Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.32 no.4 Bogotá oct./dic. 2017
https://doi.org/10.22516/25007440.177
Review articles
Structured Literature Review of Hepatic Toxicity Caused by Medicines
1Química farmacéutica. Magíster en Ciencias Farmacéuticas y Alimentarias, Facultad de Ciencias Farmacéuticas y Alimentarias; Grupo de Promoción y Prevención farmacéutica, Universidad de Antioquia, Medellín, Colombia. Correo: alejandra.canop@udea.edu.co
2Química farmacéutica. Grupo de promoción y prevención farmacéutica, Universidad de Antioquia, Medellín, Colombia
3Doctor en Farmacia. Profesor del Departamento de Farmacia, Facultad de Ciencias Farmacéuticas y Alimentarias. Grupo de promoción y prevención farmacéutica, Universidad de Antioquia, Medellín, Colombia
Objectives:
The aim of this study was to prepare an updated list of drugs that cause hepatotoxicity and identify drugs most likely to cause hepatotoxicity according to scientific evidence.
Method:
A search of PubMed/Medline was conducted using the MeSH terms: “Liver disease” and “Drug-induced Liver Injury”. The search was filtered by case reports, reviews, clinical trials, metaanalyses and letters until December 2015. The search was limited to articles in English, Spanish and French. Articles with evidence of hepatotoxicity caused by medications and relevant references were included. Articles not related to the objectives of the search were excluded. These include articles related to hepatotoxicity due to other agents, articles about other causes of liver disease and/or articles related to predictive tests or stem cells. Some aspects of hepatotoxic drugs were appearance of hepatotoxicity, type of injury, mechanisms of hepatotoxicity, risk factors and clinical manifestations. Three categories, definite, probable and possible, were established to assess probability of hepatotoxicity and type of lesion.
Results:
Six hundred ten articles were identified, 402 articles were chosen, and 208 articles were excluded. A list was prepared with 181 drugs and 17 combined pharmaceutical forms or therapeutic regimens likely to cause hepatotoxicity. Of these, methotrexate, minocycline, vancomycin, everolimus, isoniazid, and tamoxifen were categorized as definite probabilities.
Conclusions:
More than 180 hepatotoxic drugs were identified, six were categorized as definite probabilities, and most were categorized as possibilities. The consolidation of information shows that diverse categories of drugs are likely to cause liver toxicity.
Keywords: Liver disease; drug-induced liver damage
Objetivos:
elaborar un listado actualizado de medicamentos causantes de hepatotoxicidad e identificar, de acuerdo con la evidencia científica, los medicamentos con mayor probabilidad de causar hepatotoxicidad.
Método:
se realizó una búsqueda en PubMed/Medline utilizando términos Mesh: “liver disease” y “drug-induced liver injury”. La búsqueda se filtró por: reportes de casos, revisiones, ensayos clínicos, metaanálisis y cartas, hasta diciembre de 2015, en inglés, español y francés. Se incluyeron artículos con evidencia de hepatotoxicidad causada por medicamentos y referencias relevantes; fueron excluidos artículos sin relación con los objetivos de la búsqueda, relacionados con hepatotoxicidad por agentes diferentes, concernientes a otras causas de enfermedad hepática o relacionados con ensayos predictivos o células madre. Algunos aspectos de los medicamentos hepatotóxicos fueron: aparición de hepatotoxicidad, tipo de lesión, mecanismos de hepatotoxicidad, factores de riesgo y manifestaciones clínicas. Para valorar la probabilidad de aparición de hepatotoxicidad y del tipo de lesión se establecieron 3 categorías: definida, probable y posible.
Resultados:
se identificaron 610 artículos de los cuales se eligieron 402, se excluyeron 208 artículos. Se elaboró un listado con 181 medicamentos y 17 formas farmacéuticas combinadas o regímenes terapéuticos con probabilidad de causar hepatotoxicidad; de estos, 6 medicamentos tuvieron probabilidad definida (metotrexato, minociclina, vancomicina, everolimus, isoniazida y tamoxifeno).
Conclusiones:
se identificaron más de 180 medicamentos hepatotóxicos, 6 tienen una probabilidad definida, mientras que para la mayoría es posible. La consolidación de la información demostró que diversas categorías de medicamentos tienen mayor probabilidad de ser causantes de hepatotoxicidad.
Palabras clave: Enfermedad hepática; daño hepático inducido por medicamentos
Introduction
Hepatotoxicity is damage caused by exposure to a drug or non-pharmacological agents. Risk factors include idiosyncrasy, age, gender, alcohol consumption, smoking, concomitant use of other drugs, previous or underlying liver disease, genetic and environmental. 1-3 Although most lipophilic drugs can cause hepatotoxicity, 4 antibiotics, nonsteroidal anti-inflammatory drugs (NSAIDs) and anticonvulsants are the pharmacological groups which are the most frequent causes. 1,5-9. Among drugs administered intravenously, antibiotics and drugs to treat neoplasia are the groups most associated with liver toxicity.10
Hepatotoxicity can be classified into intrinsic reactions and idiosyncratic reactions:
The former are predictable, dose-dependent, and reproducible, but there is limited information on their frequency of occurrence.
Idiosyncratic reactions are either immune or metabolic and are unpredictable, not dose-dependent, and non-reproducible, but they affect only a small proportion of patients (between 1/1,000 and 1/100,000 exposed patients). 11-17
Intrinsic hepatotoxicity is less common than idiosyncratic hepatotoxicity12,18-20. Liver histology is ideal for defining patterns of liver toxicity, but in clinical practice the majority of hepatotoxic damage is classified according to biochemical tests 21. According to the international consensus of the Council for International Organizations of Medical Sciences (CIOMS), liver damage is present when liver enzymes are over two times the upper limit of normal (ULN). On the other hand, types of injuries are classified in 12,22,23:
Hepatocellular damage is defined as isolated increases of alanine aminotransferase (ALT) to over two times the ULN or an ALT/alkaline phosphatase ratio greater than five. Hy’s law defines this type of injury as ALT values greater than three times the ULN. 24,25
Cholestatic damage is defined as isolated increases of alkaline phosphatase to over two times the ULN or a ratio of less than two.
Mixed damage is defined as ALT and alkaline phosphatase over two times the ULN and a ratio greater tha two, but less than five.
