Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.32 no.1 Bogotá Jan./Mar. 2017
https://doi.org/https://doi.org/10.22516/25007440.129
Two Cases of Reversed Gastric Tube (RGT) Esophagoplasty at a Third Level Hospital in Bogota, Colombia and Literature Review
Carlos Leal Buitrago MD (1), Felipe Bernal Santos MD (2), Luis Felipe Cabrera Vargas MD. (3)
(1) General Surgeon and Gastroenterologist at Universidad El Bosque, Fundación Universitaria Ciencias de la Salud, and Clínica El Bosque in Bogotá, Colombia
(2) General Surgeon at Universidad El Bosque and Clínica El Bosque in Bogotá, Colombia
(3) Resident in General Surgery at Universidad El Bosque and Clínica El Bosque in Bogotá, Colombia. Mail: luis.felipe.cabrera@hotmail.com
Received:Â Â Â 26-04-16Â Â Accepted:Â Â Â 16-12-16
Abstract
Introduction: The principal lesions in the hypharynx and esophagus are due to caustic burns and esophageal cancer which account for 17% to 23% of all events that compromise these two structures. They account for much of the surgery, especially for the challenge of major reconstruction. This study presents our series of cases using reversed gastric tube (RGT) esophagoplasty and presents a review of the literature and a critical discussion of the main aspects of this procedure. Methods: Patients underwent RGT esophagoplasties from January 2010 to January 2015. Results: One patient developed stenosis of the cervical anastomosis which was managed with endoscopic dilations. None of the patients developed dysphagia, clinical symptoms of dumping syndrome or delayed gastric emptying as the result of dietary modifications. Gastric reflux occurred in both patients and was teated with proton pump inhibitors. Discussion: RGT esophagoplasty is not often used for reconstruction after a total esophagectomy. Compared to the conventional technique of gastric ascent, interposition of the colon and supercharged reversed gastric tube techniques, it has the advantages that it is a one-step operation and is a simple procedure requiring only one anastomosis. It can be moved to the cervical region or even to the pharyngeal esophagus to create an anastomosis. Conclusions: This technique allows the creation of a longer duct for esophageal reconstruction and has a low complication rate with no mortality.
Keywords
Reversed gastric tube, caustic esophageal burn, esophageal cancer.
INTRODUCTION
Most important lesions in the hypopharynx and esophagus are due to caustic burns and esophageal cancer. These can present and compromise the two structures from 17% to 23%, which generates the need for a great resection and, in turn, the challenge of a great reconstruction, consisting of a successful replacement of the esophagus and restoration Of pharyngeal function. In these cases, a large graft is required for cervical pharyngeal reconstruction which increases the risk of distal ischemia. Initially, interposition of the colon was the method of choice for the reconstruction of massive defects like this, but in some cases microsurgery is necessary. It presents an incidence of necrosis of approximately 5.8% and complications are difficult to manage. (1, 2)
In contrast, the use of the stomach for the graft dos not require mesenteric dissection and avoids colloidal anastomosis and gastric anastomosis. However, there is a limitation on the length of the graft of the unreversed gastric tube that is associated with great morbidity. The global incidence of complications ranges from 26% to 55%, and of these mediastinitis secondary to graft necrosis is the most catastrophic. Reversed gastric tube esophagoplasty is an option for esophageal replacement that uses a tube created from the inverted major curvature that was first described by Beck and Carrell in 1905. However, the first successful case of esophageal reconstruction using this technique in a human was reported in 1951 by Gavriliu and Georgescu. (3) They used this technique in 93.5% of 768 patients of all ages who had or malignant esophageal pathologies. In 1955, Heimlich and Winfield used this procedure in eight dogs, (4, 5), and in 1957 they corroborated the utility of this technique in esophageal reconstruction in humans. In 1972 they published a series of 53 cases which popularized the technique by presenting its advantages. These include the possibility of reaching the cervical and pharyngeal region by any of the mediastinal routes, use of only one anastomosis and satisfactory restoration of swallowing. However, the long line of gastric sutures and irregular blood supply involved may be considered disadvantages. Postoperative fistulas and stenoses at the anastomosis level were found in 7.5% of the patients in the Gavriliu series and in 12.5% ââin the Georgescu series. (3) More studies are needed to refine application of the reversed gastric tube esophagoplasty and to improve the blood supply for the reconstructed esophageal segment.
