Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.32 no.1 Bogotá Jan./Mar. 2017
https://doi.org/https://doi.org/10.22516/25007440.132
Primary Lymphoma in the Small Intestine: A Case Report and Literature Review
Helena Facundo Navia MD (1), María E. Manrique A. MD. (2)
(1) National Institute of Cancer in Bogotá, Colombia. Mail: helefacus@gmail.com
(2) National Institute of Cancer and Clinica El Country in Bogotá, Colombia
Received:Â Â Â 28-03-16Â Â Accepted:Â Â 16-12-16
Abstract
The gastrointestinal tract is the most frequent site of non-Hodgkin's lymphoma (NH) outside of the lymph nodes themselves. This tract is much more frequently compromised by tumors secondary to primary disease elsewhere in the body than by primary lymphomas of the gastrointestinal tract itself which are rare. They account for only one to four percent of malignant tumors of the gastrointestinal tract. Their development and prognoses are quite different from those of adenocarcinomas, hence their management must differ as well. It is important to understand them and keep them in mind in differential diagnosis in daily clinical practice. Young adults are most frequently affected, and men are more frequently affected than are women. We review the literature and report the case of a 47 year old woman with primary small bowel lymphoma that was diagnosed after several consultations due to abdominal symptoms.
Key words
Primary gastrointestinal lymphoma, extraganglionic Non-Hodgkin's Lymphoma, small intestine.
INTRODUCTION
Primary gastrointestinal lymphomas, particularly those in the small bowel, primarily affect young adults. They have no typical pathognomonic pattern than can be easily identified through physical examination or imaging. They are difficult to diagnose, often require multiple studies, and are frequently done in the setting of an urgent surgical intervention.
We report the case of a young woman, who happened to be a medical professional, who had been variously diagnosed before a final diagnosis of primary small bowel lymphoma was reached. We also review the literature.
We consider that this case is illustrative and indicates why a high index of suspicion is needed to reach a diagnosis in this type of case in a timely and accurate manner.
Case Presentation
The patient was a 47-year-old woman physician who had a history of several years of episodes of abdominal pain associated with vomiting, fever and, occasional diarrhea stools that resolved in 24 to 48 hours. She also suffered from asthenia, adynamia and drowsiness. She had had multiple outpatient appointments; been admitted to the emergency department; had CBC and tests for serum markers of inflammation markers; and undergone upper gastrointestinal endoscopy, colonoscopy and conventional abdominal radiology but without establishing the cause. She had been treated for irritable bowel syndrome. The only relevant antecedent was a right nephrectomy to treat pyelonephritis. There was no evidence of any risk factors for gastrointestinal lymphoma including helicobacter pylori infection, a compromised immune system, celiac disease or inflammatory bowel disease.
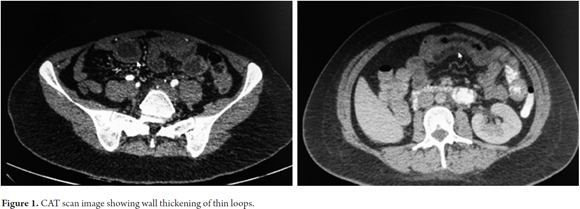
The patient was again admitted to the Emergency Department with symptoms as described above. It was decided to use contrast-enhanced computed tomography (CT) of the abdomen which showed a thickening of the thin intestinal loops but no evidence of intra-abdominal or retroperitoneal adenomegaly (Figure 1).