Hepatotoxicity is related to mitochondrial dysfunction, inhibition of cellular respiration or alteration in β oxidation of fatty acids26,27. These result in apoptosis, necrosis, autophagy and, therefore, cell death28,29. The main clinical-pathological manifestations of hepatotoxicity and its histological findings are:
Acute hepatitis (characterized by parenchymal inflammation, necrosis and Kupffer cells in the sinusoids)
Chronic hepatitis (fibrosis)
Fulminant hepatitis (necrosis and inflammation)
Cholestatic hepatitis (inflammation and liver damage)
Cholestasis (biliary plugs in zone 3)
Vanishing bile duct syndrome (damage to the bile ducts, cholestasis and inflammation)
Granulomatous hepatitis (granulomas in portal tracts or parenchyma)
Macrovesicular steatosis (lipid droplets in the cytoplasm of the hepatocyte)
Microvesicular steatosis (tiny drops of lipids in the cytoplasm of the hepatocyte)
Steatohepatitis (steatosis, lobular inflammation, engorged hepatocytes and pericellular fibrosis)12,29-31.
These manifestations are accompanied by nonspecific signs and symptoms such as fever, fatigue, nausea, abdominal pain, jaundice, dark urine, pruritus, ascites, encephalopathy and increased transaminases16,32,33.
Although some 1,100 drugs, excluding substances of abuse and natural products, have been associated with hepatotoxicity 19, identification of this adverse event is a complex process. Therefore, a meticulous investigation is required, aimed at identifying any substance and ruling out other causes of liver disease3,8,34. In addition, liver biopsy is fundamental for identifying hepatotoxicity 35. The chronological relationship between exposure to the suspect agent and the hepatotoxic reaction is key. To establish the likelihood that a drug is associated with hepatotoxicity, clinical scales such as the Roussel Uclaf Causality Assessment Method (RUCAM) and the Maria & Victorino (M&V) clinical scale have been developed. It is considered that the RUCAM scale’s content and criterion validity make it most appropriate and that it generates results compatible with medical judgment and expert opinion on hepatotoxicity. Nevertheless, due to its high cost of application, its usefulness in clinical practice is limited 36-38.
In the absence of a specific pharmacotherapy, treatment of hepatotoxicity is based on suspension of the suspect medication, treatment of symptoms and follow-up laboratory tests39. However, the use of N-acetylcysteine as an antidote for acetaminophen toxicity and hepatotoxicity due to phenytoin and carbamazepine, and the use of carnitine to treat valproic acid toxicity are exceptions40.
An updated list of hepatotoxic drugs and associated factors could help optimize identification and prevention of this adverse event. Therefore, the objectives of this review were to prepare an updated list of drugs associated with hepatotoxicity and identify, according to scientific evidence, the drugs most likely to cause hepatotoxicity. In addition, this review will systematize and specify key information such as type of injury, probability of occurrence, pathophysiological mechanisms, clinical and pathological manifestations, variation in liver enzyme levels, intrinsic or idiosyncratic reaction, risk factors and clinical outcomes.
Method
Bibliographical Search
A PubMed/Medline search was performed using the MeSH terms “liver disease” (drug effects, injuries, pathology) and “drug-induced liver injury”. The search was filtered by articles with keywords in the title or summary published until December 2015 in English, Spanish and French and for which access was available to the full text. Articles were classified as case reports, reviews, systematic reviews, clinical trials, clinical trials controlled trials, randomized clinical trials, meta-analyzes and letters to the editor. Articles with evidence of hepatotoxicity only due to medications and those considered relevant to the subject were included. Articles unrelated to the objectives of the search were excluded as were articles related to hepatotoxicity due to other substances such as natural products, dietary supplements, substances of abuse and industrial substances, those concerning other causes of liver disease and those related to predictive tests for hepatotoxicity or stem cells.
Information Analysis
Two independent reviewers determined eligibility of articles and extracted information from them while discrepancies between them were resolved by a third reviewer. The title, author, year of publication, type of study, related pharmacological group and compliance with inclusion criteria for each of the references found was recorded in a database in Excel 2010 for Windows®. In addition, the pharmacological group, ATC code (Anatomical, Therapeutic, Chemical classification), probability of occurrence of hepatotoxicity, type of injury, and probability of occurrence of that type of lesion were tabulated for each of the hepatotoxic drugs found. Mechanisms of hepatotoxicity, risk factors, clinical manifestations, management, outcome, measurements of liver enzymes and medication dosages were also recorded. Means and standard deviations were calculated for numerical data such as liver enzyme values (aspartate aminotransferase [AST], ALT, FA and total bilirubin [TB]) and dosages of drugs administered.
Assessment of Appearance of Hepatotoxicity and Type of Injury
Assessment of the appearance of hepatotoxicity and the type of injury was based on probability of occurrence. 41 Three categories were established according to the evidence found:
Definite: evidence in meta-analysis, systematic reviews or clinical trials (randomized or not)
Probable: analytical studies or description in three or more reports of clinical cases
Possible: less than 3 reported cases or recommendations from expert groups. 41
In cases of drugs for which several references and different types of study were available, articles with the highest level of evidence were used.