In this study we present two cases of the use of this technique and a review of the literature with a critical discussion of the main aspects of this procedure. (5)
PATIENTS AND METHODS
We present two patients who underwent esophageal reconstruction using reversed gastric tube esophagoplasty from January 2010 to January 2015. One patient was 22 years old, the other was 46 years old, and one was a man, and the other was a woman. Both had suffered hypopharyngeal and esophageal caustic burns. Total transhiatal esophagectomies were performed. The procedure used an esophagogastric cervical anastomosis 3 cm from the cricopharyngeal muscle for the woman patient and a pharyngogastric anastomosis for the male patient. Reconstruction used reversed gastric tube esophagoplasty due to the massive size of the resection and the large anastomoses. Patients signed informed consent forms prior to procedures. Laparoscopic gastrostomies were used to place feeding tubes towards the minor curvature of the gastric body. Clinical characteristics of patients are described in Table 1.
Preoperative Evaluation
Our patients with caustic esophageal burns suffered in suicide attempts are initially evaluated and managed with upper digestive endoscopy within the first 48 hours after admission to establish the degree of severity of the burn, define the patient's prognosis and determine treatment. Afterwards, proton pump inhibitors (PPI) and antibiotics are administered as recommended by Conitini et al. (6). There is no administration of corticosteroids, as they have not been shown to reduce short-term and medium-term risks of stenosis, as two systematic reviews by Peclova and Fulton have detailed. (7, 8).
During the first 72 hours these two patients were under continuous clinical surveillance in the intermediate intensive care unit. Metabolic support was initiated with total parenteral nutrition. An endoscopy of the upper digestive tract was performed between the second and third week to determine whether there were any stenoses. Due to occurrence of severe stenosis in our series of cases, a laparoscopic stamens gastrostomy was performed on day 21. It was placed near the lower curvature of the gastric corpus. Prior to the introduction of the feeding tube, upper digestive endoscopy was performed intraoperatively through the gastrostomy to establish the presence of any burns, the length of burns and to locate the esophageal stricture. Both patients were considered to be surgical candidates since they presented no significant comorbidities. Patients were assessed by the psychiatry service, who determined that their mental states were apt to handle major surgery, and that the patients wanted to restore normal oral intake.
