Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.33 no.1 Bogotá Jan./Mar. 2018
https://doi.org/10.22516/25007440.226
Original articles
Experience in Ocaña, Norte de Santander, with a Scale for Visualization of the Gastric Mucosa during Esophagogastroduodenoscopy in Patients Medicated with N-acetylcysteine plus Simethicone
1Médico internista, gastroenterólogo. Hospital Emiro Quintero Cañizares; Ocaña, Norte de Santander, Colombia. Miembro internacional de la American Gastroenterological Association (AGA), American Society for Gastrointestinal Endoscopy (ASGE). Correo electrónico: royerogastro@hotmail.com
Introduction:
During upper digestive tract endoscopy, visibility of the gastric mucosa can be limited by adherent mucus and bubbles.
Objectives:
This is a study of visualization of the gastric mucosa and the number of washes needed to clear bubbles and foam from the gastric surface. The modified Kuo scale by Chang was used with patients medicated prior to esophagogastroduodenoscopy.
Materials and methods:
This is a descriptive and prospective study of 120 patients who were medicated with 400 mg (10cc) of 4% N-acetylcysteine plus 133.3 mg (2cc) of simethicone (Dimethylpolysiloxane) and 100 cc of warm water 20 minutes prior to esophagogastroduodenoscopy from October to December 2016. Data were tabulated in Excel and frequencies and percentages were analyzed using the Epi Info statistical package from the Centers for Disease Control version 7.2 for Windows. Statistical significance was considered to be p <0.05.
Results:
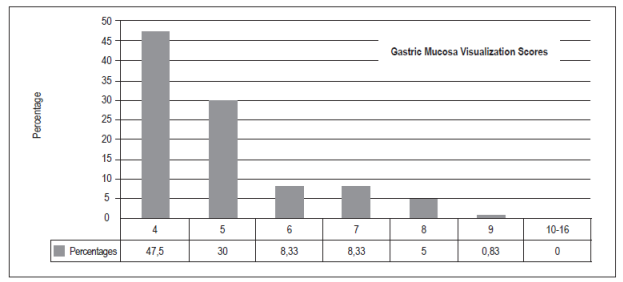
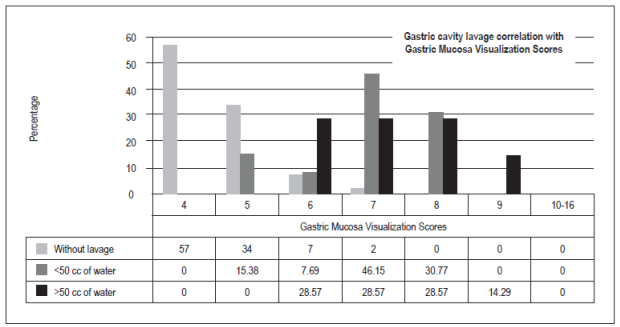
The optimal score for total visibility of four was achieved 57 patients (47.50%). Thirty-six patients (30%) had scores of five points, ten patients (8.33%) had scores of six or seven points, six patients had scores of eight points (5%), and one patient (0.83%) had a score of nine points. There were no scores from 10 to 16. Hundred patients (83,3%) did not need additional washes with water to visualize the gastric mucosa, thirteen patients (10,83%) required less than 50 cc, and seven (5,83%) required more than 50 cc (p = 0.00).
Limitations:
This study was done by a single observer which could result in detection biases. Also, the sample is small.
Conclusions:
Administration of a solution of N-acetylcysteine plus Simethicone diluted in 100 cc of warm water prior to upper digestive tract endoscopy provides for optimal visualization of the gastric mucosa in most cases. A smaller volume of water was needed to clear the gastric cavity of mucus and foam.
Keywords: Premedication; endoscopy; simethicone; N-acetylcysteine.
Introducción:
la visibilidad de la mucosa gástrica puede verse limitada por el moco adherente y la formación de burbujas durante la endoscopia de vías digestivas altas.
Objetivos:
conocer los puntajes de visualización de la mucosa gástrica y el número de lavados para aclarar la superficie gástrica de burbujas y espumas, aplicando la escala de Kuo modificada por Chang en pacientes premedicados antes de la esofagogastroduodenoscopia.
Materiales y métodos:
estudio descriptivo, prospectivo, se incluyeron 120 pacientes entre octubre y diciembre de 2016 a los que se les premedicó con N-acetilcisteína (NAC) al 4%, 400 mg (10 cc) más simeticona (SIM) (dimetilpolisiloxano) 133,3 mg (2 cc) y agua tibia 100 cc, 20 minutos antes del procedimiento; los datos se tabularon en Excel y, ulteriormente, sus frecuencias y porcentajes se analizaron con el paquete estadístico Epi Info CDC (versión 7,2 para Windows, Estados Unidos); se consideró significancia estadística una p <0,05.