Results
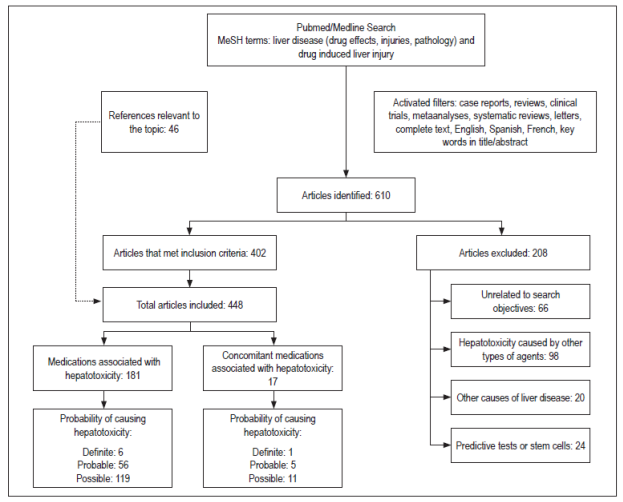
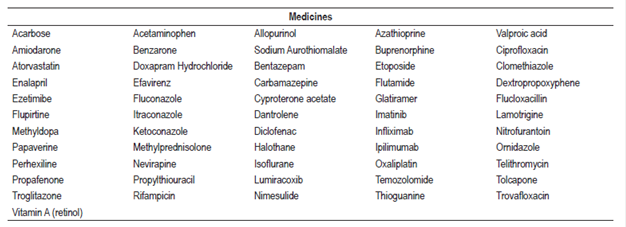
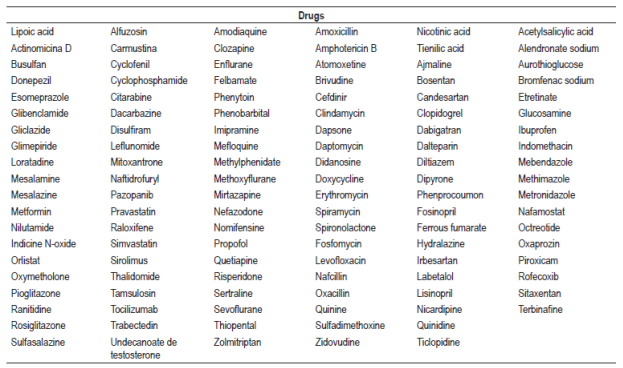
The search identified 610 articles of which 402 met the inclusion criteria and were selected while 208 did not meet the inclusion criteria and were excluded. Forty-six other articles considered relevant for the review were included (Figure 1). A list of 181 drugs and 17 combined pharmaceutical forms or therapeutic regimens likely to cause hepatotoxicity was prepared. Six of these drugs (methotrexate, minocycline, vancomycin, everolimus, isoniazid, and tamoxifen) and one therapeutic regimen (isoniazid, rifampicin plus pyrazinamide) were classified as definite, 56 drugs and five combined pharmaceutical forms or therapeutic regimens were classified as probable, and 119 drugs and 11 combined dosage forms were classified as possible.
The type of lesion caused by each drug was identified, and hepatocellular damage was found to be more common than cholestatic or mixed damage. Information found for each drug with definite probability that was tabulated included type of hepatotoxicity, type of lesion, appearance, mechanism of hepatotoxicity, risk factors, clinical manifestations and outcomes (Table 1). The drugs found were classified according to their pharmacological group and their ATC code to unify them. Drugs found to have probable probability are found in Table 2 and the drugs with probable probability are found in Table 3. Figures for liver enzymes and dosages found are in Table 4.
Table 1 Drugs with a probability of causing definite hepatotoxicity
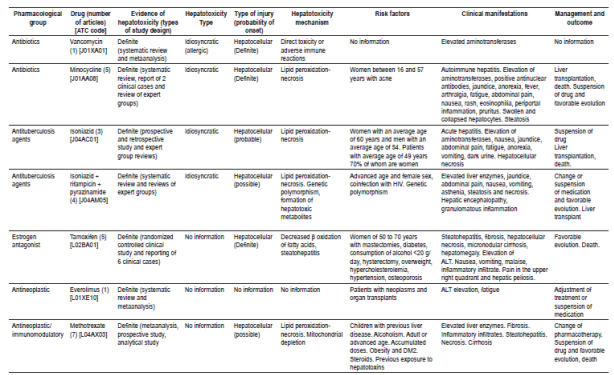
ALT: Alanine aminotransferase; ATC: Anatomical, Therapeutic, Chemical; DM2: diabetes mellitus type 2; HIV: human immunodeficiency virus.
Table 4 Liver enzyme values and dosages associated with hepatotoxic drugs
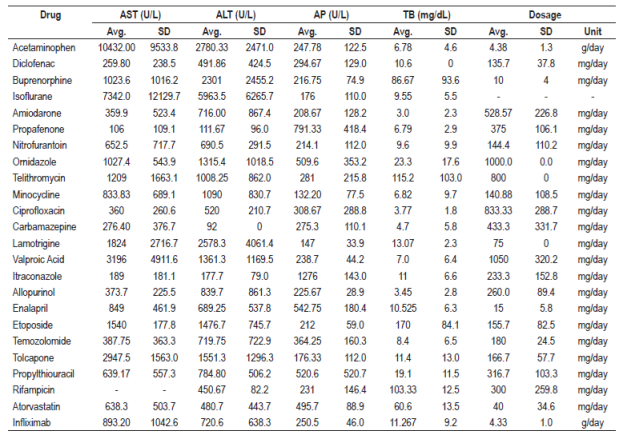
ALT: Alanine aminotransferase; AST: aspartate aminotransferase; TB: Total bilirubin; SD: standard deviation; AP: alkaline phosphatase; avg.: average
Among antidiabetic agents, the probable probability of causing hepatotoxicity of acarbose 42 and troglitazone 43,44 (withdrawn from the market) has been determined. Case reports indicate that it causes hepatocellular, cholestatic and mixed type lesions accompanied by jaundice, rashes, fevers, and other symptoms. The antiarrhythmic agents associated with probable hepatotoxicity were propafenone and amiodarone. 45 There were more reported cases for amiodarone, and they were associated with elevated liver enzymes in 15% -55% of the patients. 46 Patients improved upon suspension of medication, but there are reports of death associated with amiodarone47. Antihypertensives such as enalapril increased liver enzyme levels and produced jaundice and structural changes in the liver confirmed by biopsies which led to transplantation and death48. For methyldopa (probable), there were nine reported cases of idiosyncratic liver toxicity17. They had a pattern of hepatocellular injury, especially in women, manifested by jaundice, anorexia and nausea. In addition, liver biopsies identified necrosis and inflammatory infiltrates. 49,50 Hepatocellular lesions accompanied by elevated liver enzymes, jaundice, fever and asthenia were found to be associated with atorvastatin and ezetimibe51,52.
Propylthiouracil caused the death of one patient, affected women and girls, generated symptoms such as jaundice, pruritus and weight loss; necrosis, fibrosis, inflammatory infiltrate and ductopenia and was found in liver biopsies. Suspension of the drug improved the evolution of some patients53. Four cases of increased liver enzyme values, weakness and jaundice were identified in patients taking methylprednisolone. Symptoms improved upon suspension of the medication54.
