Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.32 no.1 Bogotá ene./mar. 2017
https://doi.org/https://doi.org/10.22516/25007440.128
A Structured Review of Hepatotoxic Medicines during Pregnancy
Alejandra Cano P. (1), Pedro Amariles PhD.(2)
(1)Pharmaceutical Chemist, MSc. student in Pharmaceutical and Food Sciences in the Faculty of Pharmaceutical and Food Sciences and the Pharmaceutical Promotion and Prevention Group at the University of Antioquia in Medellin, Colombia Mail: alejandra.canop@udea.edu.co
(2) Pharmaceutical Chemist, Doctor of Pharmacy, Professor in the Department of Pharmacy in the Faculty of Pharmaceutical and Food Sciences and the Pharmaceutical Promotion and Prevention Group at the University of Antioquia in Medellin, Colombia
Received:Â Â Â 12-08-16Â Â Â Accepted:Â Â Â 16-12-16
Abstract
Objective: The aim of this study is to identify hepatotoxic drugs and determine the main characteristics of drug hepatotoxicity in pregnancy.
Method: We conducted a structured review of PubMed/Medline, EMBASE and Web of Science using the terms âDrug induced liver injuryâ OR Hepatotoxicity AND Pregnancy. The search included articles in English and Spanish with information on hepatotoxic drugs in pregnancy that were posted between 2005 and 2015. Items not related to pregnancy or hepatotoxicity or which were related to other causes of liver disease or to hepatotoxicity due to other substances were excluded. Drug information and patient characteristics were recorded in a table. The probability of occurrence of hepatotoxicity was assessed and grouped into three categories: defined, probable, and possible. The categories of some drugs were determined with the RUCAM method.
Results: We identified 488 articles of which 46 were selected. Twelve agents that are potentially heaptotoxic for pregnant women were identified: acetaminophen, alpha-methyldopa, methotrexate, methotrexate, saquinavir, nevirapine, propylthiouracil, methimazole, carbimazole, nitrofurantoin, acetylsalicylic acid and piperidolate. Some characteristics associated with the drugs were time of reaction onset, weeks of pregnancy (3-36), risk factors (age and chronic diseases), clinical manifestations (elevation of transaminases, pruritus, and jaundice) and outcomes (liver transplantation and death of mother and/or fetus).
Conclusion: Acetaminophen, alpha-methyldopa, labetalol, methotrexate, saquinavir, nevirapine, propylthiouracil, methimazole, carbimazole, nitrofurantoin, acetylsalicylic acid and piperidolate can cause hepatotoxicity in pregnant patients. In addition to dosage and exposure time, patient age and gestation time can influence presentation and severity of hepatotoxicity.
Keywords
Hepatotoxicity, pregnancy, drug-induced liver disease.
INTRODUCTION
Prescription of drugs during pregnancy requires additional knowledge, especially about physiological changes that may lead to pharmacokinetic and pharmacodynamic variability and about possible fetal toxicity of the drugs. (1) At the time of prescription, the following fetal risk categories of medicines should be considered:
- A: No fetal risks in trimester
- B: No fetal risks have been demonstrated in animal studies
- C: Animal studies show adverse effects on the fetus
- D: There is clear evidence of fetal risk, use only after under risk-benefit assessment
- X: Contraindicated in pregnancy because of clearly defined evidence of fetal damage (2-4).
Beyond the risk of teratogenesis, hepatic toxicity is a significant risk for the fetus and even for the pregnant woman. (1, 4)
Gastrohepatic alterations characteristic of pregnancy, which are reversed after delivery, may develop. Among them are hyperemesis gravidarum, cholestasis gravidarum, toxemia gravidarum, HELLP syndrome and acute pregnancy steatosis which have nonspecific symptoms including nausea, vomiting, jaundice, pruritus and increased transaminases. (5). The physician must identify these alterations and differentiate them from hepatic lesions associated with the use of drugs. This work can be improved by the availability of accurate and reliable information on medications and other substances that may cause hepatic toxicity to the mother and/or damage to the fetus.
Although information is available about hepatic toxicity of many drugs in pregnancy including macrolides and tetracyclines, (6) antiretrovirals (nevirapine), (6-8) psychiatric drugs (lithium, carbamazepine), (9) and antithyroid drugs (Propylthiouracil and methimazole) (10, 11) this information is often incomplete and limited to characteristics related to the adverse event. The objectives of this study were to identify drugs that are hepatotoxic during pregnancy and to determine the most important characteristics associated with hepatotoxicity.