Surgical Technique
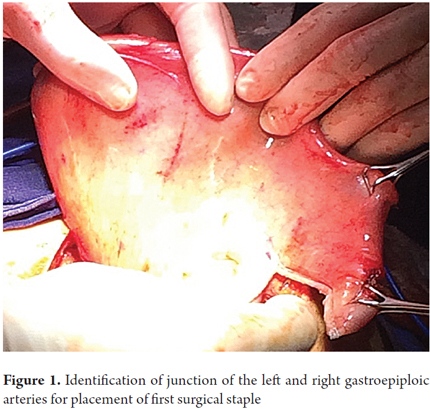
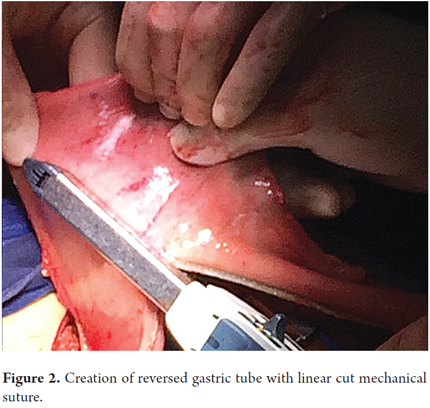
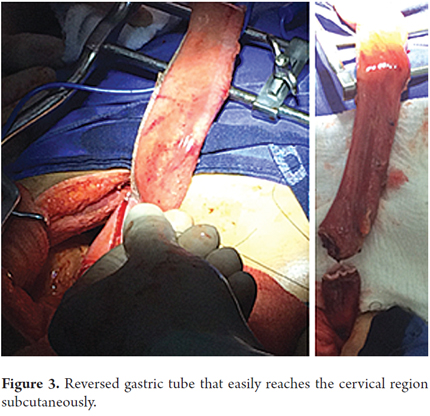
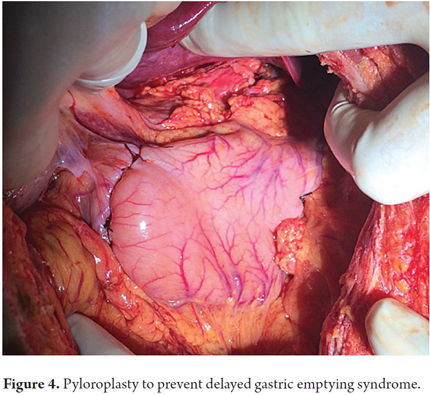
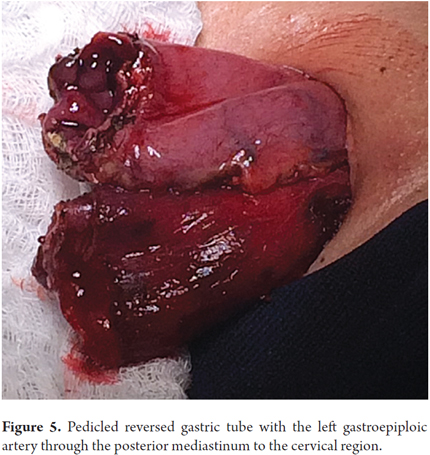
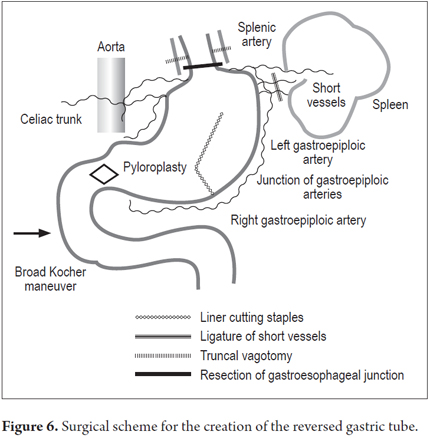
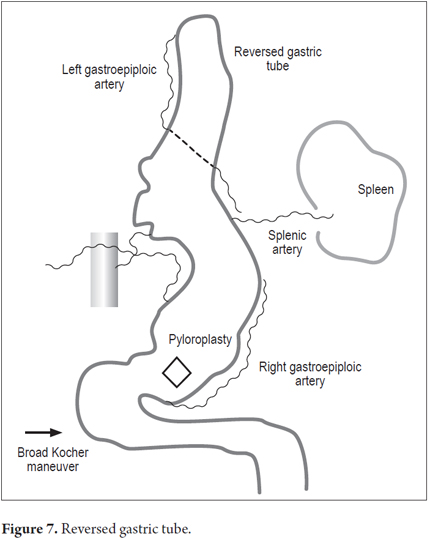
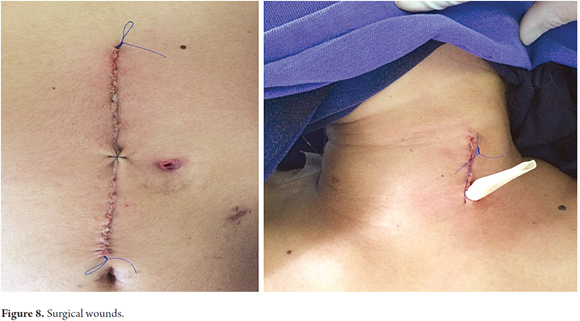
Patients were placed in a supine position and a median laparotomy was performed with dissection by planes to the abdominal cavity. The gastrostomy tube was removed and the gastric fistula was closed with 3-0 polydioxanone suture (Ethicon, Inc., Somerville, NJ, USA). The left gastroepiploic artery was dissected as close as possible to its origin in the splenic artery, preserving its irrigation to the major curvature of the stomach. The short vessels were ligated and cut with a harmonic scalpel (Ethicon, Inc., Somerville, NJ, USA) to provide more mobility to the gastric fundus. The junction between the left and right gastroepiploic arteries was identified. At this point, two 55 mm linear cutting sutures were stapled in (Ethicon, Inc., Somerville, NJ, USA) in the direction of the head at the midpoint of the gastric fundus, without sectioning, and with a margin of approximately 2 cm. Release of gastric fundus adhesions to the diaphragm created the reversed gastric tube (Figures 1, 2 and 3). The mechanical suture line was not reinforced manually. In addition, an extensive Kocher maneuver was performed to release the duodenum and head of the pancreas in order to bring the pylorus closer to the midline. Once this was done, we proceeded to the cervical stage. Through left cervicotomy, the left paravertebral space was dissected by planes and the proximal portion of the esophagus was identified. A section above the point of the burn stenosis, approximately 2 to 4 cm after the cricopharyngeal muscle was removed with a cold scalpel. The esophagus was removed through the transhiatal route and a 55 mm linear cut suture was stapled (Ethicon, Inc., Somerville, NJ, USA) at the esophagogastric junction to complete the total esophagectomy. Heineke Mikulicz pyloroplasty was then performed to prevent delayed gastric emptying syndrome caused by the trunk section of the vagus nerve (Figure 4). The reversed gastric tube was joined to the left gastroepiploic artery through the posterior mediastinum to prevent any type of torsion and