Among the antibiotics, idiosyncratic reactions were identified in association with vancomycin 55 and minocycline17,33,55,56. Minocycline affected women between 16 and 57 years of age who had been diagnosed with autoimmune hepatitis. Rifampicin caused hepatocellular lesions and especially affected women57,58. The following antibiotics were classified as probable causes of hepatotoxicity: nitrofurantoin (12% frequency of cases, idiosyncratic), 59,60, flucoxacillin (11 cases, idiosyncratic)61, telithromycin (hepatocellular lesions with elevated transaminases and fever), 62 ciprofloxacin and trovafloxacin (withdrawal from the market). In general, the outcomes varied from favorable evolutions to liver transplantation and death of the patient.
Liver damage associated with the antifungal agents itraconazole, fluconazole and ketoconazole improved with suspension of the medications. 63-66 Antiretroviral agents, especially reverse transcriptase inhibitors, nucleoside analogues and protease inhibitors, can cause dose-dependent hepatotoxicity. 67 Cases reported with efavirenz and nevirapine had elevated transaminases and an incidence between 1% and 14%. 9 Coinfection with hepatitis B or C virus can increase the level of hepatotoxicity associated with antiretroviral treatments. 68,69
Chemotherapy has increased life expectancy, but it can cause liver damage ranging from steatosis and steatohepatitis to cirrhosis. 70,71 The probability of hepatotoxicity for tamoxifen, everolimus and methotrexate is definite. Medications such as flutamide, etoposide, imatinib, ipilimumab, oxaliplatin, temozolomide, thioguanine, glatiramer, azathioprine, and infliximab were classified as probable causes of hepatotoxicity.
NSAIDs were identified as an important group that can cause liver damage, mainly idiosyncratic, in cases of abuse or overdose. 1,72,73,74 Risk factors identified included age, female gender, chronic alcohol consumption, concomitant drugs, underlying diseases, obesity, DM2 and insulin resistance. 72 Causative agents include diclofenac, lumiracoxib and nimesulide. Acetaminophen is widely recognized as an intrinsic hepatotoxic substance due to a metabolite that causes hepatic necrosis,. Fourteen case reports characterized by hepatocellular lesions were identified. When managed with N-acetylcysteine and prednisone patients improved. 75,76
Halothane was the general anesthetic most likely to cause liver toxicity. Genetic predisposition, repeated doses, obesity and advanced age were some risk factors. Women are more likely to suffer liver damage including hepatocellular lesions, increased liver enzymes, necrosis, fever, jaundice and fatigue. 77-79
Among the anticonvulsants, valproic acid had the largest number of cases of hepatotoxicity (hepatocellular type) which manifested with elevated transaminases, abdominal pain, jaundice and anorexia. In addition, microvesicular and macrovesicular steatosis, necrosis and inflammatory infiltrate were identified in liver biopsies. This medication can cause liver damage in people under 30 years of age80,81. Carbamazepine cases identified were mainly of the mixed type with the formation of granulomas82,83. Lamotrigine generated cases of idiosyncratic hepatotoxicity that required liver transplantation84,85.
We found 17 combined pharmaceutical forms or therapeutic regimens (drugs used simultaneously) capable of causing idiosyncratic hepatocellular damage. Among them is the combination isoniazid, rifampicin and pyrazinamide (definite probability). Hepatotoxicity manifested with elevated liver enzymes, abdominal pain, jaundice, asthenia, nausea, vomiting and necrosis and has been confirmed by liver biopsies. 86-88 In the case of the combined pharmaceutical forms of antibiotics, such as trimethoprim/sulfamethoxazole and amoxicillin/clavulanic acid, cases of hepatotoxicity were identified as idiosyncratic and classified as probable. 57 Hepatotoxicity occurred mainly in men and caused jaundice and pruritus. In some cases caused by amoxicillin/clavulanic acid, the outcome was liver transplantation or death. 89 The antiretroviral regimen of ritonavir, indinavir, darunavir and fosamprenavir was associated with hepatocellular damage and necrosis. 68,90 Case reports of antineoplastic agents 6-thioguanine, daunomycin, and cytosine arabinose used as part of the therapeutic regimen for myeloid leukemia in children have shown hepatomegaly, cirrhosis, and veno-occlusive disease. 91
Discussion
Antibiotics, antineoplastic agents and antituberculosis drugs were the groups of drugs identified as most likely to cause hepatotoxicity. This is in accordance with the results of previous reviews5,23,33,57. Sufficient evidence was found for the capacities of two antibiotics, vancomycin and minocycline, to cause idiosyncratic hepatotoxicity and a type of hepatocellular lesion17,33,55,56,92. They were assessed as definite. Among the other antibiotics, tetracycline was identified as being able to generate steatohepatitis, 93 but the search did not yield enough information for its inclusion.
Case reports allowed identification of tamoxifen, everolimus and methotrexate as agents that can cause liver damage, with a definite probability. In the case of methotrexate, a previously published study reported increased liver enzymes, but did not identify associated methotrexate concentrations or the probability of causing hepatotoxicity. 94 With the information found it was not possible to establish types of hepatotoxicity for these three drugs, but damage tends to be hepatocellular with elevated transaminases and outcomes that range from favorable to death.
In this review, no reports of specific cases of isoniazid hepatotoxicity were found, but some authors claim that this medication causes liver damage95. Thus, it is important to note that the concomitant use of isoniazid, rifampicin and pyrazinamide was identified as the cause of idiosyncratic hepatotoxicity and hepatocellular damage followed by suspension of the regimen86,87.
In this review, the likelihood for acetaminophen to cause liver toxicity was assessed as probable because only opinions of expert groups and case reports were identified. The absence of evidence from metaanalyses, systematic reviews and clinical trials did not allow it to be classified as a definite probability.
In the case of amiodarone, there are doubts about the type of hepatotoxicity it generates. Although information was found that supports idiosyncratic reactions, (32, 96-98) intrinsic reactions secondary to drug deposition in liver tissue have also been reported. 23
There are medications that have been withdrawn from the world market or from certain countries because they are associated with the probability of causing hepatotoxicity. 99 Some of those identified in this review are dextropropoxyphene, ketoconazole (still marketed in Colombia), nefazodone, propofol and sitaxentan.
Elevated liver enzymes were present in many of the case reports which suggests that it could be a marker for suspicion of drug hepatotoxicity. In this framework, ALT, AST, AP and TB were reported for some medications in some case reports. In addition, the available data for dosages administered doses could support the hypothesis of onset of hepatotixicty at therapeutic doses.
