Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.32 no.4 Bogotá oct./dic. 2017
https://doi.org/10.22516/25007440.178
Review articles
Chronic Liver Failure and Hemostasis
1Servicio de hematología, Hospital General de México, Ciudad de México, México
2Laboratorio de biología molecular, Hospital General de México, Ciudad de México, México.
Hepatic insufficiency is a pathology that conditions synthesis and metabolism of various biomolecules. Alterations of hemostasis is one of its first systemic consequences. Because of this and the size of the risks, it is not uncommon for clotting tests to be indispensable for formulating prognostic scales in patients with liver disease.
Knowledge about hemostasis has advanced in the last decade, and the classic cascade of activation of coagulation factors has been perfected until it has become the now-current cellular model that considers the valuable and indispensable participation of the endothelium and platelets. Thanks to this, it is possible to understand that the risk of bleeding in patients who have hepatic insufficiency is not only due to deficiencies in production of coagulation factors, and that for this reason administration of vitamin K is questionable. Even more relevant, is the fact that, thanks to this this knowledge, we can understand the sometimes contradictory and potentially life-threatening complication of thrombosis in these patients.
Keywords: Blood coagulation; liver failure; thrombosis; vitamin K
La insuficiencia hepática es un estado patológico que condiciona la síntesis y metabolismo de diversas biomoléculas, siendo las alteraciones a la hemostasia una de las primeras consecuencias a nivel sistémico que se hacen presentes; debido a esto y por la dimensión de los riesgos de esta situación, no es raro que las pruebas de coagulación sean indispensables para formular las escalas pronósticas en pacientes hepatópatas.
Los conocimientos sobre hemostasia han avanzado en la última década; la clásica cascada de activación de los factores de la coagulación ha sido perfeccionada hasta conformarse el ahora vigente modelo celular que considera la valiosa e indispensable participación del endotelio y las plaquetas. Gracias a esto es posible comprender que, en pacientes con insuficiencia hepática, el riesgo de sangrado no obedece únicamente a la deficiencia en la producción de los factores de la coagulación y, por tanto, es cuestionable la administración de vitamina K. Más relevante aún es que, gracias a estos conocimientos, se puede comprender el a veces contradictorio riesgo de trombosis en estos pacientes, complicación potencialmente mortal.
Palabras clave: Coagulación sanguínea; insuficiencia hepática; trombosis; vitamina K
Introduction
The liver is a vital organ that performs various metabolic processes ranging from transformation and elimination of toxins to storage and production of biomolecules that are indispensable for life. Liver failure, or hepatic insufficiency, occurs when liver is unable to carry out its functions of synthesis and metabolism. Initially, liver failure was classified as either acute or chronic, but a new state of acute-on-chronic liver failure (ACLF), characterized by acute exacerbation of chronic liver damage has been recently recognized1,2.
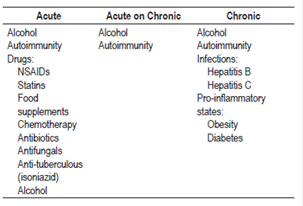
Drug-induced injury is the main cause of liver failure, but it is subdivided according to the type of damage into intrinsic or dose-dependent damage idiosyncratic, regardless of dose, damage3-5. The best example of intrinsic damage is acetaminophen related liver failure. Chronic liver failure due to alcohol is the most frequently occurring type, followed by those caused by infectious agents. Of these, 75% to 80% are associated with the hepatitis C virus (HCV). Some experts consider that the proinflammatory states associated with steatosis, such as obesity or diabetes, should also be considered as factors for the development of chronic liver failure. Autoimmune diseases deserve particular mention because they can manifest as acute liver failure but can also cause chronic deterioration (Table 1). 6,7
Physiology of Hemostasis
Hemostasis is a complex process that involves the stimulation of coagulation factors on the surface of activated platelets to achieve vascular tissue repair. 8 In general, hemostasis is divided into primary and secondary, the former is mediated by the endothelium and platelets in order to integrate the hemostatic plug while the latter involves the coagulation process. 8,9
In recent decades, understanding of hemostasis has improved. One of the most relevant changes is that platelets are no longer thought to be the sole element forming the hemostatic plug during primary hemostasis. Now it is known that they are more than 300 substances contained in intracellular granules (α granules) among which growth factors necessary for tissue regeneration should be highlighted. 10
Cellular Coagulation Model
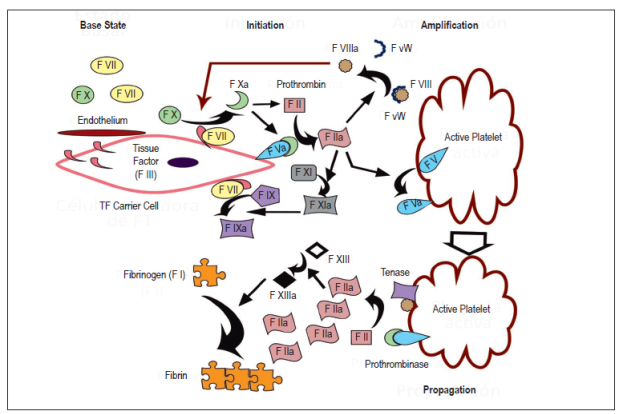
Traditionally, coagulation was understood as a cascade of ordered activation of factors, but current model proposed by Hoffman and Monroe considers interactions between factors and cellular elements. 11 The cellular model of coagulation starts from the basal state in which factors X and VII are inactive in the bloodstream. Tissue factor (TF) carrier cells including monocytes and cells from the endothelium and subendothelium and monocytes are already present before noxious stimuli cause TF to be released into the bloodstream which begins the process of coagulation. This consists of three phases:
Phase 1 (initiation): TF binds factor VII to transform and activate factors IX and X. Factor Xa is combined with Factor Va present on cell surfaces thereby transforming small amounts of prothrombin (Factor II) into thrombin (Factor IIa).