METHOD
Structured Review
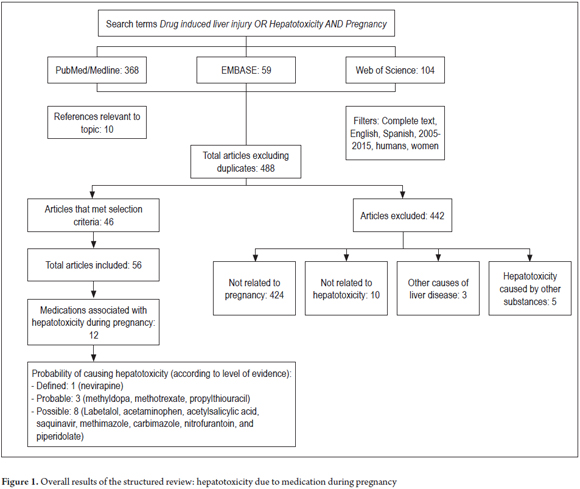
A structured review was conducted in PubMed/Medline, EMBASE and Web of Science using the terms, âDrug induced liver injury OR Hepatotoxicity AND Pregnancyâ. Search filters were used to find articles in English and Spanish, articles about humans and women, articles full text access and articles with key words in the title or abstract published between 2005 and 2015. Articles with information related to hepatotoxicity in pregnancy caused exclusively by medications were included. Articles with information unrelated to pregnancy or drug hepatotoxicity, as well as those related to other causes of liver disease or other types of substances, were excluded. In order to expand the sources of information, the references of the articles included which were considered relevant to the topic were also taken into account. Articles were reviewed and information was extracted by two reviewers.
The following information was recorded: pharmacological group, drug, the number of articles that identified an event, classification of risk in pregnancy according to the FDA, the dose (mg/day) used, the time of onset of the event , clinical manifestations, likelihood of hepatotoxicity, (12, 13) the prevalence of hepatotoxicity due to each drug, weeks of gestation, risk factors, outcome, how the event was treated, and liver enzyme values.
Assessment of the Occurrence of Hepatotoxicity
The assessment of the occurrence of hepatotoxicity was based on the probability of its occurrence. This was established in three categories, consistent with the level of evidence found:
- Defined: evidence in meta-analyzes, systematic reviews or clinical trials (randomized or not)
- Probable: analytical or descriptive studies in three or more clinical case reports
- Possible: fewer than three reported cases or recommendations from expert groups. (12)
In addition, when case reports provided sufficient information, the Roussel Uclaf Causality Assessment Method (RUCAM) was used to determine causality of the suspected drug. (13)
RESULTS
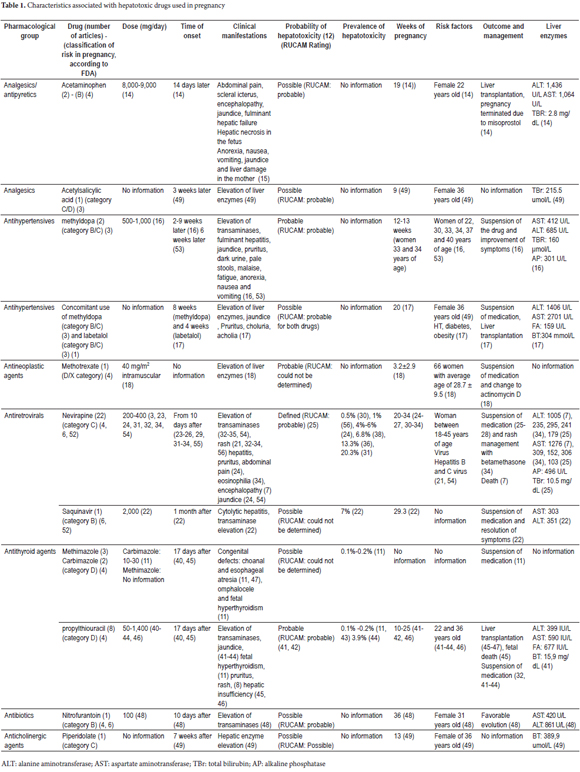
Five hundred and thirty-one articles were identified. When the duplicates were removed, 488 references were obtained. Of these, 46 were included. Ten additional articles considered relevant to the theme were also included (Figure 1). With the information from these articles, the following 12 drugs with probability of causing hepatic toxicity in pregnancy were identified: acetaminophen, methyldopa, methotrexate, saquinavir, nevirapine, propylthiouracil, methimazole, carbimazole, nitrofurantoin, acetylsalicylic acid and piperidolate. In addition, the principal characteristics associated with each of these were determined (Table 1). Relevant data on each drug identified as having probability of causing hepatotoxicity in pregnancy in the published case reports was assessed and is presented below.
