Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.32 no.1 Bogotá ene./mar. 2017
https://doi.org/https://doi.org/10.22516/25007440.126
Relationship between Hepatocyte Growth Factor (Hgf) and Stage of Cirrosis
Ismael de Jesús Yepes Barreto PhD (1), María Nicol Múnera Contreras MD (2), Amileth Suárez-Causado PhD. (3)
(1) Universidad de Cartagena in Cartagena, Colombia
(2) Pharos Biomedical research center on the coast in Cartagena, Colombia
(3) Prometheus Research Group & Biomedicine applied to clinical sciences at the Universidad de Cartagena in Cartagena, Colombia
Received:Â Â Â 05-03-16Â Â Accepted:Â Â Â 16-12-16
Abstract
Introduction: Hepatocyte growth factor (HGF) is known to be a potent mitogenic agent both in vivo and in vitro. The role of HGF in cirrhosis is not completely clear, but some studies point to it as a marker of severity in cirrhosis, acute liver failure and chronic hepatitis.
Objective: The objective of this study was to determine the relationship between HGF and the stage of hepatic cirrhosis and to identify factors associated with HGF levels in this population.
Methodology: All patients with hepatic cirrhosis treated from January to March 2014 were evaluated. At the time patients were enrolled in the study their clinical histories were taken and they underwent transient elastography and extraction of samples for measurement of HGF.
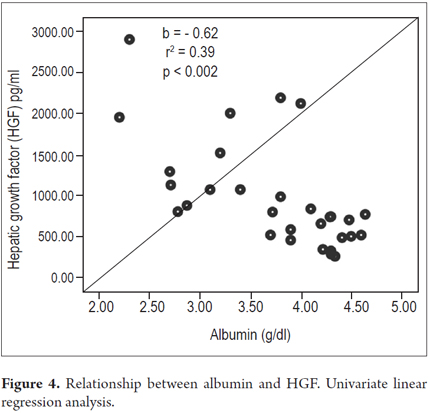
Results: No relationships were found between HGF levels and Child-Pugh classifications, however, higher levels of HGF were observed in patients with decompensated disease. A positive linear relations was found between HGF and hepatic hardness estimated by elastography (b = 0.53, r2 = 0.26, p = 0.002) and a negative linear relation was found between HGF and albumin (b = -0.62, r2 = 0.39, p <0.001). Only albumin retained this association in the multivariate analysis.
Conclusion: HGF is a marker of severity in liver cirrhosis. Albumin and the degree of fibrosis determined by transient elastography were associated with HGF levels.
Key words
Hepatic cirrhosis, hebatocyte growth factor, transient elastography, Child-Turcotte-Pugh Classification.
INTRODUCTION
Liver cirrhosis is the final stage of sustained hepatic necroinflammatory activity of viral, metabolic or autoimmune origin. Until relatively recently, cirrhosis was considered to be a single, static condition, but it is now accepted that subgroups of patients coexist within the same diagnosis, (1, 2) and that they have different degrees of liver damage with various prognostic implications within the natural history of the disease. (3)
In the liver, hepatic growth factor (HCF) is known to be a potent mitogenic agent both in vivo and in vitro, (4-6) and that it induces liver regeneration by stimulating DNA synthesis in hepatocytes. (7,8 ) Several investigators have provided direct evidence of an essential role for the HGF/Met pathway during hepatic regeneration in murine models of the liver using c-met conditional knockout mice. (9-12)
Nevertheless, clinical implications of HCF levels for cirrhosis are not yet completely clear. Despite HCF's fundamental role in hepatic regeneration processes, (13), studies have shown it to be a marker of severity in cirrhosis, in acute liver failure and in chronic hepatitis. (14,15) It has even been suggested that it as a predictor of mortality in patients with acute liver failure (ALF). (14)
Transient elastography (TE) is a novel technique for evaluating non-invasive hepatic fibrosis that has become a useful tool for studying patients with cirrhosis. (16 - 21) Although a significant association has been found between HCF and the presence of fibrosis in chronic hepatitis, (14) this relationship has never been evaluated in subjects with cirrhosis. Since TE values ââincrease as the degree of fibrosis increases across the full spectrum of liver disease severity including cirrhosis, it may be useful for evaluating this association in this group of patients.
The objectives of this study were to determine the relationship between HGF and the stage of liver cirrhosis and to identify factors associated with HGF levels in this population.
