with DNA probes in SurePrint G3 Human Gene Expression 8x60K v2 Microarrays from Agilent Technologies. The study was performed with the latest microarray version of the entire human genome which targets 50,599 different biological characteristics. (29) After hybridization, the matrices were washed and scanned in high resolution on the Agilent Microarray SureScan scanner with extended dynamic range (Figure 4).
Finally, images of the microarray (Figure 5) were visually inspected and data were obtained using the Agilent Feature Extraction software. This software generates quality control metrics for each point in the array. Any matrix that did not meet the strict quality standards were repeated to ensure that the data were of the highest quality and that the set of biomarkers were as reproducible and sensitive as possible.
Statistical Analysis
Statistical description of the variables described earlier used percentages for qualitative variables and averages for numerical variables.
Raw data was normalized throughout the array by global normalization methods for constant matrix-to-array comparison. Data analysis and identification of biomarkers included statistical visualization through hierarchical clustering, heat maps, scatter plots and Venn diagrams. (30)
RESULTS
In total, 89 patients with an average age of 59.5 years were included. Fifty-seven percent were women. Reasons for endoscopy were dyspepsia (75% of patients), anemia (4.4%), weight loss (5.56%), and upper digestive tract hemorrhaging (8.8%).
Chronic antral gastritis was found in 27 of the patients (30.4%), advanced gastric cancer in 25 (28%), chronic pangastritis in 15 (16.8%), early cancer in seven (7.8% ), suspicion of intestinal metaplasia in six (6.7%), suspicion of atrophic gastritis in three (3.3%) and peptic ulcers in two cases (2.2%).
When the pathological report was reviewed, chronic gastritis was found in 34 patients (22 women); intestinal adenocarcinoma in 20 (4 women); intestinal metaplasia in 18 (13 women); diffuse cancer in 11 (7 women); low-grade dysplasia in four patients, high-grade dysplasia in one patient, and atrophy alone in one patient. Of the 89 patients, 56 (67.2%) had Helicobacter pylori infections.
Abdominal CT scans were done of 31 patients with early or advanced gastric cancer. Six (25.8%) were found to be in Stage I (early), four in Stage II, seven in Stage III, and 14 (61%) in stage IV or with metastasis. Only four (12.9%) of the 31 had tumors located in the cardia.
Genes that were differentially expressed between men and women were removed from the analysis. Only genes that were differentially expressed more than 1.5 times and with T-test values ââof p 0.01 or less were used.
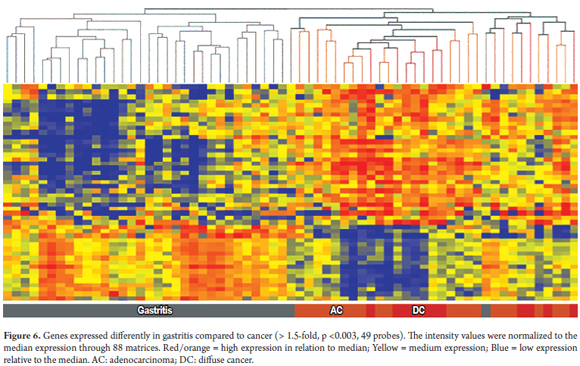
From all of the gene expressions evaluated in the blood samples, we were able to discover differentially expressed genes that can be used to distinguish patients with chronic gastritis from patients with diffuse gastric cancer and from those with intestinal adenocarcinoma. The following figures represent hierarchical trees, also called heat maps, that allow discrimination of one pathology from others based on genetic expression (Figure 6).
When we analyzed the pathological report and cross-checked all pathological samples against the 49 genes found using a class prediction algorithm, a comparison of the group of patients with chronic gastritis (n = 34) with all patients diagnosed with intestinal or diffuse cancer (n = 31) showed overall accuracy of 97% and a sensitivity of 97% and a specificity of 97%.