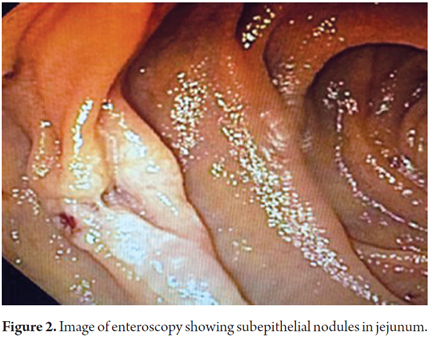
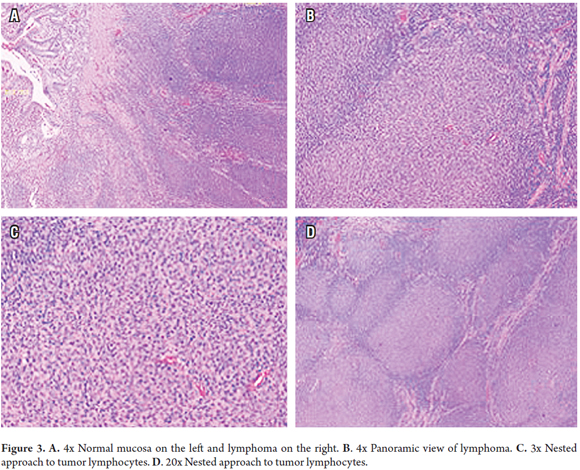
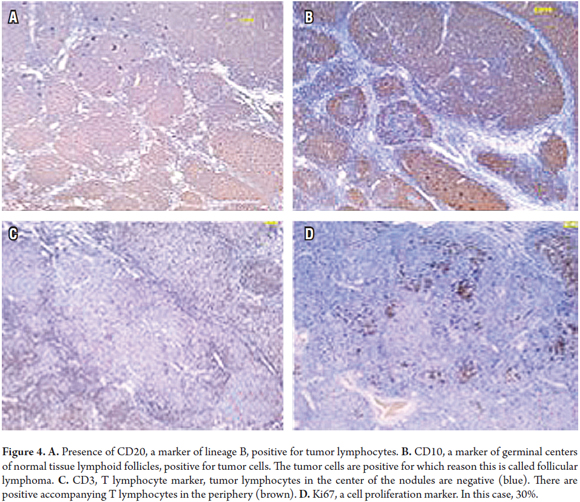
The decision was made to perform an enteroscopy. At least two subepithelial nodular lesions were found in the jejunum, and biopsies were taken (Figure 2). During follow-up and prior to receiving the pathology report, the patient developed renewed abdominal pain and underwent urgent surgery. A jejunal segment compromised by a non-specific tumor and partial obstruction of the lumen were found. Resection with intestinal anastomosis are performed. Postoperative evolution was adequate, and the patient was discharged six days later. The pathology report confirmed the diagnosis of grade I follicular lymphoma in the intestinal segment and five lymph nodes of the mesentery without involvement of tumors. Classical hematoxylin and eosin staining was done (Figure 3), and tests for immunohistochemical markers were performed (Figure 4).
The study was completed with a CT scan of the neck and thorax. Nodal and bone marrow aspiration were ruled out because there was no evidence of lymphoma.
The patient was evaluated by the hematology service which decided to initiate chemotherapy with the R-CHOP scheme (R â rituximab, a biologic [Mabthera ®], C â cyclophosphamide, H â doxorubicin [hydroxydaunomycin], O â vincristine [Oncovin ®], P â prednisolone (a steroid).
DISCUSSION
Primary intestinal lymphoma principally affects the digestive tract. In almost all cases, the intestinal lymphoma is a non-Hodgkin's lymphoma (NHL). Hodgkin's disease or Hodgkin's lymphoma has its own characteristics which are independent of its location, but it is exceptionally rare in the digestive tract. The gastrointestinal tract may be secondarily affected by lymphoma from another source, which is a different case than the subject of this review. (2)
Risk factors for the development of primary intestinal lymphoma include H. pylori infections, celiac disease, inflammatory bowel disease, and immunosuppressive states associated with human immunodeficiency virus (HIV) infections and solid organ transplantation. (3)
The Dawson criteria are the classic tests for diagnosis of gastrointestinal lymphoma. (4) They include:
- Absence of palpable adenopathy in clinical examination
- Absence of mediastinal lymphadenopathy in a chest x-ray
- Normal WBC differential count
- Disease confined to the intestine and adjacent nodes, without involvement of liver or spleen.
The gastrointestinal tract is the most frequent site of extranodal lymphoma (affecting a site or sites other than the lymph nodes). Gastrointestinal tract extranodal lymphoma accounts for 4% to 20% of all non-Hodgkin's lymphomas, most of which are lineage B cell that develop in lymphoid tissue associated with mucous membranes (MALT ). (5) The involvement of T-cell lymphomas is less frequent and has a heterogeneous presentation. (6, 7)
Classically, it has been stated that the most frequent site of gastrointestinal lymphoma is the stomach, followed by the small intestine, colon, cecum, esophagus and compromises of several parts of the GI tract. (8, 9) However, Ha's review of 61 cases found that the small intestine was compromised in 20% to 54% of primary gastrointestinal lymphomas. (10) The least frequent site is the rectum. (11)
There have been a number of classification proposals throughout history, (12, 13) but the World Health Organization (WHO) promotes the classification guided by the global consensus of 2008. (14) The most frequent types are:
- MALT Lymphoma B in the extranodal marginal zone
- Intestinal T-lymphoma associated with enteropathy
- Immunoproliferative small intestinal disease (IPSID) which includes so-called alpha heavy chain disease and Mediterranean lymphoma
- Lymphomas associated with immunosuppression.