Analgesics/antipyretics
Acetaminophen is used to control pain at considerable dosages. Hepatic toxicity was identified in a 22-year-old woman at 14 days. Liver transplantation and termination of pregnancy with misoprostol were required. (14) Toxicity was manifested by abdominal pain, jaundice, liver damage and hepatic necrosis in the fetus. (14, 15) In general, it is accepted that toxicity is due to a metabolite of acetaminophen, N-acetyl-p-benzoquinone imine.
Antihypertensives
Methyldopa administered alone has had 5 cases of reported hepatic toxicity which occurred between weeks two and nine after start of treatment in women between the ages of 22 and 40 years. The drug should be discontinued to improve elevation of transaminases and symptoms such as jaundice, itching, fatigue, anorexia, nausea, and vomiting. (16)
Methyldopa administered together with labetalol has had one case reported. A woman who used these medications concomitantly to treat high blood pressure had symptoms including jaundice and pruritus at 20 weeks of pregnancy which developed into liver failure that required transplantation. It was not possible to establish which medication was responsible for the liver damage. (17)
Antineoplastics
Methotrexate is used to treat gestational trophoblastic disease. It was associated with hepatotoxicity in 7 case reports which were characterized by increased hepatic enzymes. The drug was suspended and replaced with other alternatives. (18)
Antiretrovirals
The objectives of pharmacotherapy with antiretroviral drugs include preventing transmission of the human immunodeficiency virus (HIV) from mother to child. (7, 19, 20) Hepatic toxicity due to antiretrovirals occurs in 5% of cases. (21)
Saquinavir has had one case of hepatotoxicity reported. One month after starting antiretroviral therapy (2,000 mg/day), a patient who was 29 weeks pregnant developed a hepatocellular lesion characterized by elevation of transaminases and hepatitis. Suspension of medication was recommended in order to improve the symptoms (22).
Nevirapine is a drug with defined likelihood of causing hepatotoxicity. However, the risk of toxicity in neonates from indirect exposure has not yet been defined. (20) Toxic reactions may occur between 10 and 280 days after initiation of treatment. (23-26) Hepatotoxicity has been reported in patients aged 18-45 years, (21, 24-27) with elevated transaminases and rashes. (23, 26-37) One case of ductopenia characterized by a type of cholestatic lesion has been reported. (25) Management is based on the suspension of nevirapine and change of pharmacotherapy. (23, 26-29) The frequency of hepatotoxicity due to this drug varies between 0.5% and 20%. (24, 30, 31, 34, 37-39)
Antithyroid agents
These medicines have been used to treat pregnant women with problems of hyperthyroidism and Graves' disease. The frequency of hepatotoxicity for this group of drugs ranges from 0.1%Â to 0.2%, and is idiosyncratic. (11)
Propylthiouracil has been reported to cause edema, jaundice, pruritus and rashes in women who are 10-14 weeks pregnant. (40-42) Management has been through suspension of the drug and the treatment of symptoms with pharmacotherapy. (40-44) Hepatic toxicity was idiosyncratic, with consequences such as hepatic failure, liver transplantation, maternal death and neonatal death. (42, 45-47) Nevertheless, its use may be chosen during the first trimester of pregnancy because it is associated with a lower incidence of birth defects than methimazole. (45, 47)
Methimazole and carbimazole can cause cholestatic hepatic toxicity of type as well as choanal and esophageal atresia, (11, 47) omphalocele, and fetal hyperthyroidism. (11) In general, its use should be avoided during the first trimester of pregnancy because of the high incidence of birth defects. (45)
Antibiotics
Nitrofurantoin has been reported to have caused elevation of transaminases in a 36-weeks pregnant woman patient who was being treated for a urinary tract infection. After suspensión of the medication, symptoms resolved. (48)
Other Medications
Acetylsalicylic acid and piperidolate have had two cases of 36-year-old women who developed liver damage from these drugs reported. Both had elevated hepatic enzymes, one at 9 weeks of pregnancy due to acetylsalicylic acid, and the other at 13 weeks due to piperidolate. (49)
DISCUSSION
This study identified twelve drugs from seven pharmacological groups which are likely to cause hepatic toxicity during pregnancy: acetaminophen and acetylsalicylic acid (analgesics), methyldopa and labetalol (antihypertensive agents), methotrexate (antineoplastic agents), saquinavir and nevirapine (antiretroviral agents), propylthiouracil, methimazole and carbimazole (antithyroid agents), nitrofurantoin (antibiotics) and piperidolate (anticholinergic agent). Although hepatic toxicity due to psychiatric medications has been reported, (9) this structured review found no evidence of a clear causal relationship between the use of these drugs and hepatotoxicity during pregnancy. This could be due to limited information about this topic.