A limitation of this review is that it used only one database: PubMed/Medline. This may decrease the number of drugs identified as likely to cause liver toxicity and could influence assessment of the probabilities found for medications tabulated. To reduce the risk of information biases, a procedure proposed by Amariles et al. that is based on the probability of the occurrence of hepatotoxicity was used. 41 Three categories were established, definite, probable and possible, according to the types of published studies (level of evidence) for each drug. To decrease the confusion bias between and among drugs that were used at the same time, a difference is made between combined pharmaceutical forms (several drugs in a single pharmaceutical form) and therapeutic regimens.
Conclusions
We identified more than 180 drugs associated with hepatotoxicity. Of these, six have definite probabilities while most of the rest have possible probabilities. It is noteworthy that more than 50% of the drugs found are associated with idiosyncratic hepatotoxicity and that female sex is the main risk factor. The age range of people affected is broad. In addition, the elevation of liver enzymes, jaundice and fever the symptoms that occur most often. They can lead to hepatocellular lesions followed by liver necrosis. In most cases, adequate patient evolution occurs after identifying and suspending the causative agent. The consolidation of information on hepatotoxicity shows that several groups of drugs have greater evidence of being substances that cause liver toxicity. Nevertheless, precise understanding of the mechanisms that explain the development and onset of hepatotoxicity is scarce.
REFERENCES
1. Ibáñez L, Pérez E, Vidal X, et al. Prospective surveillance of acute serious liver disease unrelated to infectious, obstructive, or metabolic diseases: epidemiological and clinical features, and exposure to drugs. J Hepatol. 2002;37(5):592-600. https://doi.org/10.1016/S0168-8278(02)00231-3 [ Links ]
2. Fernández-Castañer A, García-Cortés M, Lucena M, et al. An analysis of the causes, characteristics, and consequences of reexposure to a drug or compound responsible for a hepatotoxicity event. Rev Esp Enferm Dig. 2008;100(5):278-84. [ Links ]
3. Lee W. Drug-Induced Hepatotoxicity. N Engl J Med. 2003;349(5):474-85. https://doi.org/10.1056/NEJMra021844 [ Links ]
4. Chen M, Borlak J, Tong W. High lipophilicity and high daily dose of oral medications are associated with significant risk for drug-induced liver injury. Hepatology. 2013;58(1):388-96. https://doi.org/10.1002/hep.26208 [ Links ]
5. Tejada F. Hepatotoxicidad por fármacos. Rev clínica Med Fam. 2010;3(3):177-91. [ Links ]
6. Sgro C, Clinard F, Ouazir K, et al. Incidence of drug-induced hepatic injuries: A French population-based study. Hepatology . 2002;36(2):451-5. https://doi.org/10.1053/jhep.2002.34857 [ Links ]
7. Martí L, Olmo J, Tosca J, et al. Clinical evaluation of drug-induced hepatitis. Rev Esp Enfermedades Dig. 2005;97(4):258-65. https://doi.org/10.4321/S1130-01082005000400006 [ Links ]
8. Hernández N, Bessone F, Sánchez A, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13(2):231-9. [ Links ]
9. Liss G, Lewis J. Drug-induced liver injury: what was new in 2008? Expert Opin Drug Metab Toxicol. 2009;5(8):843-60. https://doi.org/10.1517/17425250903018904 [ Links ]
10. Ghabril M, Fontana R, Rockey D, et al. Drug induced liver injury caused by intramuscular administered medications: the drug induced liver injury network (DILIN) experience. J Clin Gastroenterol. 2013;47(6):553-8. https://doi.org/10.1097/MCG.0b013e318276bf00 [ Links ]
11. Larrey D. Epidemiology and individual susceptibility to adverse drug reactions affecting the liver. Semin Liver Dis. 2002;22(2):145-55. https://doi.org/10.1055/s-2002-30101 https://doi.org/10.1055/s-2002-30105 [ Links ]
12. Ramachandran R, Kakar S. Histological patterns in drug-induced liver disease. J Clin Pathol. 2009;62(6):481-92. https://doi.org/10.1136/jcp.2008.058248 [ Links ]
13. Fisher K, Vuppalanchi R, Saxena R. Drug-induced liver injury. Arch Pathol Lab Med. 2015;139(7):876-87. https://doi.org/10.5858/arpa.2014-0214-RA [ Links ]
14. Castell J, Miñana M. Hepatitis inducida por tóxicos. Mecanismos de toxicidad y patrones de lesión. GH Contin. 2003;2(5):190-6. [ Links ]
15. Lee WM. Drug induced hepatotoxicity. N Engl J Med . 1995;333(17):1118-27. https:// doi.org/ 10.1056/ NEJM199510263331706 [ Links ]
16. Kaplowitz N. Drug-induced liver injury. Clin Infect Dis. 2004;38(Suppl 2):44-8. https://doi.org/10.1086/381446 [ Links ]
17. Adams D, Ju C, Ramaiah S, et al. Mechanisms of immune-mediated liver injury. Toxicol Sci. 2010;115(2):307-21. https://doi.org/10.1093/toxsci/kfq009 [ Links ]
18. Grattagliano I, Bonfrate L, Diogo C, et al. Biochemical mechanisms in drug-induced liver injury: Certainties and doubts. World J Gastroenterol. 2009;15(39):4865-76. https://doi.org/10.3748/wjg.15.4865 [ Links ]
19. García-Cortés M, Andrade R, Lucena M, et al. Hepatotoxicidad secundaria a fármacos de uso común. Gastroenterol Hepatol. 2005;28(8):461-72. https://doi.org/10.1157/13079002 [ Links ]