Phase 2 (amplification): Thrombin maintains production of Factor Xa through intervention in the intrinsic pathway (Factors XI and IX), and, more importantly, through releasing plasma transporter and activating Factor VIII. Factor VIIIa is a very efficient Factor X catalyst. Overall, these pathways maintain and substantially increase thrombin production and create an environment conducive to recruiting and activating more platelets.
Phase 3 (propagation): Once platelets are activated, they release chemoattractants such as adenosine diphosphate (ADP) and thromboxane A2 and change their physical form to one which facilitates creation of the hemostatic plug. This form expresses negatively charged phospholipids such as phosphatidylserine on the platelet cell surface. In conjunction with calcium, these phospholipids serve as a template for the formation of tenase complexes (Factors VIIIa and IXa) and prothrombinase complexes (factors Xa and Va), which have the capacity to generate large amounts of thrombin. Finally, thrombin conditions the polymerization of fibrinogen fragments in fibrin fibers that stabilize the initial clot. In addition, thrombin activates Factor XIII which stabilizes fibrin and provides greater resistance to the lysis process (Figure 1). 9,12,13
Physiopathology of Hemostasis during Hepatic Insufficiency
Alterations in Primary Hemostasis
From the early stages of liver disease massive accumulations of lipids develop in hepatocytes and induce the release of various inflammatory mediators, among which are interleukin (IL) and tumor necrosis factors (TNF) 1 and 6. Expression of Toll-like receptor 4 is stimulated14,15, and these pathways trigger dysregulation of the endothelium by activating asymmetric dimethylarginine (ADMA), an endogenous inhibitor of endothelial nitric oxide synthetase (eNOS)16,17. Animal models have shown that decreased activity of eNOS is a fundamental part of the pathophysiology of portal hypertension18,19.
In addition to serving as cellular elements in the hemostatic plug, platelets possess intracellular granules whose elements are capable of modifying inflammatory and immunological processes within the liver. 10 The α-granules contain growth factors whose targets are the Akt pathway (also known as the PI3K-Akt pathway) and extracellular signal-regulated kinase (ERK) 1 and 2 pathways all of which are transcendental for hepatocytes 20 to the degree that recent research suggests that platelet transfusion may improve liver regeneration. 20,21 The dense granules contain adenine nucleotides that inactivate the stellar cells of the sinusoidal space and, consequently, slow profibrotic mechanisms. 21,22
Thrombocytopenia is one of the most frequently occurring manifestations of liver disease. Approximately 76% to 85% of patients develop thrombocytopenia, defined as less than 150,000 thrombocytes (platelets)/μL of blood, although 13% of these patients have less than 50,000 thrombocytes/μL23. It should be remembered that the main stimulus for formation of platelets is thrombopoietin (TPO), a hormone produced primarily by hepatocytes. 24 TPO binding with its c-Mpl receptor promotes activation of cascades of the Janus 2 kinase (JAK2) and tyrosine kinase 2 (TYK2) and synergizes with other stimulants such as IL-3, IL-11 and stem-cell factor resulting in the proliferation and maturation of megakaryocytes24,25. Panasiuk et al. have shown decreased production of TPO in patients with liver cirrhosis and, consequently, decreased numbers of platelets. They also found a direct association between the platelet count and hepatocyte growth factor (HGF) (p <0.01)26. In this context, clinical trials began for TPO mimetics for treatment of liver failure, especially for patients with chronic viral hepatitis. McHutchison et al. evaluated the addition of eltrombopag, a TPO receptor agonist. The four week trial was conducted with HCV carriers who could not be treated with antivirals due to severe thrombocytopenia. Dosages of 30, 50 and 75 mg were compared to controls who recieved placebos. The best response was observed in the group treat with 75 mg doses of eltrombopag, 95% of whose platelet counts increased to acceptable levels 27. Afdhal et al. obtained similar favorable results in the Endothelin Antagonist Used To Decrease Cardiac Events (ENABLE) 1 and 2 test cohorts which treated patients with bosentan and which had nine weeks of follow-up 28. Afdhal et al. had reported the benefits of using eltrombopag for elevated platelet counts before carrying out invasive procedures in an earlier study called Early Levosimendan Vs. Usual Care In Advanced Chronic Heart Failure (ELEVATE). It compared levosimendan with usual care in advanced chronic heart failure using 75 mg doses for 14 days before each procedure. This reduced requirements for transfusions by 72% over those of patients who received placebos, although case patients experienced more portal vein thrombosis than did controls 29.