METHODOLOGY
We evaluated all patients who had been unequivocally diagnosed with hepatic cirrhosis by clinical, radiological or histological criteria from January to March 2014 in the outpatient department of Comprehensive Solutions in Gastroenterology and Hepatology, a first level hepatology referral center for the four largest EPS (Colombian health maintenance organizations) in Cartagena. All patients signed informed consent forms prior to inclusion in the study. For each patient, estimation of liver stiffness through TE, collection of clinical and analytical information, and extraction of a sample for determination of HGF were all done on the same morning. Patients diagnosed with hepatocellular carcinoma and those who had been hospitalized due to decompensation of hepatic disease during the 30 days prior to the evaluation were excluded from the study.
Liver Disease Staging
Child- Turcotte - Pugh Score
The Child-Pugh or Child- Turcotte - Pugh score is used for staging cirrhosis. Patients with Child-Pugh A scores were considered to be in the compensated phase and those with scores of B-C were considered to be in the decompensated phase.
Determination of Hepatic Growth Factor
For determination of serum hepatic growth factor, a blood sample was collected in sterile vacutainer tubes (Becton-Dickimson Vacutainer System) and centrifuged at 3500 rpm for 15 minutes at 4 ° C. For quantification of HGF, an ELISA kit (Cat No. KAC2211, Invitrogen) was used according to the manufacturer's instructions, based on indirect sandwich antigen capture and detection of immunocomplexes with a detection limit of 20 μg/mL.
Transient Elastography
Transient elastography with fibroscan (Ecosense) series 502 was used for estimation of liver stiffness. This apparatus emits a vibrating wave that crosses the hepatic parenchyma. The speed at which this wave travels through the organ allows estimation of the degree of fibrosis expressed in kilopascals (Kpa). Higher velocities indicate higher degrees of fibrosis, and vice versa. All patients fasted for at least 8 hours prior to testing. The procedure was performed in supine position with the right arm at maximum abduction. Determinations were made on the median axillary line. The median value of 10 estimates was used as the measure of liver stiffness, and results that did not meet the quality criteria of the manufacturer were not used in the analysis. An interquartile range of less than 30% and a success rate (number of valid measurements/total measurements) of greater than 60% were considered indispensable for evaluating the reliability of the result. All patients underwent the procedure free of charge as part of the study protocol to estimate the relationship between elastography and quality of life. In no case was the procedure used to confirm a diagnosis of cirrhosis.
Statistical Analysis
Statistical analysis was done with PASW stastistics 15. (SPSS, Inc, Chicago). All p values ââwere calculated bilaterally. The level of statistical significance was set at p <0.05. To describe patient characteristics, medians were used for quantitative variables, and percentages were used for categorical variables. Student's t tests, ANOVA and Chi-square tests were used to compare groups. Factors associated with HGF levels were initially identified through univariate linear regression analysis. Those variables identified in this phase were included in the multivariate analysis.
RESULTS
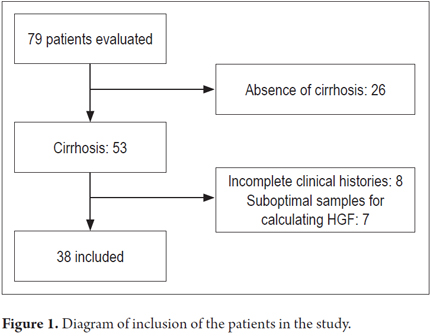
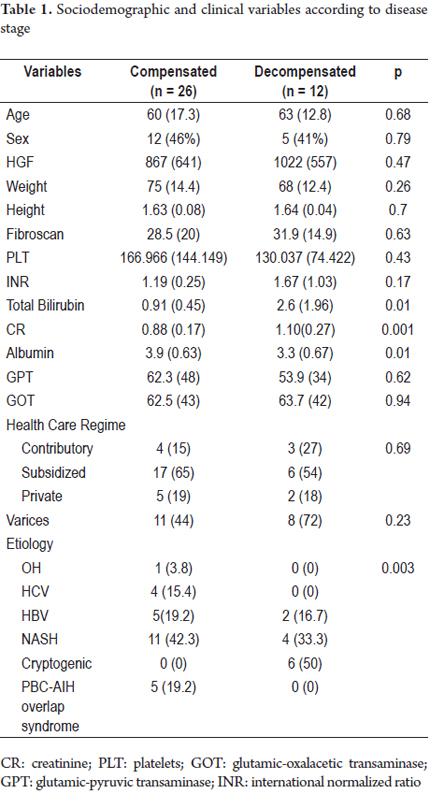
Thirty-eight patients were available for analysis (Figure 1): 28 with compensated disease and 12 in the decompensated phase. The sample consisted of similar proportions of men and women. None of the patients evaluated had ascites at the time of inclusion. All of the elastography estimates included in the analysis met the quality criteria stipulated by the manufacturer (interquartile range <30% of the median value, success rate> 60%). The means of age, TE, and hepatic growth factor were 61.5 years (DE 15.8), 29.6 Kpa (DE 18.5) and 916 pg/mL (DE 612), respectively. There were no significant differences between compensated and decompensated subjects related to sex, health status, anthropometric variables or transaminase levels. However, alcoholic and viral etiologies was more frequent among compensated patients (p = 0.003). The characteristics of the population according to the stage of the disease are described in Table 1.