avoid excessive tension in the distal vascular pedicle by finger dissection to the cervical region (Figure 5). A termino-terminal anastomosis was created between the esophagus and stomach in the mucosal and seromuscular layers with 3-0 polydioxanone sutures (Ethicon, Inc., Somerville, NJ, USA). Under vision and direct control, we advanced a nasogastric tube for decompression and a nasojejunal probe for early enteral nutrition (Figures 6 and 7). A Penrose drain was left as a control at the site of the anastomosis (Figure 8). Advanced enteral nutrition was started on the third postoperative day. Both patients required postoperative monitoring in the intensive care unit (ICU). On the seventh postoperative day, routine radiography of the upper digestive tract was performed to establish whether or not there was any leakage and to determine if oral feeding could be resumed. Endoscopic inspection of the upper digestive tract two weeks after surgery checked the anastomosis and the suture line of the inverted stomach in order to rule out fistulas or poorly perfused mucosa.
RESULTS
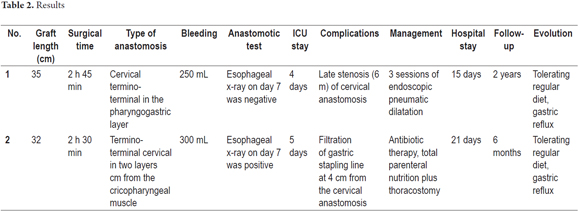
The average length of the reversed gastric tube graft measured endoscopically at two weeks following surgery was 32.5 cm. One patient was in the ICU for four days while the other was in the ICU for five 5 days. Neither patient died, and both procedures were performed without intraoperative complications. The female patient developed a postoperative fistula of the mechanical suture line in the reversed gastric tube graft at 4 cm from the esophageal-gastric junction which was resolved with medical management through total parenteral nutrition and broad spectrum antibiotic therapy and closed thoracostomy for drainage of the right pleural space. There were no other clinical repercussions after two weeks. It is noteworthy that neither of our patients developed perioperative pneumothorax. The male patient presented a late stricture of the cervical pharyngeal-gastric anastomosis which resolved completely with endoscopic pneumatic dilations without long-term dysphagia. The Penrose drain was removed on the seventh day and a normal diet was resumed on the tenth day after feeding tubes had been removed without complications. Neither of the patients showed dysphagia when their diets were modified, nor did they develop clinical symptoms of Dumping syndrome or delayed gastric emptying. However, both patients did experience gastric reflux which requires continuous use of PPIs and a 30-degree head elevation at night (Table 2).