The pathophysiology of thrombocytopenia includes increased destruction of platelets mediated by antibodies or due to hypersplenism. The two mechanisms can be differentiated because hypersplenism is accompanied by moderate thrombocytopenia accompanied by a decrease in leukocytes due to splenic sequestration 30,31. Two large series point to traditional or laparoscopic splenectomies as therapeutic measures that help elevate platelet numbers to over 100,000/μL) while also increasing the leukocyte counts of patients with liver cirrhosis 32,33. In animal models of hepatic fibrosis, splenectomies have has been shown to reduce the levels of transforming growth factor β (TGF-β) making it is possible to limit and even reduce the areas of cirrhosis while also changing the immunological profile by increases of cytotoxic T cells 34,35.
Alterations in Secondary Hemostasis
Most procoagulant factors including fibrinogen, prothrombin and factors V, VII, IX, X, XI and XII are synthesized in the liver, so their serum levels depend on the degree of liver damage 36,37. Factors V and VIII are the first to decrease because they have the shortest half-lives (12 and 4-6 hours, respectively) 38. It is common for cirrhotic patients to suffer moderate reductions of factor VII which can be identified through the significant relationship between factor VII levels and prothrombin time (PT) (r = 0.76, p <0.001) 39.
Ruling out congenital defects is important for evaluating coagulation tests in patients with liver disease. The most frequent congenital defect is factor VII deficiency (one case per 500,000 births) 40. Factor VII deficiencies have been described in a number of clinical entities including pregnancy, heart disease and obstructive pulmonary disease. A small number of cases have been reported in cirrhotic patients 41.
Thrombosis and Hepatic Insufficiency
Thrombin’s molecular pathways and their ability to generate states of microthrombosis and consequent ischemic states in the sinusoids have all been mentioned. Expression of pro-fibrogenic factors results from these processes and in turn accelerates and perpetuates damage to the parenchyma of the liver 42. It is not uncommon for patients with hepatic insufficiency, even when they are prone to hemorrhaging, to suffer from highly localized thrombophilia in the splanchnic venous system. Clinical, this condition is expressed as Budd-Chiari Syndrome (SBC), mesenteric thrombosis (one case per million people) and extrahepatic portal vein thrombosis (four cases per million people)43. It should be remembered that up to 40% of patients with liver cirrhosis suffer from portal vein thrombosis 19,44-46. Some experts suggest that reduction of portal vein flow velocity is a predictor of thrombotic events 45, so that, once detected, an antithrombotic strategy should be initiated. Among the possibilities are anticoagulant therapy, angioplasty, stenting, intrahepatic transjugular shunts and even liver transplantation 47,48.
Von Willebrand Factor (vWF)
vWF is a glycoprotein stored in Weibel-Palade bodies inside endothelial cells. Once secreted, vWF favors platelet adhesion and binds to factor VIII to prevent its early degradation 49. As liver damage progresses, coagulation factors decrease, but, contrary to what had been previously thought, there is also parallel elevation of VWF and factor VIII 50. The underlying mechanism seems to be very high levels of endothelial activity at the site of vWF synthesis where the cells involved in hepatic fibrosis processes are located that will lead to the reduction of proteins C and S (anticoagulant factors) and ADAMTS13, (A Disintegrin And Metalloproteinase With Thrombospondin Type 1 Motif # 13) 44. This protease is synthesized by stellate cells and is responsible for dividing vWF into small multimers capable of binding factor VIII 51,52. Decreases of ADAMTS13 activity to less than 10% caused by mutations, autoantibodies or by reduced production due to liver failure results in the production of ultra-long vWF multimers (UL-FvW) that cannot bind to factor VIII. This leads to increased amounts of factor VIII in its free and active form 52,53. A group of researchers from the University of Nara in Japan have shown that, the activity of ADAMTS13 decreases and serum levels of UL-FvW increase in patients with hepatic impairment due to alcohol. More importantly, these events correlate with the degree of severity of liver damage and might be useful as prognostic markers 53,54.
Proteins C and S
Protein C is one of the main anticoagulants produced in the liver and is a zymogen that is activated in the presence of the thrombin-thrombomodulin complex on endothelial surfaces. Activated protein C (APC) inhibits factors Va and VIIIa, two of the main cofactors of coagulation 55.
The S protein is encoded by the PROS1 gene and is produced by hepatocytes, endothelial cells, megakaryocytes and osteoblasts. It is fundamental to coagulation but is also key to atherosclerosis, angiogenesis and the progression of neoplastic cells 56. Dependent on vitamin K, it acts by stimulating the production of APC to regulate coagulation 57.