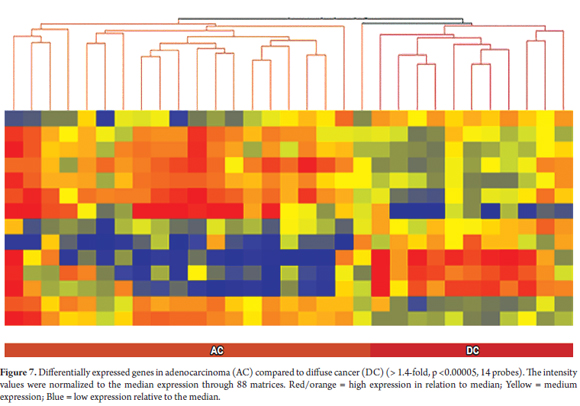
In addition, we found that the gene expression profiles in the blood of the two types of cancer are distinct from those of gastritis. By also creating a hierarchical grouping of gene expressions of diffuse cancer and adenocarcinoma, we found 14 different genes that can almost completely differentiate between patients with diffuse cancer (n = 11) and those with adenocarcinoma (n = 20). The exception was one patient with adenocarcinoma (Figure 7).
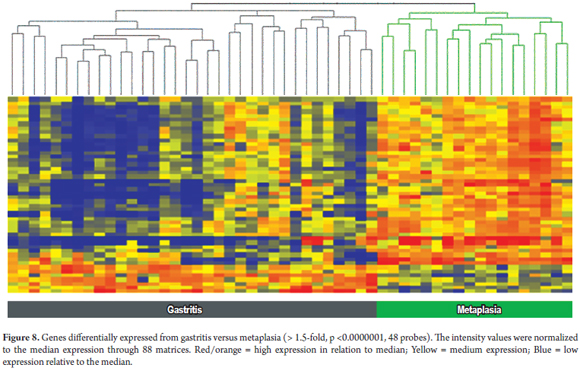
To differentiate patients with chronic gastritis from patients with intestinal metaplasia, an expression profile of 48 different genes was found. A comparison of this group of genes with the pathological diagnosis found that the accuracy of the hierarchical grouping of these genes was 100%, with no false positives or false negatives (Figure 8). (15)
DISCUSSION
Gastric cancer is the fifth cause of cancer in the world, but the second cause of mortality indicating ââits high case fatality rate. There are two main types of GC: diffuse and adenocarcinoma. The latter occurs most frequently and is known to go through a sequence of events from chronic gastritis to advanced cancer in the Correa cascade. (11) H. pylori infections, other environmental factors and genetic factors are involved in this cascade of events leading to cancer.
It takes a long time for advanced cancer to develop. During this period, doctors can intervene to detect the disease in initial stages of atrophy, metaplasia or dysplasia. However, as we have seen, there is almost no evidence that allows us to simply diagnose these stages. Some methods have been described, but except for pepsinogen these are not available in clinical practice. Moreover, pepsinogen only allows assessment of whether the patient has atrophy. In Colombia, pepsinogen does not seem to be very useful according to the results recently reported by Martínez et al. from the Colombian Cancer Institute. (23, 31)
It is clear that traditional cancer markers such as CAE and CA19-9 are not useful for diagnosis of gastric cancer. (3) In the stomach, precise detection of malignant and premalignant lesions, particularly atrophy, intestinal metaplasia (IM) and dysplasia, always requires upper digestive endoscopy with biopsies according to a standardized protocol that is frequently not followed in routine clinical practice. In addition, mass screening programs with endoscopy or fluoroscopy as in Japan are not widely accepted. This may be one of the explanations of why the disease is detected in more advanced states with poor prognoses in the western world. (32)
For these reasons, the information that new biomarkers can stratify the risk of gastric cancer is very important for large-scale applications, especially in high risk populations such as Colombia's. The intention of this work is to look for and find these biomarkers. To the knowledge of the authors, this is the first such study in Colombian population which has identified a blood gene expression profiles which clearly differentiate patients with gastritis from those with gastric cancer. The identification of these 49 genes should allow design of a test to diagnose whether or not a patient has gastric cancer (Figure 1).