DISCUSSION
Eighty percent of caustic esophageal burns are the result of accidental ingestion by children, but the remaining 20% ââare suicide attempts by adults, as in the two cases we have presented here. Reconstruction through total esophagectomies with the interposition of the colon are performed routinely because the colon is long enough to replace the resected segment. Nevertheless, the complicated surgical manipulation involved is a disadvantage of this technique, as has been reported by Knezevic (9) and colleagues. Early complications such as anastomosis filtration, colonic necrosis and pneumothorax) occur in up to 26.4% of the patients who undergo this procedure, and late complications such as stenosis of the anastomosis, peptic ulcer of the colon, intestinal obstruction and compression of the thoracic outlet occur in an additional 14%. To correct these types of anatomic defects after esophageal resection, the stomach is widely used as the first option, and the colon is used only for patients who have previously undergone gastrectomies, patients requiring simultaneous gastrectomy and esophagectomy, and those with extensive caustic burns of the gastric mucosa. On the other hand, difficulties increase with the height of the anastomosis, with more frequent leaks and stenoses. To minimize the problems of anastomotic dehiscence and graft necrosis due to increased tension and poor blood supply, other techniques have been designed to increase the vascularization of the reconstructed region. These includes the reversed gastric tube and the supercharged reversed gastric tube. This technique employs microvascular anastomosis at the cervical level using the left gastric artery and gastric conditioning procedures based on the delay phenomenon as described by Akiyama et al. (10) Patients undergo percutaneous embolization of the gastric vessels in order to generate collateral blood circulation by gastric diffusion and thus improve the irrigation of the ascending gastric tube. (11)
Although the reversed gastric tube technique is not often used for reconstruction after a total esophagectomy, it may have advantages over the conventional techniques of gastric ascent, colonic interposition or overloaded reversed gastric tube since it is a simple procedure requiring only a cervical anastomosis. It can be transferred to the cervical region or even to the pharyngeal esophagus to create an anastomosis. Because the caliber of the reversed gastric tube varies, it may be closer to that of the proximal esophagus or even to the oropharynx which decreases the possibility of postoperative stenosis of the anastomosis as has been reported to occur in up to 42% of esophageal reconstructions by Honkoop et al. (12) In addition, the reversed gastric tube technique facilitates a more physiological swallowing process and allows for a greater esophageal clearance. The vascular pedicle of the reversed gastric tube given by the left gastroepiploic artery avoids the need for microvascular surgery and reduces the risks of necrosis and leakage of the anastomosis (which has been observed to be as high as 13% by Orringer et al.). (13) One of our patients developed leakage at the mechanical suture line of the reversed gastric tube proximal to the anastomosis, but this resolved with medical management with thoracostomy for drainage which was subsequently closed. The patient did not require reoperation. Part of the greater omentum that ascends with the greater curvature of the reversed gastric tube provides ideal graft coverage and fills all dead spaces in the posterior mediastinum. Because of its great length, the graft can ascend through the posterior or retrosternal mediastinum or even subcutaneously, although the latter pathway presents a high risk of obstruction due to the compression of the bony prominences, as was used in our patients. It is important to make clear that surgical time and bleeding are similar to those in conventional gastric ascents. (14, 15)
Disadvantages of the reversed gastric tube include excessive secretion of gastric acid even though the esophagogastric anastomosis of the reversed gastric tube involves a portion of the stomach with low levels of acid secretion. In these cases, gastric reflux requires prolonged administration and 30 degree nighttime head elevation as was need for our patients. (16, 17)
Most studies that have been published about this technique discuss its use in the pediatric population. Dr. Ein (18) from Toronto published a 32-year case series of 11 patients who had undergone esophageal replacement with gastric tubes. Eight of these were reversed, and three were not. Initially, six patients required dilations, but 30-year long-term follow-up revealed that 9 of 11 patients were swallowing normally. In our results analysis, only one patient required endoscopic pneumatic dilatation and both patients are currently tolerating a regular diet with no symptoms of dysphagia and gastric reflux is controlled with PPIs without the need for parenteral or enteral nutritional support. It is important to emphasize that the great length of the gastric graft obtained with this technique allows use of any of the thoracic routes (posterior mediastinum, retrosternal and subcutaneous). In addition, it is a feasible technique which is reproducible by any general surgeon since it does not require microvascular surgery as does jejunal transposition. Nevertheless, it does require constant care that an acceptable diameter (3 cm) be maintained when forming the reversed gastric tube. Long-term follow-up requires less expertise and can be done through upper digestive endoscopy at medium to low complexity medical centers. (19-21)
CONCLUSIONS
This procedure produced favorable results in these two cases, and we can conclude that the reversed gastric tube technique allows the creation of a longer tube for the replacement of the whole esophagus without mortality and with moderate hospitalization time. Although experience with this procedure in adults is minimal, it is a safe, reproducible reconstruction procedure and a promising option for pharyngeal-esophageal reconstruction surgery.