Liver damage results in decreased activation of these proteins, or even complete absence of activation, which must be taken into account in diagnosing patients who have suffered thrombosis. Other concomitant clinical situations must also be ruled out. These include resistance to APC, hereditary coagulation defects including deficiencies of proteins C and S, Factor V Leiden, Factor II, mutations in the methylene tetrahydrofolate reductase (MTHF) gene, hyperhomocysteinemia, and antiphospholipid syndrome57,58. Neoplasms must also be considered because there are homologs of the S protein in some hepatic tumors59,60.
Evaluation of Hemostasis in Patients with Hepatopathy
Hemostasis equilibrates the great weight of procoagulant and anticoagulant factors. When any of these are deficient or over abundant, the balance will move in favor of the dominant side. In patients with liver disease, this balance is precarious due to decreases of factors on both sides: the smallest increase or decrease of any of element can cause erratic movements of the balance needle until it finds and marks the dominant site (Figure 2)23,36,61. This should be taken into account in assessment of the hemostatic state of patients with liver disease at the beginning of routine tests such as complete blood counts and the basic coagulation tests of thrombin time, prothrombin time (PT), international normalized ratio (INR) and partial thromboplastin time (PTT). These tests only evaluate procoagulant factors 23,36, so evaluation should be complemented with determinations of coagulation factors unless there is a strong suspicion of a congenital defect 40,61.
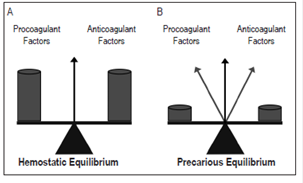
Figure 2 A. Hemostatic balance in healthy people. B. Hemostatic balance in patients with liver disease
It is important to say that PT and PTT are poor predictors of bleeding in liver patients: measurements within the usual range do not guarantee protection against bleeding, and high measurements are not synonymous with uncontrollable bleeding requiring corrective or preventive therapeutic measures61,62. The search for a reliable method for determining the real hemostatic state of patients with liver failure continues. Two of the most promising possibilities are thrombin generation testing and thromboelastography although both are far from becoming routine tests 61,62.
Vitamin K
Vitamin K (from the German word koagulationsvitamin, vitamin coagulant) is composed of a group of fat-soluble molecules derived from 2-methyl-1,4-naphthoquinone 63. Phytomenadione, known as vitamin K1, is of vegetable origin while Menaquinone, known as vitamin K2, is of bacterial origin. The water-soluble forms are menadione and menadiol which are known as vitamin K3 and vitamin K4, respectively 63,64.
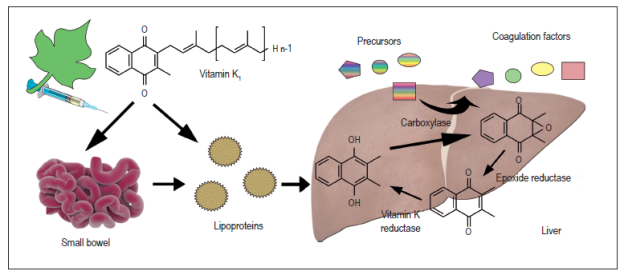
When it is ingested orally, vitamin K is absorbed in the intestine, passes to the lymph system and then into the bloodstream where 75% to 90% of it is transported to the liver by various high-density lipoproteins, mainly apolipoprotein E (ApoE) (Figure 3). This complex not only serves as transport but also facilitates binding and internalization from the Disse space to the interior of the hepatocyte 65. Rapid uptake of phytomenadione in the liver is evidenced by excretion of its metabolites in bile salts (30% -40%). Other excretion routes are urine (15%) and stool 65,66.
In hemostasis, vitamin K catalyzes carboxylation of hepatic synthesis coagulation factors VII, IX, X and prothrombin by forming calcium binding sites in the glutamic acid residues on the sides of the protein chain. Proteins C and S which are natural anticoagulants also require vitamin K for their activity 66,67. Because of this, it is easy to understand that patients with significant chronic liver disease do not respond adequately to vitamin K supplementation and coagulation disorders become difficult to control.
Administration of vitamin K
Vitamin K deficiencies in newborns are the most frequent reason for administration of the substance even though this rare alteration occurs in only 0.1% of all live newborns. It is a potentially lethal condition but can be prevented with oral administration of 2 mg of the vitamin in all newborns 65,68,69. The second most frequent therapeutic indications are anticoagulation regimens with vitamin K antagonists for active bleeding and high risk of bleeding for which the vitamin is used as an antidote. Usually vitamin K1 is slowly administered intravenously or intramuscularly in a period of not less than 30 seconds at an average dose of 10-20 mg in conjunction with fresh frozen plasma or prothrombin concentrates 70,71. Usually administration requires no more than two hours allows to reduce INR to usual levels, but in severe cases a similar second dose can be administered eight to twelve hours later 66,72.