In general, the establishment of causality of hepatotoxicity in pregnancy requires determination of whether the cause is the drug cause or other pathologies typical of pregnancy. These include intrahepatic cholestasis in the third trimester which is associated with hormonal changes and polymorphisms. (50-56)
Drug typing together with characterization of the most influential factors in this health problem could contribute to early identification of problems related to these pharmacological agents. Teenage women and women in their twenties may be at increased risk for liver problems due to the use of medications such as methotrexate, methyldopa and propylthiouracil. (16, 18, 40, 46). Dosages used by patients presenting with hepatotoxicity during pregnancy were within the usual limits, except for acetaminophen. In that case the patient erroneously believed that 8-9 g/day was safe. (14),
Information about the frequency of toxic reactions in the liver during gestation is scarce for acetaminophen, acetylsalicylic acid, piperidolate, nitrofurantoin, methotrexate and methyldopa whereas for antithyroid drugs, hepatic toxicity has been found to occur in 0.1% to 0.2% of the pregnant population who use these drugs. (11) The time to onset of toxic reactions after administration of antithyroid drugs and acetaminophen has been estimated in days, (14, 40) whereas for saquinavir, methyldopa and piperidolate it has been estimated in months. (16, 22, 49) Hepatotoxic reactions to methotrexate have occurred when patients were in or around the third week of pregnancy but have occurred in the tenth week or later with acetaminophen, methyldopa, nitrofurantoin, nevirapine, saquinavir and antithyroid agents. (16, 18, 22, 33, 46, 48)
According to the FDA's risk classification, antithyroid drugs (D) and methotrexate (D/X) are the drugs with the highest risk of causing harm to the fetus and may even cause death. (4, 45) Nevertheless, this review found one case of hepatotoxicity in a pregnant woman due to acetaminophen (classified in category B) has been reported to have resulted in maternal liver transplantation and fetal death. (4, 14)
Elevation of transaminases is a frequent and important manifestation of liver damage caused by drugs used during pregnancy. (11, 16, 18, 22, 32-35). In the cases of acetaminophen, nitrofurantoin, methyldopa and nevirapine, levels of aspartate-aminotransferase (AST) and alanine-aminotransferase (ALT) were more than 10 times the upper limit of normal. (7 , 14, 16, 48) Other nonspecific symptoms such as jaundice, vomiting, pruritus and anorexia may also appear in these patients. (15, 16, 34, 40) On the other hand, acetaminophen may cause hepatic necrosis in the fetus while methimazole and carbimazole may cause birth defects (11, 15, 47). Management of hepatotoxicity in pregnant women has been suspension of the suspected drug which has improved symptoms in some cases.
There are doubts about the safety of nevirapine during pregnancy as documented by the varied incidence reported in studies. (30, 31, 36) In this sense, it is important to emphasize that use of nevirapine can cause liver toxicity in pregnant women even though it does not seem to cause birth defects. (7, 8, 34)
The probability of causing hepatotoxicity of the drugs identified in this review varies. Most of them presented differences in the assessments from the two different methods used according to the level of evidence and the RUCAM scale.