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
1. Tiedeken J, Uradomo L, Anderson K. Minimally invasive repair of a late stricture in a reversed gastric tube. J Pediatr Surg. 2012;47:2321-6. [ Links ]
2. Dale WA, Sherman DC. Late reconstruction of congenital esophageal atresia by intrathoracic colon transplantation. J Thorac Surg. 1955;29:344-56. [ Links ]
3. Gavriliu D, Georgescue L. Esophagoplastic direction a material gastric. Rev Stiintelor Med (Bucharest). 1955;3:33. [ Links ]
4. Heimlich HJ. The use of a gastric tube to replace the esophagus as performed by Dr. Dan Gavriluiu of Bucharest, Romania. Surgery. 1957;42:693-5. [ Links ]
5. Heimlich HJ, Winfield JM. The use of a gastric tube to replace or bypass the esophagus. Surgery. 1955;37:549-59. [ Links ]
6. Contini S, Scarpignato C. Caustic injury of the upper gastrointestinal tract: A comprehensive review. World J Gastroenterol. 2013;19(25):3918-30. [ Links ]
7. Pelclová D, Navrátil T. Do corticosteroids prevent oesophageal stricture after corrosive ingestion? Toxicol Rev. 2005;24(2):125-9. [ Links ]
8. Fulton JA, Hoffman RS. Steroids in second degree caustic burns of the esophagus: a systematic pooled analysis of fifty years of human data: 1956-2006. Clin Toxicol (Phila). 2007;45(4):402-8. [ Links ]
9. Knezevi JD, Radovanovi NS, Simi AP, et al. Colon interposition in the treatment of esophageal caustic strictures: 40 years of experience. Dis Esophagus. 2007;20(6):530-4. [ Links ]
10. Reavis K, Chang E, Jobe B, et al. Utilization of the delay phenomenon improves blood flow and reduces collagen deposition in esophagogastric anastomoses. Ann Surg. 2005;241(5):736-47. [ Links ]
11. Hölscher A, Schneider P, Gutschow C, et al. Laparoscopic ischemic conditioning of the stomach for esophageal replacement. Ann Surg. 2007;245(2):241-6. [ Links ]
12. Honkoop P, Siersema PD, Tilanus HW, et al. Benign anastomotic strictures after transhiatal esophagectomy and cervical esophagogastrostomy: risk factors and management. J Thorac Cardiovasc Surg. 1996;111(6):1141-6. [ Links ]
13. Orringer MB, Marshall B, Iannettoni MD. Transhiatal esophagectomy for treatment of benign and malignant esophageal disease. World J Surg. 2001;25(2):196-203. [ Links ]
14. Harlak A, Yigit T, Coskun K. Surgical treatment of caustic esophageal strictures in adults. Int J Surg. 2013;11(2):164-8. [ Links ]
15. Gupta L, Bhatnagar V, Gupta AK. Long-term follow-up of patients with esophageal replacement by reversed gastric tube. Eur J Pediatr Surg. 2011;21(2):88-93. [ Links ]
16. Huang P, Chen C, Yang T. Supercharged reversed gastric tube technique: a microvascular anastomosis procedure for pharyngo-oesophageal reconstruction after total laryngopharyngo-oesophagectomy. Eur J Cardiothorac Surg. 2013;44(2):258-62. [ Links ]
17. Murakami M, Sugiyama A, Ikegami T, et al. Revascularization using the short gastric vessels of the gastric tube after subtotal esophagectomy for intrathoracic esophageal carcinoma. J Am Coll Surg. 2000;190:71-7. [ Links ]
18. Ein SH, Shandling B, Stephens CA. Twenty one year experience with the pediatric gastric tube. J Pediatr Surg. 1987;22:77-81. [ Links ]
19. Sitges AC, Sanchez-ortega JM, Sitges AS. Late complications of reversed gastric tube esophagoplasty. Thorax. 1986;41:61-5. [ Links ]
20. Guzmán-Chávez OR, Bautista-González S, Ramírez-Solís A. Manejo quirúrgico de reconstrucción esofágica en pacientes con estenosis esofágica por cáusticos. Rev Med MD 2012;3(4):211-6. [ Links ]
21. Mendez M, Perez A, Perez D. Quemadura esofágica por ingesta de cáusticos en una población pediátrica Hospital de la Misericordia – Bogotá 1996 al 2009. CIRUPED. 2012;2(2). [ Links ]











 text in
text in