In addition, this study identified another gene expression profile with 14 genes that discriminates whether a tumor is diffuse or is intestinal adenocarcinoma.
It is also noteworthy that, although the initial intent was to include a group of 20 patients with atrophy, during the comparison of pathologies, there was only one patient with atrophy. Many patients who endoscopically appeared to have atrophy had pathologies compatible with gastritis or intestinal metaplasia. This confirms that endoscopy is not a very sensitive method for detection of either atrophy or intestinal metaplasia and must therefore always be accompanied by a biopsy.
As discussed, pepsinogen is used for diagnosis of atrophy, but in the literature no blood tests are described for the diagnosis of metaplasia. This work shows that a gene expression profile made up of 48 genes (Figure 3) made it possible to clearly differentiate patients with gastritis from those with metaplasia. This seems fundamental, since every passing day shows that intestinal metaplasia is more important than atrophy. It seems that this is the point of no return in the Correa cascade after which the risk becomes much higher for cancer rather than atrophy. This makes it not only in detection, but follow-up, even more important. (33, 34)
Another interesting aspect of this study is that it allows identification of patients with gastric cancer by means of a blood test and then differentiates them into the two main types: intestinal and diffuse. This is something that had not been reported in the literature and that traditionally could only be done with a gastric biopsy. This seems important because of the potential for identifying gastric cancer or risk GC in a patient with a blood test. This would be fundamental if one takes into account that many patients with gastric cancer are asymptomatic when diagnosed.
This group intends to use these gene expression profiles and their classifying algorithms as diagnostic tools. This may be done either in the current form using RNA isolated from whole blood, or in a faster and more economical test using measurements of expression levels of a subset of genes or their protein products isolated from serum or blood cells. Because early diagnosis of cancer or premalignant lesions is critical for clinical decisions, the use of these trials could have profound implications for prevention and management of this very common disease which generates a high mortality rate.
REFERENCES
1. IARC/WHO. Globocan 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. Available at: http://globocan.iarc.fr/. Accessed March 31, 2014. [ Links ]
2. Krew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354-62. [ Links ]
3. Hamashima C, Shibuya D, Yamazaki H, et al. The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol. 2008;38:259-67. [ Links ]
4. Piñeros M, Hernández G, Bray F. Increasing mortality rates of common malignancies in Colombia. Cancer. 2004;101:2285-92. [ Links ]
5. Correa P, Piazzuelo MB, Camargo MC. Overview and pathology of gastric cancer. En: Wang T, Fox J, Giraud A (editores). The biology of gastric cancers. Springer Science Business Media. 2009. p. 21-446. [ Links ]
6. Smith MG, Hold GL, Tahara E, et al. Cellular and molecular aspects of gastric cancer. World J Gastroenterol. 2006;12:2979-90. [ Links ]
7. Moss SF, Malfertheiner P. Helicobacter and gastric malignancies. Helicobacter. 2007;12(1):23-30. [ Links ]
8. Otero W, Gómez M, Castro D. Carcinogénesis gástrica. Rev Colomb Gastroenterol. 2009;24:314-29. [ Links ]
9. Jarvi O, Lauren P. On the role of heterotopias of the intestinal epithelium in the pathogenesis of gastric cancer. Acta Pathol Microbiol Scand. 1951;29:26-44. [ Links ]