Some histological subtypes show a predilection for certain locations: MALT lymphoma for the stomach;
Mantle cell lymphoma for the terminal ileum, jejunum, and colon; T-cell lymphoma is associated with enteropathy in the jejunum and follicular lymphoma in the duodenum. (15)
The distribution of gastrointestinal NHL varies in different populations. In North America, MALT lymphomas and diffuse large B-cell lymphoma predominate while in the Middle East and the Mediterranean basin IPSID predominates. In Africa, Burkitt lymphoma, and NHL, is fifty times more frequent than in the United States (2).
In the small intestine, B-cell NHL predominates in the ileum and T-cell NHL associated with celiac disease is more common in the jejunum. Different basic forms are seen in radiology studies: single or multiple mucous nodules, focal or diffuse thickening of the intestinal wall or aneurysmal dilatation of a segment, with or without mesenteric adenomegalies. Differential imaging diagnoses must decide primarily between adenocarcinoma and stromal tumors. A generalized multifocal appearance, absence of obstruction despite large tumor volume, and absence of hypervascularization are signs that point towards a lymphoma. In the colon, particularly in the cecum and rectum, lesions may be polypoid masses, circumferential infiltrations, ulcerated masses, thickened haustra or mucous nodules. (16) Capsule endoscopy is a useful tool when a primary pathology of the small intestine is suspected and when there is suspicion that the small intestine has been affected by a systemic pathology as in the case of Crohn's disease. It has been shown to be slightly superior to enteroscopy fore detection of tumors, although with no statistically significant difference. (17) In the case presented here, enteroscopy was preferred because of its immediate availability.
In general, although this did not apply to the case presented here, when there is suspicion of intestinal lymphoma, capsule endoscopy has limitations because there are no pathognomonic images for diagnosis and because biopsies cannot be taken. (18) It is also important to consider the risk of intestinal obstruction due to capsule retention, which occurs in 0.75% of cases to 3.6% of cases. Risk factors include stenosis, swallowing disorders, fistulae, and previous clinic signs suggestive of obstruction. (19)
Computed tomography (CT) enterography is a first-line diagnostic method for suspicion of small bowel pathologies. Advantages include availability, ease of use by technicians at many institutions, and familiarity of images for clinicians. (20) A high quality alternative that does not expose the patient to radiation is magnetic resonance (MR) enterography. Both studies perform well for diagnosis of small bowel lesions and particularly for the detection of parietal alterations in ileum, the segment most frequently affected in NHL. (21)
The two most common types of primary small-bowel lymphomas are MALT lymphoma and diffuse large B-cell lymphoma (DLBC). These can compromise the duodenum, ileum and less frequently the jejunum as in the case presented here. The enteroscopic appearance of lesions varies and includes partially healed ulcers, multiple polypoid lesions, and single jejunal stenoses. Five patterns of ileal compromise have been described: thickened mucosal folds, nodular, infiltrative, ulcerative and mosaic. At least five patterns have been described for DLBC: polypoid, ulcerative, multiple polyposis, diffuse and mixed infiltrate. This lymphoma is more frequently associated with perforation in the small intestine than are other types. (22)Â Â Â Â Â Â Â Â Â Â Â Â Â Â Â
There are also several proposals regarding staging of gastrointestinal lymphomas. We present only the Ann Arbor system as modified by Musshoff which has been widely used in many previous reports. (23, 24)
- Stage I: tumor limited to the digestive tract, either single location or multiple contiguous lesions
- Stage II: tumor with intra-abdominal extension to nodes
II1: local, gastric region or intestinal lymph nodes
II2: distant nodes (mesenteric, aortic region, vena cava region, pelvic or inguinal regions)
- Stage IIIE: penetration of the serosa, affected by contiguity of adjacent organs or tissues
- Stage IV: diffuse intestinal compromise, compromise outside of the area of the diaphragm, spinal compromise.