20. Tarantino G, Di Minno M, Capone D. Drug-induced liver injury: Is it somehow foreseeable? World J Gastroenterol . 2009;15(23):2817-33. https://doi.org/10.3748/wjg.15.2817 [ Links ]
21. Bakke O, Manochia M, De Abajo F, et al. Drug safety discontinuations in the United Kingdom, the United States, and Spain from 1974 through 1993: a regulatory perspective. Clin Pharmacol Ther. 1995;58(1):108-17. https://doi.org/10.1016/0009-9236(95)90078-0 [ Links ]
22. Bénichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol . 1990;11(2):272-6. https://doi.org/10.1016/0168-8278(90)90124-A [ Links ]
23. Kaplowitz N. Drug Induced - Hepatotoxicity. Ann Intern Med. 1986;104(6):826-39. https://doi.org/10.7326/0003-4819-104-6-826 [ Links ]
24. Reuben A. Hy’s Law. Hepatology . 2004;39(2):574-8. https://doi.org/10.1002/hep.20081 [ Links ]
25. Björnsson E. Drug-induced liver injury: Hy’s rule revisited. Clin Pharmacol Ther . 2006;79(6):521-8. https://doi.org/10.1016/j.clpt.2006.02.012 [ Links ]
26. Pessayre D, Fromenty B, Berson A, et al. Central role of mitochondria in drug-induced liver injury. Drug Metab Rev. 2012;44(1):34-87. https://doi.org/10.3109/03602532.2011.604086 [ Links ]
27. Pessayre D, Mansouri A, Berson A, et al. Mitochondrial involvement in drug-induced liver injury. Handb Exp Pharmacol. 2010;(196):311-65. https://doi.org/10.1007/978-3-642-00663-0_11 [ Links ]
28. Kass G, Price S. Role of mitochondria in drug-induced cholestatic injury. Clin Liver Dis. 2008;12(1):27-51. https://doi.org/10.1016/j.cld.2007.11.005 [ Links ]
29. Labbe G, Pessayre D, Fromenty B. Drug-induced liver injury through mitochondrial dysfunction: mechanisms and detection during preclinical safety studies. Fundam Clin Pharmacol. 2008;22(4):335-53. https://doi.org/10.1111/j.1472-8206.2008.00608.x [ Links ]
30. Amacher D. A toxicologist’s guide to biomarkers of hepatic response. Hum Exp Toxicol. 2002;21(5):253-62. https://doi.org/10.1191/0960327102ht247oa [ Links ]
31. Brunt E. Nonalcoholic steatohepatiiis (NASH): further expansion of this clinical entity? Liver. 1999;19(4):263-4. https://doi.org/10.1111/j.1478-3231.1999.tb00047.x [ Links ]
32. Gunawan B, Kaplowitz N. Clinical perspectives on xenobiotic-induced hepatotoxicity. Drug Metab Rev . 2004;36(2):301-12. https://doi.org/10.1081/DMR-120034148 [ Links ]
33. Hayashi P, Fontana R. Clinical features, diagnosis, and natural history of drug-induced liver injury. Semin Liver Dis . 2014;34(2):134-44. https://doi.org/10.1055/s-0034-1375955 [ Links ]
34. Hewitt M, Enoch S, Madden J, et al. Hepatotoxicity: a scheme for generating chemical categories for read-across, structural alerts and insights into mechanism(s) of action. Crit Rev Toxicol. 2013;43(7):537-58. https://doi.org/10.3109/10408444.2013.811215 [ Links ]
35. Kleiner D. The pathology of drug-induced liver injury. Semin Liver Dis . 2009;29(4):364-72. https://doi.org/10.1055/s-0029-1240005 [ Links ]
36. Regev A, Seeff L, Merz M, et al. Causality assessment for suspected DILI during clinical phases of drug development. Drug Saf. 2014;37(S1):S47-56. https://doi.org/10.1007/s40264-014-0185-4 [ Links ]
37. Lucena M, Camargo R, Andrade R, et al. Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology . 2001;33(1):123-30. https://doi.org/10.1053/jhep.2001.20645 [ Links ]
38. Lucena M, Andrade R, Rodrigo L, et al. Trovafloxacin-Induced Acute Hepatitis. Clin Infect Dis . 2000;30(2):400-1. https://doi.org/10.1086/313680 [ Links ]
39. Andrade R, López-Ortega S. Hepatitis tóxicas. Rev Española Enfermedades Dig. 2006;98(9):701. DOI: 10.4321/S1130-01082006000900009. [ Links ]
40. Björnsson E. Hepatotoxicity associated with antiepileptic drugs. Acta Neurol Scand. 2008;118(5):281-90. https://doi.org/10.4321/S1130-01082006000900009 [ Links ]
41. Amariles P, Giraldo N, Faus M. Interacciones medicamentosas: Aproximación para establecer y evaluar su relevancia clínica. Med Clin (Barc). 2007;129(1):27-35. https://doi.org/10.1157/13106681 [ Links ]
42. Fujimoto Y, Ohhira M, Miyokawa N, et al. Acarbose-induced hepatic injury. Lancet. 1998;351(9099):340. https://doi.org/10.1016/S0140-6736(05)78337-9 [ Links ]
43. Schiano TD, Bellary S V, Cassidy MJ, et al. Subfulminant liver failure and severe hepatotoxicity caused by loratadine use. Ann Intern Med . 1996;125(9):738-40. https://doi.org/10.7326/0003-4819-125-9-199611010-00006 [ Links ]
44. Li H, Heller D, Leevy C, et al. Troglitazone-induced fulminant hepatitis. Report of a case withautopsy findings. J Diabetes Complications. 2000;14(3):175-7. https://doi.org/10.1016/S1056-8727(00)00076-3 [ Links ]
45. Cocozzella D, Curciarello J, Corallini O, et al. Propafenone hepatotoxicity: report of two new cases. Dig Liver Dis. 2003;48(2):354-7. [ Links ]
46. Flaharty K, Chase S, Yaghsezian H, et al. Hepatotoxicity associated with amiodarone therapy. Pharmacotherapy. 1989;9(1):39-44. https://doi.org/10.1002/j.1875-9114.1989.tb04102.x [ Links ]
47. Shepherd N, Dawson A, Crocker P, et al. Granular cells as a marker of early amiodarone hepatotoxicity: a pathological and analytical study. J Clin Pathol . 1987;40(4):418-23. https://doi.org/10.1136/jcp.40.4.418 [ Links ]
48. Jeserich M, Ihling C, Allgaier H, et al. Acute liver failure due to enalapril. Herz. 2000;25(7):689-93. https://doi.org/10.1007/PL00001983 [ Links ]