Vitamin K is frequently prescribed by some specialists to reverse alterations of hemostasis in patients with liver damage under the hypothesis that there is a deficiency of coagulation factors dependent on vitamin K 73. Nevertheless, it is important to point out that most hemorrhages are not caused by deficiencies of secondary hemostasis, but rather are caused by rupture of esophageal varices or gastric ulcers secondary to portal hypertension due to localized thrombophilia 74. According to the systematic review by Martí-Carvajal et al., there is no benefit from the administration of vitamin K in patients with hepatic insufficiency and hemorrhaging. This statement is based on series of cases or observational studies and still lacks the support of randomized clinical trials 75.
Final Considerations
Understanding the latest scientific evidence about the bleeding-coagulation process allows us to better understand why patients can develop hemostatic disorders from early stages of liver disease. For this reason it is essential to assess secondary hemostasis through TP or INR which are also necessary for the usual prognostic scales for Hepato-pathologies.
Nevertheless, it is now known that fibrinolysis is the main risk factor associated with persistent hemorrhaging. In the event of hemorrhaging, appropriate transfusion support is equally or more important because it has been shown to have a positive impact on short-term prognosis. Transfusion support is indicated prophylactically for patients without bleeding who are going to undergo liver biopsies or other invasive procedures as well as for therapeutic purposes such as treatment of gastrointestinal bleeding. In the latter case, greater pathophysiological support than empirical administration of vitamin K is needed. Before starting transfusion support, it is important to consider complications inherent in transfusion such as formation of alloantibodies as well as hemodynamic alterations typical of patients with liver failure such as Transfusion-Related Acute Lung Injury (TRALI) and volume overload. It is important to remember that the transfusion of plasma and platelets are the main causes of TRALI 76,77. Mention was also made of the apparent usefulness of TPO mimetic drugs for reducing thrombocytopenia and avoiding the use of transfusion support, but, since the evidence is still limited, widespread use cannot yet be recommended.
Referencias
1. Bernal W, Jalan R, Quaglia A, et al. Acute-on-chronic liver failure. Lancet. 2015;386(10003):1576-87. https://doi.org/10.1016/S0140-6736(15)00309-8 [ Links ]
2. Wang Q, Yang F, Miao Q, et al. The clinical phenotypes of autoimmune hepatitis: A comprehensive review. J Autoimmun. 2016;66:98-107. https://doi.org/10.1016/j.jaut.2015.10.006 [ Links ]
3. Licata A. Adverse drug reactions and organ damage: The liver. Eur J Intern Med. 2016;28:9-16. https://doi.org/10.1016/j.ejim.2015.12.017 [ Links ]
4. Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc. 2014;89(1):95-106. https://doi.org/10.1016/j.mayocp.2013.09.016 [ Links ]
5. Rangnekar AS, Fontana RJ. An update on drug induced liver injury. Minerva Gastroenterol Dietol. 2011;57(2):213-29. [ Links ]
6. Dugum M, McCullough A. Diagnosis and management of alcoholic liver disease. J Clin Transl Hepatol. 2015;3(2):109-16. https://doi.org/10.14218/JCTH.2015.00008 [ Links ]
7. Modaresi Esfeh J, Ansari-Gilani K. Steatosis and hepatitis C. Gastroenterol Rep (Oxf). 2016;4(1):24-9. [ Links ]
8. Versteeg HH, Heemskerk JW, Levi M, et al. New fundamentals in hemostasis. Physiol Rev. 2013;93(1):327-58. https://doi.org/10.1152/physrev.00016.2011 [ Links ]
9. Stassen JM, Arnout J, Deckmyn H. The hemostatic system. Curr Med Chem. 2004;11(17):2245-60. https://doi.org/10.2174/0929867043364603 [ Links ]
10. Golebiewska EM, Poole AW. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev. 2015;29(3):153-62. https://doi.org/10.1016/j.blre.2014.10.003 [ Links ]
11. Roberts HR, Hoffman M, Monroe DM. A cell-based model of thrombin generation. Semin Thromb Hemost. 2006;32 Suppl 1:32-8. https://doi.org/10.1055/s-2006-939552 [ Links ]
12. Nilsson IM. Coagulation and fibrinolysis. Scand J Gastroenterol Suppl. 1987;137:11-8. https://doi.org/10.3109/00365528709089754 [ Links ]