This review has shown that, in addition to dosage and time of exposure to a possibly hepatotoxic drug, the the age of the pregnant woman and the time of gestation should be variables used in assessment and clinical follow-up of these women. This practice could help control the risk of fatal outcomes associated with hepatotoxicity for the mother and/or fetus. Nevertheless, these measures require further study to explore this effect. In this sense, it is important to emphasize that the risk of hepatotoxicity for the group of drugs identified varies from defined to probable to possible and that this information could be used to improve risk/benefit analysis.
CONCLUSION
Acetaminophen, methyldopa, methotrexate, saquinavir, nevirapine, propylthiouracil, methimazole, carbimazole, nitrofurantoin, acetylsalicylic acid and piperidolate may cause hepatotoxicity in pregnant patients. In addition to the dose and time of exposure to the drug, age and gestation time may influence the presentation and severity of hepatotoxicity. The available information on hepatic toxicity of drugs during pregnancy is limited.
Financing
The Pharmaceutical Promotion and Prevention group received funding from the 2014-2015 Sustainability Call of the Research Development Committee (CODI) of the University of Antioquia, Medellín, Colombia.
REFERENCES
1. Vázquez-Benitez E. El uso de algunos farmacos y sus riesgos durante el embarazo. Gac Méd Mex. 1996;132(5):541-3. [ Links ]
2. Lorente S, Serrano T. Enfermedades hepáticas propias del embarazo. Rev Esp Enf Dig. 2010;102(8):505-6. [ Links ]
3. Gallego M, Delgado L, Campos M, et al. Actualización del uso de fármacos durante el embarazo: categorías de riesgo. Farm Hosp. 2014;38(4):364-78. [ Links ]
4. Abad F, Pons J, Micó M, et al. Categorías de riesgo de los medicamentos utilizados durante el embarazo: Guía rápida de consulta. Farm Aten Prim. 2005;3(2):49-61. [ Links ]
5. Pérez A, Allende M, Agustín M, et al. Teratogénesis: clasificaciones. Farm Hosp. 2002;26(3):171-7. [ Links ]
6. Vallano A, Arnau J. Antimicrobianos y embarazo. Enferm Infecc Microbiol Clin. 2009;27(9):536-42. [ Links ]
7. Lyons F, Hopkins S, Kelleher B, et al. Maternal hepatotoxicity with nevirapine as part of combination antiretroviral therapy in pregnancy. HIV Med. 2006;7(4):255-60. [ Links ]
8. Coster L, Kumar P. Contemporary role of nevirapine in HIV treatment. AIDS Rev. 2012;14(2):132-44. [ Links ]
9. Gómez E, Lizbeth C. Manejo del trastorno bipolar en el embarazo. Rev Med MD. 2012;3(3):154-62. [ Links ]
10. Petit D. The use of antithyroid drugs. Calif Med. 1951;74(2):99-104. [ Links ]
11. Cassina M, Doná M, Di Gianantonio E, et al. Pharmacologic treatment of hyperthyroidism during pregnancy. Birth Defects Res Clin Mol Teratol. 2012;94(8):612-9. [ Links ]
12. Amariles P, Giraldo N, Faus M. Interacciones medicamentosas: aproximación para establecer y evaluar su relevancia clínica. Med Clin (Barc). 2007;129(1):27-35. [ Links ]
13. Danan G, Benichou C. Causality assessment of adverse reactions to drugs-I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46(11):1323-30. [ Links ]
14. Thornton S, Minns A. Unintentional chronic acetaminophen poisoning during pregnancy resulting in liver transplantation. J Med Toxicol. 2012;8:176-8. [ Links ]
15. Wilkes J, Clark L, Herrera J. Acetaminophen overdose in pregnancy. South Med J. 2005;98(11):1118-23. [ Links ]
16. Slim R, Ben Salem C, Hmouda H, et al. Hepatotoxicity of alpha-methyldopa in pregnancy. J Clin Pharm Ther. 2010;35:361-3. [ Links ]
17. Firoz T, Webber D, Rowe H. Drug-induced fulminant hepatic failure in pregnancy. Obstet Med. 2015;8(4):190-2. [ Links ]