Relationship between Hepatic Growth Factor and Stage of Hepatic Cirrhosis
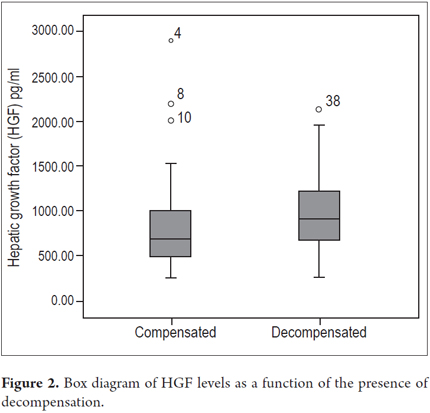
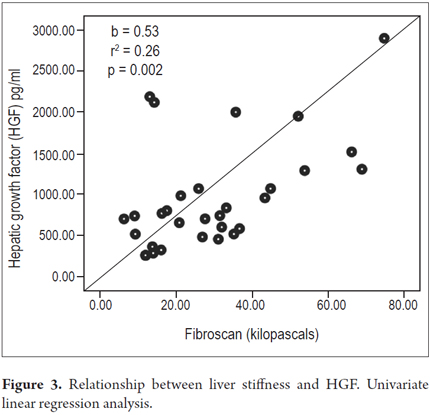
Higher levels of hepatic growth factor (HGF) were observed in patients with decompensated liver disease than in compensated patients (867 versus 1022 pg/mL) (Figure 2). However, these differences did not reach statistical significance (p = 0.47). The analysis of other factors related to the stage of the disease found a positive linear association between HGF and liver stiffness estimated by elastography (b = 0.53, r2 = 0.26, p = 0.002) (Figure 3), and a negative linear association with albumin (b = -0.62, r2 = 0.39, p <0.001) (Figure 4). HGF levels decreased as albumin levels increased. This relationship was maintained in the multivariate analysis, in which albumin was the only independent predictor of HGF levels (Tables 2 and 3).
DISCUSSION
As previously described, our data indicate a relationship between HGF and the stage of hepatic cirrhosis (14, 15). Albumin and TE, both markers of severity in cirrhosis (3, 19-21), had linear and significant relationships with plasma levels of HGF. HGF was initially identified in the serum of rats subjected to hepatectomy, (4) and its role in liver regeneration processes was demonstrated by stimulating DNA synthesis in hepatocytes in vivo and in vitro. (7, 8, 22-24)
In liver disease, serum HGF levels may be increased due to increased production and decreased liver clearance, since the liver is the organ through which most of the HGF is removed from circulation. (25) When HGF levels increase in chronic hepatic injury, one of its physiological actions is to induce the activation of liver progenitor cells, which favors tissue restoration (26) through the increase of cyclin itself and of cyclin-dependent kinases which play a critical role in the progression of the cell cycle under conditions of liver damage. (8)
This suggests that there is an association of severity of liver disease with HGF that has a double two-directional effect on the HGF/cMet pathway through which regeneration is stimulated. It is possible that in chronic liver injury, increased oxidative stress, reactive oxygen species generated in hepatocyte mitochondria, and the level of per-oxidized lipids from necrotic hepatic tissue maintain constant activation of the hepatic stellate cells thus favoring fibrogenesis in hepatic tissue. (27) This microenvironment could activate signals that inhibit the regenerative process and lead to blockade of DNA synthesis in the hepatocyte which in turn stimulates production of HGF and which would explain the elevation of plasma levels as the severity of the disease increases.
However, the effects of HGF on the cirrhotic liver have not been clearly established, and more studies are needed to identify the signaling pathways that activate HGF in cirrhosis and the mechanisms that prevent the development of an efficient process of liver regeneration.
Available evidence points to HGF as a marker of liver disease severity. In fact, some studies have even found positive associations between HGF and the Child-Pugh score, bilirubin, albumin, prothrombin time, AST and some proinflammatory cytokines. (14, 15)
HGF levels appear to also have prognostic implications as elevated levels of HGF in FHF decrease patient survival. (14). HGF has also been associated with the progression of fibrosis and the need for liver transplantation in patients with biliary atresia who have received surgical treatment and with the appearance of hepatocellular carcinoma. (28, 29)
Recently, the behavior of HGF and interleukin 6 (IL-6) has been studied in 60 patients with cirrhosis of alcoholic origin. (15) HGF levels were significantly higher in Child-Pugh B and C patients than in Child-Pugh A, cirrhotic patients and the control group. This study, which includes the largest number of cirrhotic patients to date, provides b evidence of the relationship between HGF and disease stage.