13. Chan AK, Paredes N. The coagulation system in humans. Methods Mol Biol. 2013;992:3-12. https://doi.org/10.1007/978-1-62703-339-8_1 [ Links ]
14. Tilg H, Moschen AR, Szabo G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2016;64(3):955-65. https://doi.org/10.1002/hep.28456 [ Links ]
15. Kawaratani H, Tsujimoto T, Douhara A, et al. The effect of inflammatory cytokines in alcoholic liver disease. Mediators Inflamm. 2013;2013:495156. https://doi.org/10.1155/2013/495156 [ Links ]
16. Yang YY, Lee TY, Huang YT, et al. Asymmetric dimethylarginine (ADMA) determines the improvement of hepatic endotelial dysfunction by vitamin E in cirrhotic rats. Liver Int. 2012;32(1):48-57. https://doi.org/10.1111/j.1478-3231.2011.02651.x [ Links ]
17. Vairappan B. Endothelial dysfunction in cirrhosis: Role of inflammation and oxidative stress. World J Hepatol. 2015;7(3):443-59. https://doi.org/10.4254/wjh.v7.i3.443 [ Links ]
18. Laleman W, Omasta A, Van de Casteele M, et al. A role for asymmetric dimethylarginine in the pathophysiology of portal hypertension in rats with biliary cirrhosis. Hepatology . 2005;42(6):1382-90. https://doi.org/10.1002/hep.20968 [ Links ]
19. Pareja J, Restrepego JC. Métodos diagnósticos en hipertensión portal. Rev Col Gastroenterol. 2016;31:135-45. [ Links ]
20. Kmieć Z. Cooperation of liver cells in health and disease. Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. https://doi.org/10.1007/978-3-642-56553-3_7 [ Links ]
21. Murata S, Maruyama T, Nowatari T, et al. Signal transduction of platelet-induced liver regeneration and decrease of liver fibrosis. Int J Mol Sci. 2014;15(4):5412-25. https://doi.org/10.3390/ijms15045412 [ Links ]
22. Kurokawa T, Zheng YW, Ohkohchi N. Novel functions of platelets in the liver. J Gastroenterol Hepatol. 2016;31(4):745-51. https://doi.org/10.1111/jgh.13244 [ Links ]
23. Gangireddy VG, Kanneganti PC, Sridhar S, et al. Management of thrombocytopenia in advanced liver disease. Can J Gastroenterol Hepatol . 2014;28(10):558-64. https://doi.org/10.1155/2014/532191 [ Links ]
24. Kaushansky K. Thrombopoiesis. Semin Hematol. 2015;52(1):4-11. https://doi.org/10.1053/j.seminhematol.2014.10.003 [ Links ]
25. Hitchcock IS, Kaushansky K. Thrombopoietin from beginning to end. Br J Haematol. 2014;165(2):259-68. https://doi.org/10.1111/bjh.12772 [ Links ]
26. Panasiuk A, Prokopowicz D, Zak J, et al. Reticulated platelets as a marker of megakaryopoiesis in liver cirrhosis; relation to thrombopoietin and hepatocyte growth factor serum concentration. Hepatogastroenterology. 2004;51(58):1124-8. [ Links ]
27. McHutchison JG, Dusheiko G, Shiffman ML, et al. Eltrombopag for thrombocytopenia in patients with cirrhosis associated with hepatitis C. N Engl J Med. 2007;357(22):2227-36. https://doi.org/10.1056/NEJMoa073255 [ Links ]
28. Afdhal NH, Dusheiko GM, Giannini EG, et al. Eltrombopag increases platelet numbers in thrombocytopenic patients with HCV infection and cirrhosis, allowing for effective antiviral therapy. Gastroenterology. 2014;146(2):442-52.e1. https://doi.org/10.1053/j.gastro.2013.10.012 [ Links ]
29. Afdhal NH, Giannini EG, Tayyab G, et al. Eltrombopag before procedures in patients with cirrhosis and thrombocytopenia. N Engl J Med . 2012;367(8):716-24. https://doi.org/10.1056/NEJMoa1110709 [ Links ]
30. Peck-Radosavljevic M. Thrombocytopenia in liver disease. Can J Gastroenterol. 2000;14 Suppl D:60D-6D. https://doi.org/10.1155/2000/617428 [ Links ]
31. Boyer TD, Habib S. Big spleens and hypersplenism: fix it or forget it? Liver Int . 2015;35(5):1492-8. https://doi.org/10.1111/liv.12702 [ Links ]
32. Kedia S, Goyal R, Mangla V, et al. Splenectomy in cirrhosis with hypersplenism: improvement in cytopenias, Child’s status and institution of specific treatment for hepatitis C with success. Ann Hepatol. 2012;11(6):921-9. [ Links ]
33. Akahoshi T, Tomikawa M, Kawanaka H, et al. Laparoscopic splenectomy with interferón therapy in 100 hepatitis-C-virus-cirrhotic patients with hypersplenism and thrombocytopenia. J Gastroenterol Hepatol . 2012;27(2):286-90. https://doi.org/10.1111/j.1440-1746.2011.06870.x [ Links ]
34. Nakamura T, Sakata R, Ueno T, et al. Inhibition of transforming growth factor beta prevents progression of liver fibrosis and enhances hepatocyte regeneration in dimethylnitrosamine-treated rats. Hepatology . 2000;32(2):247-55. https://doi.org/10.1053/jhep.2000.9109 [ Links ]