18. Gilani M, Fariba B, Behtash N, et al. The WHO score predicts treatment outcome in low risk gestational trophoblastic neoplasia patients treated with weekly intramuscular methotrexate. J Cancer Res Ther. 2013;9(1):38-43. [ Links ]
19. Solís I, Muñoz E, Tomás J, et al. Características maternas en una cohorte de gestantes con infección por el VIH-1. Med Clin (Barc). 2006;127(4):121-5. [ Links ]
20. Minniear T, Zeh C, Polle N, et al. Rash, Hepatotoxicity and hyperbilirubinemia among Kenyan infants born to hiv-infected women receiving triple-antiretroviral drugs for the prevention of mother-to-child HIV transmission. Pediatr Infect Dis J. 2012;31(11):1155-7. [ Links ]
21. Snijdewind I, Smit C, Godfried M, et al. HCV coinfection, an important risk factor for hepatotoxicity in pregnant women starting antiretroviral therapy. J Infect. 2012;64(4):409-16. [ Links ]
22. Brunet C, Reliquet V, Jovelin T, et al. Effectiveness and safety of saquinavir / ritonavir in HIV-infected pregnant women: INEMA cohort. Med Mal Infect. 2012;42:421-8. [ Links ]
23. Bottaro E, Huberman M, Iannella M, et al. Nevirapine-associated toxicity in clinical practice in Buenos Aires, Argentina. J Int Assoc Physicians AIDS Care. 2010;9(5):306-12. [ Links ]
24. Peters P, Polle N, Zeh C, et al. Nevirapine-associated hepatotoxicity and rash among HIV-infected pregnant women in Kenya. J Int Assoc Physicians AIDS Care. 2012;11(2):142-9. [ Links ]
25. Kochar R, Nevah M, Lukens F, et al. Vanishing bile duct syndrome in human immunodeficiency virus : Nevirapine hepatotoxicity revisited. World J Gastroenterol. 2010;16(26):3335-8. [ Links ]
26. Jamisse L, Balkus J, Hitti J, et al. Antiretroviral-associated toxicity among HIV-1 – Seropositive pregnant women in Mozambique receiving nevirapine-based regimens. Acquir Immune Defic Syndr. 2007;44(4):371-6. [ Links ]
27. Timmermans S, Tempelman C, Godfried M, et al. Nelfinavir and nevirapine side effects during pregnancy. AIDS Rev. 2005;19:795-9. [ Links ]
28. Phanuphak N, Apornpong T, Teeratakulpisarn S, et al. Nevirapine-associated toxicity in HIV-infected Thai men and women, including pregnant women. HIV Med. 2007;8:357-66. [ Links ]
29. Coffie P, Tonwe-Gold B, Tanon A, et al. Incidence and risk factors of severe adverse events with nevirapine-based antiretroviral therapy in HIV-infected women. MTCT-Plus program, Abidjan, Côte dIvoire. BMC Infect Dis. 2010;10:188. [ Links ]
30. Kilewo C, Karlsson K, Ngarina M, et al. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: The Mitra Plus study. J Acquir Immune Defic Synd. 2009;52(3):406-16. [ Links ]
31. Kondo W, Carraro E, Prandel E, et al. Nevirapine-induced side effects in pregnant women – experience of a Brazilian university hospital. BJID. 2007;11(6):544-8. [ Links ]
32. Natarajan U, Pym A, Mcdonald C, et al. Safety of nevirapine in pregnancy. HIV Medicine. 2007;8:64-9. [ Links ]
33. Marazzi M, Germano P, Liotta G, et al. Safety of nevirapine-containing antiretroviral triple therapy regimens to prevent vertical transmission in an African cohort of HIV-1-infected pregnant women. HIV Medicine. 2006;7:338-44. [ Links ]
34. Joy S, Poi M, Hughes L, et al. Third-trimester maternal toxicity with nevirapine use in pregnancy. Obstet Gynecol. 2005;106(5):1032-8. [ Links ]
35. Ouyang D, Shapiro D, Lu M, et al. Increased risk of hepatotoxicity in HIV-infected pregnant women receiving antiretroviral therapy independent of nevirapine exposure. AIDS Rev. 2009;23(18):2425-30. [ Links ]