49. Thomas E, Rosenthal W, Zapiach L, et al. Spectrum of methyldopa liver injury. Am J Gastroenterol. 1977;68(2):125-33. [ Links ]
50. Puppala A, Steinheber F. Fulminant hepatic failure associated with methyldopa. Am J Gastroenterol . 1977;68(6):578-81. [ Links ]
51. Pelli N, Setti M, Ceppa P, et al. Autoimmune hepatitis revealed by atorvastatin. Eur JGastroenterol Hepatol . 2003;15(8):921-4. https://doi.org/10.1097/00042737-200308000-00014 [ Links ]
52. Stolk M, Becx M, Kuypers K, et al. Severe hepatic side effects of ezetimibe. Clin Gastroenterol Hepatol . 2006;4(7):908-11. https://doi.org/10.1016/j.cgh.2006.04.014 [ Links ]
53. Jonas M, Eidson M. Propylthiouracil hepatotoxicity: two pediatric cases and review of literature. J Pediatr Gastroenterol Nutr. 1988;7(5):776-8. https://doi.org/10.1097/00005176-198809000-00027 [ Links ]
54. Melamud B, Lurie Y, Goldin E, et al. Methylprednisolone-induced liver injury: a diagnostic challenge. IMAJ. 2014;16(3):180-1. [ Links ]
55. Chen Y, Yang X, Zeckel M, et al. Risk of hepatic events in patients treated with vancomycin in clinical studies: a systematic review and meta-analysis. Drug saf. 2011;34(1):73-82. https://doi.org/10.2165/11539560-000000000-00000 [ Links ]
56. Lawrenson R, Seaman H, Sundström A, et al. Liver damage associated with minocycline use in acne: a systematic review of the published literature and pharmacovigilance data. Drug Saf . 2000;23(4):333-49. https://doi.org/10.2165/00002018-200023040-00006 [ Links ]
57. Navarro V, Senior J. Drug-related hepatotoxicity. N Engl J Med . 2006;354(7):731-9. https://doi.org/10.1056/NEJMra052270 [ Links ]
58. Prince M, Burt A, Jones D. Hepatitis and liver dysfunction with rifampicin therapy for pruritus in primary biliary cirrhosis. Gut. 2002;50(3):436-9. https://doi.org/10.1136/gut.50.3.436 [ Links ]
59. Hydes T, Wright M, Jaynes E, et al. Nitrofurantoin immune-mediated drug-induced liver injury: a serious complication of a commonly prescribed medication. BMJ Case Rep. 2014;2014. https://doi.org/10.1136/bcr-2013-203136 [ Links ]
60. Edoute Y, Karmon Y, Roguin A, et al. Fatal liver necrosis associated with the use of nitrofurantoin. Isr Med Assoc J. 2001;3(5):382-3. [ Links ]
61. Koek G, Striker B, Blok A, et al. Flucloxacillin-associated hepatic iniurv. Liver. 1994;14(5):225-9. https://doi.org/10.1111/j.1600-0676.1994.tb00079.x [ Links ]
62. Clay K, Hanson J, Pope S, et al. Telithromycin: a possible cause of severe liver damage? Ann Intern Med . 2006;144(6):I42. [ Links ]
63. Adriaenssens B, Roskams T, Steger P, et al. Hepatotoxicity related to itraconazole: report of three cases. Acta Clin Belg. 2001;56(6):364-9. https://doi.org/10.1179/acb.2001.055 [ Links ]
64. Jacobson M, Hanks D, Ferrell L. Fatal acute hepatic necrosis due to fluconazole. Am J Med. 1994;96(2):188-90. https://doi.org/10.1016/0002-9343(94)90141-4 [ Links ]
65. Chien R, Yang L, Lin P, et al. Hepatic injury during ketoconazole therapy in patients with onychomycosis: a controlled cohort study. Hepatology . 1997;25(1):103-7. https://doi.org/10.1002/hep.510250119 [ Links ]
66. Van Parys G, Evenepoel C, Van Damme B, et al. Ketoconazole-induced hepatitis: a case with a definite cause-effect relationship. Liver . 1987;7(1):27-30. https://doi.org/10.1111/j.1600-0676.1987.tb00311.x [ Links ]
67. Akhtar M, Mathieson K, Arey B, et al. Hepatic histopathology and clinical characteristics associated with antiretroviral therapy in HIV patients without viral hepatitis. Eur JGastroenterol Hepatol . 2008;20(12):1194-204. https://doi.org/10.1097/MEG.0b013e328305b9e0 [ Links ]
68. Macías J, Neukam K, Mallolas J, et al. Liver toxicity of initial antiretroviral drug regimens including two nucleoside analogs plus one non-nucleoside analog or one ritonavir-boosted protease inhibitor in HIV/HCV-coinfected patients. HIV Clin Trials. 2012;13(2):61-9. https://doi.org/10.1310/hct1302-61 [ Links ]
69. Clarke S, Harrington P, Condon C, et al. Late onset hepatitis and prolonged deterioration in hepatic function assciated with nevirapine therapy. Int J STD AIDS. 2000;11(5):336-7. https://doi.org/10.1177/095646240001100511 [ Links ]
70. McWhirter D, Kitteringham N, Jones R, et al. Chemotherapy induced hepatotoxicity in metastatic colorectal cancer: a review of mechanisms and outcomes. Crit Rev Oncol Hematol. 2013;88(2):404-15. https://doi.org/10.1016/j.critrevonc.2013.05.011 [ Links ]
71. Choti M. Chemotherapy-associated hepatotoxicity: do we need to be concerned? Ann Surg Oncol. 2009;16(9):2391-4. https://doi.org/10.1245/s10434-009-0512-7 [ Links ]
72. Agúndez J, Lucena M, Martínez C, et al. Assessment of nonsteroidal anti-inflammatory drug-induced hepatotoxicity. Expert Opin Drug Metab Toxicol . 2011;7(7):817-28. https://doi.org/10.1517/17425255.2011.574613 [ Links ]
73. Boelsterli U, Zimmerman H, Kretz-Rommel A. Idiosyncratic liver toxicity of nonsteroidal antiinflammatory drugs: molecular mechanisms and pathology. Crit Rev Toxicol . 1995;25(3):207-35. https://doi.org/10.3109/10408449509089888 [ Links ]
74. Prescott L. Hepatotoxicity of mild analgesics. Br J Clin Pharmacol. 1980;10(Suppl 2):373S-9S. https://doi.org/10.1111/j.1365-2125.1980.tb01825.x [ Links ]