35. Nomura Y, Kage M, Ogata T, et al. Influence of splenectomy in patients with liver cirrhosis and hypersplenism. Hepatol Res. 2014;44(10):E100-9. https://doi.org/10.1111/hepr.12234 [ Links ]
36. Monroe DM, Hoffman M. The coagulation cascade in cirrhosis. Clin Liver Dis. 2009;13(1):1-9. https://doi.org/10.1016/j.cld.2008.09.014 [ Links ]
37. Prelipcean CC, Fierbinteanu-Braticevici C, Drug VL, et al. Liver cirrhosis--procoagulant stasis. Rev Med Chir Soc Med Nat Iasi. 2011;115(3):678-85. [ Links ]
38. Muciño-Bermejo J, Carrillo-Esper R, Uribe M, et al. Coagulation abnormalities in the cirrhotic patient. Ann Hepatol . 2013;12(5):713-24. [ Links ]
39. Donaldson GW, Davies SH, Darg A, et al. Coagulation factors in chronic liver disease. J Clin Pathol. 1969;22(2):199-204. https://doi.org/10.1136/jcp.22.2.199 [ Links ]
40. Mariani G, Bernardi F. Factor VII deficiency. Semin Thromb Hemost . 2009;35(4):400-6. https://doi.org/10.1055/s-0029-1225762 [ Links ]
41. Zhang C, Wang Q, Zhang T, et al. Liver cirrhosis with factor VII deficiency: a case report and literature review. Zhonghua Gan Zang Bing Za Zhi. 2014;22(1):73-4. [ Links ]
42. De Stefano V, Martinelli I. Splanchnic vein thrombosis: clinical presentation, risk factors and treatment. Intern Emerg Med. 2010;5(6):487-94. https://doi.org/10.1007/s11739-010-0413-6 [ Links ]
43. Darwish Murad S, Plessier A, Hernandez- Guerra M, Faris F, Eapen CE, Bahr MJ, Trebicka J, et al. Etiology, management and outcome of the Budd-Chiari syndrome. Ann Intern Med. 2009;151(3):167-175. [ Links ]
44. Bianchini M, De Pietri L, Villa E. Coagulopathy in liver diseases: complication or therapy? Dig Dis. 2014;32(5):609-14. https://doi.org/10.1159/000360514 [ Links ]
45. Zocco MA, Di Stasio E, De Cristofaro R, et al. Thrombotic risk factors in patients with liver cirrhosis: correlation with MELD scoring system and portal vein thrombosis development. J Hepatol. 2009;51(4):682-9. https://doi.org/10.1016/j.jhep.2009.03.013 [ Links ]
46. Valla D. Splanchnic vein thrombosis. Semin Thromb Hemost . 2015;41(5):494-502. https://doi.org/ 10.1055/s-0035-1550439 [ Links ]
47. Wani ZA, Bhat RA, Bhadoria AS, et al. Extrahepatic portal vein obstruction and portal vein thrombosis in special situations: Need for a new classification. Saudi J Gastroenterol. 2015;21(3):129-38. https://doi.org/10.4103/1319-3767.157550 [ Links ]
48. Monereo Muñoz M, Aguilera García SG, de la Barreda Heusser R, et al. Cryptogenetic liver cirrhosis and prothrombotic mutations - A mere association? Rev Esp Enferm Dig. 2016;108(9):588-91. [ Links ]
49. Lenting PJ, Christophe OD, Denis CV. von Willebrand factor biosynthesis, secretion, and clearance: connecting the far ends. Blood. 2015;125(13):2019-28. https://doi.org/10.1182/blood-2014-06-528406 [ Links ]
50. Kujovich JL. Coagulopathy in liver disease: a balancing act. Hematology Am Soc Hematol Educ Program. 2015;2015:243-9. https://doi.org/10.1182/asheducation-2015.1.243 [ Links ]
51. Uemura M, Fujimura Y, Ko S, et al. Pivotal role of ADAMTS13 function in liver diseases. Int J Hematol. 2010;91(1):20-9. https://doi.org/10.1007/s12185-009-0481-4 [ Links ]
52. Zheng XL. ADAMTS13 and von Willebrand factor in thrombotic thrombocytopenic purpura. Annu Rev Med. 2015;66:211-25. https://doi.org/10.1146/annurev-med-061813-013241 [ Links ]
53. Uemura M, Matsuyama T, Ishikawa M, et al. Decreased activity of plasma ADAMTS13 may contribute to the development of liver disturbance and multiorgan failure in patients with alcoholic hepatitis. Alcohol Clin Exp Res. 2005;29(12 Suppl):264S-71S. https://doi.org/10.1097/01.alc.0000192326.08931.cb [ Links ]
54. Matsuyama T, Uemura M, Ishikawa M, et al. Increased von Willebrand factor over decreased ADAMTS13 activity may contribute to the development of liver disturbance and multiorgan failure in patients with alcoholic hepatitis. Alcohol Clin Exp Res . 2007;31(1 Suppl):S27-35. https://doi.org/10.1111/j.1530-0277.2006.00283.x [ Links ]
55. D’Alessio S, Genua M, Vetrano S. The protein C pathway in intestinal barrier function: challenging the hemostasis paradigm. Ann N Y Acad Sci. 2012;1258:78-85. https://doi.org/10.1111/j.1749-6632.2012.06557.x [ Links ]