36. Ouyang D, Brogly S, Lu M, et al. Lack of increased hepatotoxicity in HIV-infected pregnant women receiving nevirapine compared to other antiretrovirals. AIDS Rev. 2010;24(1):109-14. [ Links ]
37. Pillay P, Black V. Safety, strength and simplicity of efavirenz in pregnancy. South Afr J HIV Med. 2012;13(1):28-33. [ Links ]
38. Ford N, Calmy A, Andrieux-Meyer I, et al. Adverse events associated with nevirapine use in pregnancy: a systematic review and meta-analysis. AIDS Rev. 2013;27:1135-43. [ Links ]
39. Aaron E, Kempf M-C, Criniti S, et al. Adverse events in a cohort of HIV infected pregnant and non-pregnant women treated with nevirapine versus non-nevirapine antiretroviral medication. PLoS One. 2010;5(9):e12617. [ Links ]
40. Ekiz B, Soysal N, Kirkizlar O, et al. Effectiveness of preoperative plasmapheresis in a pregnancy complicated by hyperthyroidism and anti-thyroid drug-associated angioedema. Gynecol Endocrinol. 2013;29(5):508-10. [ Links ]
41. Miyamura T, Kanda T, Minemura S, et al. Acute liver failure associated with propylthiouracil in a pregnant 26-year-old woman. Case Rep Gastroenterol. 2013;7(2):240-4. [ Links ]
42. Sequeira E, Wanyonyi S, Dodia R. Severe propylthiouracil-induced hepatotoxicity in pregnancy managed successfully by liver transplantation: A case report. J Med Case Rep. 2011;5:461. [ Links ]
43. Lo J, Rivkees S, Chandra M, et al. Gestational thyrotoxicosis, antithyroid drug use and neonatal outcomes within an integrated healthcare delivery system. Thyroid. 2015;25(6):698-705. [ Links ]
44. Yoshihara A, Noh J, Watanabe N, et al. Frequency of adverse events of antithyroid drugs administered during pregnancy. J Thyroid Res. 2014;2014:952352. [ Links ]
45. Akmal A, Kung J. Propylthiouracil, and methimazole, and carbimazole-related hepatotoxicity. Expert Opin Drug Saf. 2014;13(10):1397-406. [ Links ]
46. Glinoer D, Cooper D. The propylthiouracil dilemma. Curr Opin Endocrinol Diabetes Obes. 2012;19(5):402-7. [ Links ]
47. Azizi F, Amouzegar A. Management of hyperthyroidism during pregnancy and lactation. Eur J Endocrinol. 2011;164:871-6. [ Links ]
48. Aksamija A, Horvat G, Habek D, et al. Nitrofurantoin-induced acute liver damage in pregnancy. Arh Hig Rada Toksikol. 2009;60:357-61. [ Links ]
49. Tsuyoshi H, Nishijima K, Takahashi J, et al. Management of druginduced hyperbilirubinaemia in early pregnancy. S Afr J Obstet Gynaecol. 2013;19(1):22-3. [ Links ]
50. Dietrich C, Geier A. Effect of drug transporter pharmacogenetics on cholestasis. Expert Opin Drug Metab Toxicol. 2014;10(11):1533-51. [ Links ]
51. Magnus P, Meier P, Stieger B. Genetic determinants of drug-induced cholestasis and intrahepatic cholestasis of pregnancy. Semin Liver Dis. 2010;30(2):147-59. [ Links ]
52. Peña J, Ramos J, Pedrol P, et al. Tratamiento de la mujer embarazada infectada por el VIH. Enferm Infecc Microbiol Clin. 2002;20(Suppl 2):29-34. [ Links ]
53. Ali T, Srinivasan N, Le V, et al. Alpha-methyldopa hepatotoxicity in pregnancy. J Coll Physicians Surg Pak. 2009;19(2):125-6. [ Links ]
54. Andreotti M, Pirillo M, Liotta G, et al. The impact of HBV or HCV infection in a cohort of HIV-infected pregnant women receiving a nevirapine-based antiretroviral regimen in Malawi. BMC Infect Dis. 2014;14:180. [ Links ]
55. Bera E, Mia R. Safety of nevirapine in HIV-infected pregnant women initiating antiretroviral therapy at higher CD4 counts: A systematic review and meta-analysis. S Afr Med. 2012;102(11):855-9. [ Links ]
56. Cecchini D, Urueña A, Trinidad P, et al. HIV and pregnancy: maternal and neonatal evolution. Medicina (B Aires). 2011;71(5):432-6. [ Links ]











 texto en
texto en