75. Black M. Acetaminophen hepatotoxicity. Ann Rev Med. 1984;35:577-93. https://doi.org/10.1146/annurev.me.35.020184.003045 [ Links ]
76. Quartuccio L, Maset M, Soardo G, et al. Acetaminophen-induced liver injury in a woman with febrile flare of systemic lupus erythematosus. J Clin Rheumatol. 2014;20(6):349-51. [ Links ]
77. Miller D, Dwyer J, Klatskin G. Halothane hepatitis: benign resolution of a severe lesion. Ann Intern Med . 1978;89(2):212-5. https://doi.org/10.7326/0003-4819-89-2-212 [ Links ]
78. Munro H, Snider S, Magee J. Halothane-associated hepatitis in a 6-year-old boy: evidence for native liver regeneration following failed treatment with auxiliary liver transplantion. Anesthesiology. 1998;89(2):524-7. https://doi.org/10.1097/00000542-199808000-00033 [ Links ]
79. Camilleri M, Victorino R, Hodgson H. Halothane aggravation of chronic liver disease. Acta Med Port. 1984;5(6):194-6. [ Links ]
80. Colleti R, Trainer T, Krawisz B. Reversible valproate fulminant hepatic failure. J Pediatr Gastroenterol Nutr . 1986;5(6):990-4. https://doi.org/10.1097/00005176-198611000-00032 [ Links ]
81. Bicknese A, May W, Hickey W, et al. Early childhood hepatocerebral degeneration misdiagnosed as valproate hepatotoxicity. Ann Neurol. 1992;32(6):767-75. https://doi.org/10.1002/ana.410320610 [ Links ]
82. Levy M, Goodman M, Van Dyne B, et al. Granulomatous hepatitis secondary to carbamazepine. Ann Intern Med . 1981;95(1):64-5. https://doi.org/10.7326/0003-4819-95-1-64 [ Links ]
83. Horowitz S, Patwardhan R, Marcus E. Hepatotoxic reactions associated with carbamazepine therapy. Epilepsia. 1988;29(2):149-54. [ Links ]
84. Bhayana H, Appasami S, Thapa B, et al. Lamotrigine-induced vanishing bile duct syndrome in a child. J Pediatr Gastroenterol Nutr . 2012;55(6):e147-8. https://doi.org/10.1097/MPG.0b013e31823c2500 [ Links ]
85. Mecarelli O, Pulitano P, Mingoia M, et al. Acute hepatitis associated with lamotrigine and managed with the molecular adsorbents recirculating system (MARS). Epilepsia . 2005;46(10):1687-9. https://doi.org/10.1111/j.1528-1167.2005.00269.x [ Links ]
86. Tostmann A, Boeree M, Aarnoutse R, et al. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol . 2008;23(2):192-202. https://doi.org/10.1111/j.1440-1746.2007.05207.x [ Links ]
87. Cramer J, Lohse A, Burchard G, et al. Low N-acetyltransferase 2 activity in isoniazid-associated acute hepatitis requiring liver transplantation. Transpl Int. 2010;23(2):231-3. https://doi.org/10.1111/j.1432-2277.2009.00921.x [ Links ]
88. Yew W, Leung C. Antituberculosis drugs and hepatotoxicity. Respirology. 2006;11(6):699-707. https://doi.org/10.1111/j.1440-1843.2006.00941.x [ Links ]
89. Lucena M, Andrade J, Fernández M, et al. Determinants of the clinical expression of amoxicillin- clavulanate hepatotoxicity: a prospective series from Spain. Hepatology . 2006;44(4):850-6. https://doi.org/10.1002/hep.21324 [ Links ]
90. Ortu F, Weimer L, Floridia M, et al. Raltegravir, tenofovir and emtricitabine in an HIV-infected patient with HCV chronic hepatitis, NNRTI intolerance and protease inhibitors-induced severe liver toxicity. Eur J Med Res. 2010;15(2):81-3. [ Links ]
91. D’Cruz C, Wimmer R, Harcke T, et al. Veno-occlusive disease of the liver in children following chemotherapy for acute myelocytic leukemia. Cancer. 1983;52(10):1803-7. https://doi.org/10.1002/1097-0142(19831115)52:10<1803::AID-CNCR2820521007>3.0.CO;2-D [ Links ]
92. Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov. 2005;4(6):489-99. https://doi.org/10.1038/nrd1750 [ Links ]
93. Schumacher J, Guo G. Mechanistic review of drug-induced steatohepatitis. Toxicol Appl Pharmacol. 2015;289(1):40-7. https://doi.org/10.1016/j.taap.2015.08.022 [ Links ]
94. Fathi N, Mitros F, Hoffman J, et al. Longitudinal measurement of methotrexate liver concentrations does not correlate with liver damage, clinical efficacy, or toxicity during a 3.5 year double blind study in rheumatoid arthritis. J Rheumatol. 2002;29(10):2092-8. [ Links ]
95. Hayashi P, Fontana R, Chalasani N, et al. Under-reporting and poor adherence to monitoring guidelines for severe cases of isoniazid hepatotoxicity. Clin Gastroenterol Hepatol . 2015;13(9):1676-82. https://doi.org/10.1016/j.cgh.2015.02.024 [ Links ]
96. Rigas B, Rosenfeld L, Barwick K, et al. Amiodarone hepatotoxicity. A clinicopathologic study of five patients. Ann Intern Med . 1986;104(3):348-51. https://doi.org/10.7326/0003-4819-104-3-348 [ Links ]
97. Jain D, Bowlus C, James A, et al. Granular cells as a marker of early amiodarone hepatotoxicity. J Clin Gastroenterol . 2000;31(3):241-3. https://doi.org/10.1097/00004836-200010000-00012 [ Links ]
98. Lupon-Rosés J, Simó-Canonge R, Lu-Cortez L, et al. Probable early acute hepatitis with parenteral amiodarone. Clin Cardiol. 1986;9(5):223-5. https://doi.org/10.1002/clc.4960090512 [ Links ]
99. Onakpoya I, Heneghan C, Aronson J. Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: a systematic review of the world literature. BMC Med. 2016;14(10):1-11. Ann Intern Med . 1986;104:348-51. https://doi.org/10.1186/s12916-016-0553-2 [ Links ]
Received: October 13, 2016; Accepted: October 06, 2017











 texto en
texto en