10. Lauren P. The two histological main types of gastric carcinoma: diffuse and so called intestinal-type carcinoma. An attempt at a histoclinical classification. Acta Pathol Microbiol Scand. 1951;64:31-49. [ Links ]
11. Correa P, Carneiro F. Classification of gastric carcinomas. Curr Diagn Pathol. 1997;4:51-9. [ Links ]
12. Ekstrom AM, Serafini M, Nyren O, et al. Dietary antioxidant intake and the risk of non-cardia cancer of the intestinal and diffuse types: a population based case control study in Sweden. Int J Cancer. 2000;87:133-40. [ Links ]
13. El-Omar EM, Lochhead P. Gastric cancer. Br Med Bull. 2008;85:87-100. [ Links ]
14. Henson DE, Dittus C, Younes M, et al. Differential trends in the intestinal and diff use topless of gastric carcinoma in the United States-2000: increase in the signet ring cell type. Arch Pathol Lab Med. 2004;128:765-70. [ Links ]
15. Oliveira C, Seruca R, Carneiro F. Hereditary gastric cancer. Best Pract Res Clin Gastroenterol. 2009;23:147-57. [ Links ]
16. Alberts SR, Cervantes A, van de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol. 2003;14(2):ii31-6. [ Links ]
17. Guilford P, Hopkins J, Harraway J, et al. E-cadherin germ line mutations in familial gastric cancer. Nature. 1998;392:402-5. [ Links ]
18. Cisco RM, Ford JM, Norton JA. Hereditary diff use gastric cancer. Cancer. 2008;113(7):1850-6. [ Links ]
19. Masciari S, Larsson N, Senz J, et al. Germline E-cadherin mutations in familial lobular breast cancer. J Med Genet. 2007;44:726-31. [ Links ]
20. Malfertheiner P, Megraud F, O´Morain CA, et al. Management of Helicobacter pylori infection-The Maastricht IV/Florence Consensus Report. Gut. 2012;61:646-64. [ Links ]
21. Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19(1):S37-S43. [ Links ]
22. Bornschein J, Malfetheiner P. Gastric carcinogensis. Langenbecks Arch Surg. 2011;396:729-42. [ Links ]
23. Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007;133:659-72. [ Links ]
24. Graham DY, Asaka M. Eradication of gastric cancer and more efficient gastric cancer surveillance in Japan: two peas in a pod. J Gastroenterol. 2010;45:1-8. [ Links ]
25. Piazuelo B, Epplein M, Correa P. Gastric cancer: an infectious disease. Infect Dis Clin N Am. 2010;24:853-69. [ Links ]
26. Guo J, Miao Y, Xiao B, et al. Differential expression of micro RNA species in human gastric cancer versus non-tumorous tissues. J Gastroenterol Hepatol. 2009;24:652-7. [ Links ]
27. Liu Y, Zeng G. Cancer and innate immune system interactions: translational potentials for cancer immunotherapy. J Immunother. 2012;35(4):299-308. [ Links ]
28. Grivennikov I, Florian G, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883-99. [ Links ]
29. MAQC Consortium. The Microarray Quality Control (MAQC) project shows inter- and intraplatform reproducibility of gene expression measurements. Nat Biotechnol. 2006;24:1151-61. [ Links ]
30. Palayoor T, Aryankalayil M, Makinde A, et al. Differential expression of stress and immune response pathway transcripts and miRNAs in normal human endothelial cells subjected to fractionated or single-dose radiation. Mol Cancer Res. 2014;12(7):1002-15. [ Links ]
31. Martínez T, Bravo M, Núnez D, et al. Niveles séricos de pepsinógeno y su capacidad diagnóstica de atrofia gástrica en diferentes poblaciones colombianas. Rev Colomb Cancerol. 2014;18(4). [ Links ]
32. Danesh J. Helicobacter pylori infection and gastric cancer: systematic review of the epidemiological studies. Aliment Pharmacol Ther. 1999;13:851-6. [ Links ]
33. Cassaro M, Rugge M, Gutierrez O, et al. Topographic patterns of intestinal metaplasia and gastric cancer. Am J Gastroenterol. 2000;95:1431-8. [ Links ]
34. El-Zimaity HM, Ramchatesingh J, Saeed MA, et al. Gastric intestinal metaplasia: subtypes and natural history. J Clin Pathol. 2001;54:679-83. [ Links ]











 text in
text in