The clinical presentation of gastrointestinal lymphoma is frequently associated with pain, masses, perforations and obstructions. This nonspecific picture, which is part of the acute abdomen scenario, is a diagnostic challenge, since there are no pathognomonic clinical features and imaging findings vary and are not specific. (25) A diagnosis is often only made after surgical exploration. (26-28)
Treatment of gastrointestinal tract lymphomas is related to each patient's particular characteristics, tumor type and stage. Surgical resection and chemotherapy have been and remain the mainstays of treatment. Radiation therapy historically had some role for local lesions of significant volume and for lesions which cannot be resected, but, particularly in the case of the small intestine, it has been abandoned due to frequent involvement of several segments and the risks of post-radiation enteritis. Biological management with rituximab has shown benefits, especially for diffuse B-cell lymphoma. (15) It should be noted that grade I follicular lymphoma, which was what our patient suffered from, tends to have an indolent course although it can become DLBC. For DLBC, chemotherapy is most frequently based on the CHOP scheme combination of cyclophosphamide, doxorubicin, vincristine and prednisone. When rituximab is added, this becomes the R-CHOP regimen. Rituximab is a genetically engineered chimeric monoclonal antibody that binds specifically to CD20 antigen, a phosphoprotein that is expressed in B lymphocytes. (28, 29)
Patient prognosis is related to factors that include age, histological subtype, stage at diagnosis and the presence or absence of systemic symptoms. Although the literature reports that the disease stage is the most important prognostic factor for survival, it has been observed that perforation at the onset of the disease is a deleterious factor which is clearly associated with early mortality during the course of treatment. (30-32)
CONCLUSION
The gastrointestinal tract is the most frequent site of extra lymphatic non-Hodgkin's lymphoma. The stomach is most frequently affected, followed by the small intestine and colon. Although there is usually one primary site of involvement, multi-segment involvement is common. Presentation is bizarre and depends on location but is most frequently associated with pain, obstruction, perforation and bleeding. This combination makes these lymphoma clinically indistinguishable from other more frequent digestive tract neoplasias and even from subacute and chronic inflammatory pathologies. When associated with constitutional systemic symptoms, the diagnosis is more likely to target neoplastic pathology. It is necessary to maintain a high index of suspicion to reach this diagnosis in a timely manner.
Acknowledgement
We would like to thank Dr. Marta Lucía Cadena, pathologist, for her invaluable help and advice.
REFERENCES
1. Papaxoinis G, Papageorgiou S, Rontogianni D, et al. Primary gastrointestinal non-Hodgkins lymphoma: A clinicopathologic study of 128 cases in Greece. A Hellenic Cooperative Oncology Group study (HeCOG). Leuk Lymphoma. 2006;47(10):2140-6. [ Links ]
2. López A, Villarubia J. Linfomas intestinales. En: Ponce J. Tratamiento de las enfermedades gastroenterológicas. Asociación Española de Gastroenterología. Valencia: Fundación AstraZeneca; 2011. p. 327-34. [ Links ]
3. Pellisé M, Castells A. Tumores del intestino delgado. En: Montoro M, Garcia JC. Gastroenterología y hepatología. Asociación Española de Gastroenterología. Madrid: Jarpyo Editores; 2012. p. 435-42. [ Links ]
4. Dawson MP, Cornes JS, Morson BC. Primary malignant lymphoid tumours of the intestinal tract. Report of 37 cases with a study of factors influencing prognosis. Br J Surg. 1961;49:80-9. [ Links ]
5. DAmore F, Brincker H, Grønbaek K, et al. Non-Hodgkins lymphoma of the gastrointestinal tract: a population-based analysis of incidence, geographic distribution, clinicopathologic presentation features, and prognosis. Danish Lymphoma Study Group. J Clin Oncol. 1994;12(8):1673-84. [ Links ]
6. Molina R, Jiménez A, López JL, et al. Linfoma T primario intestinal: a propósito de dos casos con revisión de la literatura. An Med Int (Madrid). 2002;19:457-9. [ Links ]