Although in our study patients with decompensated liver disease had higher levels of HGF, this relationship could not be confirmed. (p = 0.47) The size of the sample and the small number of subjects included in the decompensated phase are probably the reason for these results. On the other hand, the group of patients included in other studies of this association had more severe disease stages than did our sample, with higher bilirubin, INR and albumin levels. This circumstance has caused the differences in the levels of HGF between compensated and decompensated patients to be more pronounced which facilitates the identification of the relationship between HGF and Child-Pugh scores by these authors. (14, 15)
Albumin, which is a Child-Pugh component, is routinely used to assess hepatic biosynthesis capacity and has been shown to be a prognostic marker of progression in patients with compensated cirrhosis. (3) In our study it was tightly bound to HGF levels, so that as albumin decreased, HGF levels increased. These findings are similar to those found previously and support the role of HGF as a marker of severity in chronic liver disease. (14)
Cirrhosis has ceased to be considered a single, static stage of chronic liver disease and is now recognized as a broad spectrum from the histological, clinical and hemodynamic points of view with cirrhosis with different prognoses for progression and mortality. (1, 30)
A study that analyzed the association between hepatic histology and the presence of clinically significant portal hypertension showed that, in terms of cirrhosis, there may be different degrees of histological damage in patients classified at the same stage of the disease. (1, 30)
TE is able to determine the degree of hepatic fibrosis through the estimation of tissue stiffness using a vibrating wave. Values ââincrease parallel to the degree of hepatic damage. (19-21)
Among patients with established cirrhosis, elastography is able to identify a subpopulation of patients with more severe histological damage, with greater risks of decompensation, and can anticipate the appearance of hepatocellular carcinoma. (19-21) The positive linear relationship between TE and HGF observed in this study reflects its association with the severity of liver disease. Similar findings have been obtained with other biochemical and clinical markers characteristic of the more advanced stages of cirrhosis. (14, 31)
On the other hand, this study is the first to evaluate the relationship between TE and HGF. Although previous studies have described a relationship between the degree of fibrosis and HGF in patients with chronic hepatitis, (14) this relationship had not previously been evaluated in patients with established cirrhosis. In this context, TE is a useful tool for avoiding the risk of bleeding and other complications associated with hepatic biopsies while it can provide precise and objective estimates of the degree of histological damage without the bias common to qualitative scales that measure the degree of fibrosis. It thus increases the reproducibility and the robustness of the results.
Finally, by establishing a linear relationship between TE and HGF within a sample of patients with cirrhosis that is similar to that observed for albumin, bilirubin and prothrombin time, all markers of severity of chronic liver disease, our findings support the growing body of scientific information that recognizes that different degrees of histological damage can coexist in cirrhosis. (1, 14, 15, 30)
CONCLUSION
Hepatic growth factor (HGF) is a marker of severity in liver cirrhosis. Albumin and the degree of fibrosis determined by transient elastography (TE) were associated with HGF levels.
Acknowledgements
The authors would like to thank the University of Cartagena and Colciencias, for supporting this research project through the Young Investigators program.