56. Suleiman L, Négrier C, Boukerche H. Protein S: a multifunctional anticoagulant vitamin K-dependent protein at the crossroads of coagulation, inflammation, angiogenesis, and cancer. Crit Rev Oncol Hematol. 2013;88(3):637-54. https://doi.org/10.1016/j.critrevonc.2013.07.004 [ Links ]
57. Dahlbäck B. Progress in the understanding of the protein C anticoagulant pathway. Int J Hematol . 2004;79(2):109-16. https://doi.org/10.1532/IJH97.03149 [ Links ]
58. Denninger MH, Chaït Y, Casadevall N, et al. Cause of portal or hepatic venous thrombosis in adults: the role of multiple concurrent factors. Hepatology . 2000;31(3):587-91. https://doi.org/10.1002/hep.510310307 [ Links ]
59. van der Meer JH, van der Poll T, van ‘t Veer C. TAM receptors, Gas6, and protein S: roles in inflammation and hemostasis. Blood . 2014;123(16):2460-9. https://doi.org/10.1182/blood-2013-09-528752 [ Links ]
60. Pobbati AV, Hong W. Emerging roles of TEAD transcription factors and its coactivators in cancers. Cancer Biol Ther. 2013;14(5):390-8. https://doi.org/10.4161/cbt.23788 [ Links ]
61. Colomo A, Puente A. Corrección de la coagulación en la hemorragia por varices en el paciente con cirrosis. ¿Es útil? Gastroenterol Hepatol Contin 2011;10:175-9. https://doi.org/10.1016/S1578-1550(11)70039-0 [ Links ]
62. Muciño-Bermejo J, Carrillo-Esper R, Uribe M, et al. Coagulation abnormalities in the cirrhotic patient. Ann Hepatol . 2013;12(5):713-24. [ Links ]
63. Vermeer C, Schurgers LJ. A comprehensive review of vitamin K and vitamin K antagonists. Hematol Oncol Clin North Am. 2000;14(2):339-53. https://doi.org/10.1016/S0889-8588(05)70137-4 [ Links ]
64. Booth SL, O’Brien-Morse ME, Dallal GE, et al. Response of vitamin K status to different intakes and sources of phylloquinone-rich foods: comparison of younger and older adults. Am J Clin Nutr. 1999;70(3):368-77. [ Links ]
65. Shearer MJ. Vitamin K deficiency bleeding (VKDB) in early infancy. Blood Rev . 2009;23(2):49-59. https://doi.org/10.1016/j.blre.2008.06.001 [ Links ]
66. Shearer MJ, Fu X, Booth SL. Vitamin K nutrition, metabolism, and requirements: current concepts and future research. Adv Nutr. 2012;3(2):182-95. https://doi.org/10.3945/an.111.001800 [ Links ]
67. Larson AE, Friedman PA, Suttie JW. Vitamin K-dependent carboxylase. Stoichiometry of carboxylation and vitamin K 2,3-epoxide formation. J Biol Chem. 1981;256(21):11032-5. [ Links ]
68. von Kries R, Hachmeister A, Göbel U. Can 3 oral 2 mg doses of vitamin K effectively prevent late vitamin K deficiency bleeding? Eur J Pediatr. 1999;158 Suppl 3:S183-6. https://doi.org/10.1007/PL00014352 [ Links ]
69. Cornelissen M, von Kries R, Loughnan P, et al. Prevention of vitamin K deficiency bleeding: efficacy of different multiple oral dose schedules of vitamin K. Eur J Pediatr . 1997;156(2):126-30. https://doi.org/10.1007/s004310050570 [ Links ]
70. Gonsalves WI, Pruthi RK, Patnaik MM. The new oral anticoagulants in clinical practice. Mayo Clin Proc . 2013;88(5):495-511. https://doi.org/10.1016/j.mayocp.2013.03.006 [ Links ]
71. Bounameaux H. The novel anticoagulants: entering a new era. Swiss Med Wkly. 2009;139(5-6):60-4. [ Links ]
72. Ageno W, Gallus AS, Wittkowsky A, et al. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e44S-88S. [ Links ]
73. Wada H, Usui M, Sakuragawa N. Hemostatic abnormalities and liver diseases. Semin Thromb Hemost . 2008;34(8):772-8. https://doi.org/10.1055/s-0029-1145259 [ Links ]
74. Lisman T, Leebeek FW. Hemostatic alterations in liver disease: a review on pathophysiology, clinical consequences, and treatment. Dig Surg. 2007;24(4):250-8. https://doi.org/10.1159/000103655 [ Links ]
75. Martí-Carvajal AJ, Solà I. Vitamin K for upper gastrointestinal bleeding in patients with acute or chronic liver diseases. Cochrane Database Syst Rev. 2012;(9):CD004792. https://doi.org/10.1002/14651858.CD004792.pub4 [ Links ]
76. Barton CA. Treatment of coagulopathy related to hepatic insufficiency. Crit Care Med. 2016;44(10):1927-33. https://doi.org/10.1097/CCM.0000000000001998 [ Links ]
77. Ditisheim S, Goossens N, Spahr L, et al. Coagulation and cirrhosis: new insight. Rev Med Suisse. 2012;8(352):1652, 1654-6. [ Links ]
Received: October 31, 2016; Accepted: October 06, 2017











 texto en
texto en