7. Vaquero L, Alvarado M, Arias L, et al. Linfoma intestinal de células T asociado a enteropatía y sin relación con enfermedad celíaca. Gastroenterol Hepatol. 2012;35(1):17-21. [ Links ]
8. Varghese C, Jose C, Subhashini J, et al. Primary small intestinal lymphoma. Oncology. 1992;49:340-2. [ Links ]
9. Shirsat HS, Vaiphei K. Primary gastrointestinal lymphomas-A study of 81 cases from a tertiary healthcare centre. Indian J Cancer. 2014;51(3):290-2. [ Links ]
10. Ha C, Cho MJ, Allen P, et al. Primary nonHodgkins lymphoma of the small bowel. Radiology. 1999;211:183-7. [ Links ]
11. Fernández MM, Macho FM, Vela CG, et al. Linfomas gastrointestinales: Revisión en el Hospital Universitario Marqués de Valdecilla de Santander (1992-2005). Conceptos morfológicos, inmunohistoquímicos y de patología molecular aplicados a su diagnóstico. 7.º Congreso Virtual Hispanoamericano de Anatomía Patológica 2005. Disponible en: http://www.conganat.org/7congreso/PDF/541.pdf. [ Links ]
12. Shepherd NA, Hall PA, Coates PJ, et al. Primary malignant lymphoma of the colon and rectum. A histopathological and immunohistochemical analysis of 45 cases with clinicopathological correlations. Histopathology. 1988;12(3):235-52. [ Links ]
13. Isaacson PG. Gastrointestinal lymphoma. Human Pathol. 1994;25(10):1020-9. [ Links ]
14. Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood- 2011;117(19):5019-32. [ Links ]
15. Ghimire P, Wu GY, Zhu L. Primary gastrointestinal lymphoma. World J Gastroenterol. 2011;17(6):697-707. [ Links ]
16. Frampas E. Lymphomas: basic points that radiologists should know. Diagn Interv Imaging. 2013;94:131-44. [ Links ]
17. Caunedo A, Herrerias JM. Papel de la cápsula endoscópica en el diagnóstico de las enfermedades digestivas. Med Clin (Barc). 2005;124(11):427-33. [ Links ]
18. Olds G, McLoughlin R, O´Morian C, et al. Celiac disease for the endoscopist. Gastrointest Endosc. 2002;56(3):407-15. [ Links ]
19. Delvaux M, Gay G. Capsule endoscopy: technique and indications. Best Pract Res Clin Gastroenterol. 2008;22(5):813-37. [ Links ]
20. Upegui D, Derek O, Segura W, et al. Uso de entero-TC para evaluar patología del intestino delgado: experiencias y hallazgos en 90 pacientes. Rev Col Radiol. 2010;21(1):2818-25. [ Links ]
21. O´Brien A. Evaluación imaginológica del intestino delgado por TC y RM. Rev Med Clin Las Condes. 2013;24(1):109-15. [ Links ]
22. Vetro C, Romano A, Amico I, et al. Endoscopic features of gastro-intestinal lymphomas: from diagnosis to follow-up. World J Gastroenterol 2014;20(36):12993-13005. [ Links ]
23. Yin L, Chen CQ, Peng CH, et al. Primary small-bowel non-Hodgkins lymphoma: a study of clinical features, pathology, management and prognosis. J Int Med Res. 2007;35(3):406-15. [ Links ]
24. Guzmán M, Quispe M, Quiroga G, et al. Linfoma primario de Colon descendente: comunicación de un caso. Rev Gastroenterol Perú. 2005;25(2):210-5. [ Links ]
25. Ghai S, Pattison J, Ghai S, et al. Primary gastrointestinal lymphoma: spectrum of imaging findings with pathologic correlation. Radiographics. 2007;27(5):1371-88. [ Links ]
26. Ramia JM, Sancho E, Lozano Ó, et al. Linfoma primario de intestino delgado. Cir Esp. 2007;81(1):46-8. [ Links ]
27. Paz R, Herrera R. Linfoma intestinal. Caso clínico. Rev Diag Imagem. 2008;3(1):52-4. [ Links ]
28. Grande C. Actualización del tratamiento del linfoma difuso de células grandes B. Cuadernos de Hematología. Hospital Universitario Doce de Octubre [internet]. 2011. Disponible en: www.leucemiaylinfoma.com/modulos/CH/2011/Capitulo_3-II_2011.pdf. [ Links ]
29. Assar AN. Primary small intestinal lymphoma presenting as a groin abscess. Saudi J Gastroenterol. 2009;15(1):64-5. [ Links ]
30. Campos Rojas A, Monterroso V, Regocez Z, et al. Linfomas del tracto gastrointestinal: reporte de 19 casos. Rev Costarricense Cien Med. 1994;15(1/2):3-14. [ Links ]
31. Albújar B, Diaz P, Tantaleán R. Linfomas gastrointestinales primarios: cuadro clínico patológico y sobrevida. Rev Gastroenterol Perú. 1995;15(2):141-51. [ Links ]
32. Vaidya R, Habermann TM, Donohue JH, et al. Bowel perforation in intestinal lymphoma: incidence and clinical features. Ann Oncol. 2013;24:2439-43. [ Links ]











 text in
text in