REFERENCES
1. Nagula S, Jain D, Groszmann RJ, et al. Histological-hemodynamic correlation in cirrhosis-a histological classification of the severity of cirrhosis. J Hepatol. 2006;44:111-7. [ Links ]
2. Sethasine S, Jain D, Groszmann RJ, et al. Quantitative histological-hemodynamic correlations in cirrhosis. Hepatology. 2012;55:1146-53. [ Links ]
3. Ripoll C, Groszmann R, Garcia-Tsao G, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481-8. [ Links ]
4. Nakamura T, Nawa K, Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984;122:1450-9. [ Links ]
5. Russell WE, McGowan JA, Bucher NL. Partial characterization of a hepatocyte growth factor from rat platelets. J Cell Physiol. 1984;119:183-92. [ Links ]
6. Fabregat I, de Juan C, Nakamura T, et al. Growth stimulation of rat fetal hepatocytes in response to hepatocyte growth factor: modulation of c-myc and c-fos expression. Biochem Biophys Res Commun. 1992;189:684-90. [ Links ]
7. Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60-6. [ Links ]
8. Fausto N. Liver regeneration. J Hepatol. 2000;32:19-31. [ Links ]
9. Huh CG, Factor VM, Sanchez A, et al. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci. 2004;101:4477-82. [ Links ]
10. Borowiak M, Garratt AN, Wustefeld T, et al. Met provides essential signals for liver regeneration. Proc Natl Acad Sci. 2004;101:10608-13. [ Links ]
11. Phaneuf D, Moscioni AD, LeClair C, et al. Generation of a mouse expressing a conditional knockout of the hepatocyte growth factor gene: demonstration of impaired liver regeneration. DNA Cell Biol. 2004;23:592-603. [ Links ]
12. Suarez-Causado A, Caballero-Diaz D, Bertran E, et al. HGF/c-Met signaling promotes liver progenitor cell migration and invasion by an epithelial-mesenchymal transition-independent, phosphatidyl inositol-3 kinase-dependent pathway in an in vitro model. Biochim Biophys Acta. 2015;1853:2453-63. [ Links ]
13. Fujimoto J, Kaneda Y. Reversing liver cirrhosis: impact of gene therapy for liver cirrhosis. Gene Ther. 1999;6:305-6. [ Links ]
14. Shiota G, Okano J, Kawasaki H, et al. Serum hepatocyte growth factor levels in liver diseases: clinical implications. Hepatology. 1995;21:106-12. [ Links ]
15. Prystupa A, Kicinski P, Sak J, et al. Proinflammatory cytokines (IL-1alpha, IL-6) and hepatocyte growth factor in patients with alcoholic liver cirrhosis. Gastroenterol Res Pract. 2015;2015:532615. [ Links ]
16. Pavlov CS, Casazza G, Nikolova D, et al. Transient elastography for diagnosis of stages of hepatic fibrosis and cirrhosis in people with alcoholic liver disease. Cochrane Database Syst Rev. 2015;1:CD010542. [ Links ]
17. Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-13. [ Links ]
18. Ciccarelli N, Fabbiani M, Grima P, et al. Liver fibrosis is associated with cognitive impairment in HIV-positive patients. J Int AIDS Soc. 2014;17:19722. [ Links ]
19. Kitson MT, Roberts SK, Colman JC, et al. Liver stiffness and the prediction of clinically significant portal hypertension and portal hypertensive complications. Scand J Gastroenterol. 2015;50:462-9. [ Links ]
20. Pang JX, Zimmer S, Niu S, et al. Liver stiffness by transient elastography predicts liver-related complications and mortality in patients with chronic liver disease. PLoS One. 2014;9:e95776. [ Links ]
21. Singh S, Fujii LL, Murad MH, et al. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1573-84. [ Links ]
22. Tajima H, Nakamura T. Function, molecular structure and gene expression regulation of hepatocyte growth factor and its receptor. Nihon Rinsho. 1992;50:1918-25. [ Links ]
23. Kinoshita T, Tashiro K, Nakamura T. Marked increase of HGF mRNA in non-parenchymal liver cells of rats treated with hepatotoxins. Biochem Biophys Res Commun. 1989;165:1229-34. [ Links ]
24. Hamanoue M, Kawaida K, Takao S, et al. Rapid and marked induction of hepatocyte growth factor during liver regeneration after ischemic or crush injury. Hepatology. 1992;16:1485-92. [ Links ]
25. Porowski D, Wirkowska A, Hryniewiecka E, et al. Liver failure impairs the intrahepatic elimination of interleukin-6, tumor necrosis factor-alpha, hepatocyte growth factor, and transforming growth factor-beta. Biomed Res Int. 2015;2015:934065. [ Links ]
26. Vessey CJ, de la Hall PM. Hepatic stem cells: a review. Pathology. 2001;33:130-41. [ Links ]
27. Jiang JX, Venugopal S, Serizawa N, et al. Reduced nicotinamide adenine dinucleotide phosphate oxidase 2 plays a key role in stellate cell activation and liver fibrogenesis in vivo. Gastroenterology. 2010;139:1375-84. [ Links ]
28. Uchida K, Inoue M, Otake K, et al. The significance of serum hepatocyte growth factor levels in planning follow-up of postoperative jaundice-free patients with biliary atresia. J Pediatr Surg. 2006;41:1657-62. [ Links ]
29. Shiota G, Rhoads DB, Wang TC, et al. Hepatocyte growth factor inhibits growth of hepatocellular carcinoma cells. Proc Natl Acad Sci. 1992;89:373-7. [ Links ]
30. Garcia-Tsao G, Friedman S, Iredale J, et al. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445-9. [ Links ]
31. DAmico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-31. [ Links ]











 texto en
texto en