Resultados:
la puntuación total de visibilidad de la mucosa gástrica considerada como óptima con un puntaje de 4 fue 57 (47,50%), con 5 puntos fueron 36 (30%), con 6 y 7 puntos 10 (8,33%), con 8 puntos 6 (5%) y, por último, con 9 puntos 1 (0,83%); no hubo casos en las puntuaciones de 10 a 16. 100 (83,3%) pacientes no necesitaron lavados adicionales con agua para visualizar la mucosa gástrica, contra 13 (10,83%) que requirieron menos de 50 cc y 7 (5,83%) que necesitaron más de 50 cc (p = 0,00).
Limitaciones:
un solo observador realizó el estudio, lo que pudo generar sesgos de detección; además, la muestra es pequeña.
Conclusiones:
con la administración de una solución de NAC más SIM diluidas en 100 cc de agua tibia previa a la endoscopia de vías digestivas altas se obtuvo una visualización óptima de la mucosa gástrica en la mayoría de los casos y se observó la necesidad de un menor volumen de agua para aclarar la cavidad gástrica de moco y espuma.
Palabras clave: Premedicación; endoscopia; simeticona; N-acetilcisteína
Introduction
Upper gastrointestinal endoscopy is sometimes performed to detect early gastric cancer, 1 but this diagnostic tool may be limited by adherent mucus and bubbles that prevent adequate visualization of the gastric mucosa. 2. One strategy to overcome this disadvantage is to use antifoaming and mucolytic substances. One of these substances is Pronase, a product of culturing streptomyces griseus. It serves as the basis for a preparation of digestive and anti-inflammatory enzymes. 3 A review of the effectiveness of Pronase for improving visualization of the gastric mucosa has been published recently by Kim, 4 but these benefits are of no use in our environment because Pronase is not available in the Western Hemisphere. A second similar substance is simethicone (SIM) which is a mixture of polydimethylsiloxane and silica gel. It is physiologically inactive, non-toxic and is not absorbed in the gastrointestinal tract. Its reduces the adhesion force of air bubbles, does not interact with other medications, and no complications due to its use have been reported. 5 Finally, there is N-acetylcysteine (NAC), a mucolytic with antioxidant and anti-inflammatory properties. It contains a free thiol or sulfhydryl group that breaks the disulfide bond in the mucin monomer to reduce the viscosity of mucus adhering to mucosal surfaces. 6 The properties of these drugs have been quantified using several scales for visualization of the gastric mucosa with good results. 4,7,8
Several guidelines for detection of early gastric cancer strongly recommending cleaning the gastric surface of patients who are about to undergo an upper endoscopy. 9,10. In Bogotá, two massive campaigns conducted by Emura to screen for preneoplastic lesions and early gastric cancer used Pronase and polydimethylsiloxane to remove adherents from the epithelium and dissolve saliva bubbles. 11 This substantially improved visualization of the gastric mucosa. Another study about endoscopic treatment of early gastric cancer by the same author mentioned use of NAC plus SIM prior to submucosal dissection in 53 patients. 12 Pronase is not available in Colombia, and no studies have been reported quantifying the effect of mucolytics and antifoams for cleaning of the gastric mucosa. For these reasons, our objective is to show the results of application of a visualization scale of the gastric mucosa during the upper endoscopy following premedication of patients with a solution of SIM plus NAC diluted in warm water. We include visualization scores of the gastric mucosa and the volume of water used in washing the gastric surface to remove bubbles and foam.
Materials and methods
We designed a retrospective descriptive study based on information collected from 120 consecutive patients who had undergone upper endoscopy after being medicated with a solution of 400 mg (10 cc) of 4% NAC, (Fluimucil®, Zambon Laboratory, Bogotá , Colombia) plus 133.3 mg (2 cc) of SIM (polydimethylsiloxane), (Siligas®, Incobra SA laboratory, Barranquilla, Colombia) and 100 cc of warm water twenty minutes prior to performance of the procedure. Endoscopic procedures were performed in an open access endoscopy unit attached to the local hospital. Premedication had become part of patient care one year prior to the study. The study was conducted between October and December 2016. Patients over 15 years of age who required upper endoscopy on an outpatient basis were included while those excluded consisted of patients with histories of gastrointestinal surgery (because the cleaning visualization scale could not be applied), pregnant women because of the precaution on prescribing NAC to this group), patients experiencing upper digestive tract hemorrhaging as indicated by blood from the gastric cavity, patients who had not fasted, and patients with known disorders of gastric emptying and allergies to NAC or SIM.
The study obtained the approval of the ethics committee of the local hospital, and all participants provided informed consent. Patients who met the above criteria were included in the study. Endoscopic procedures were performed by a single endoscopist with extensive experience who used Pentax i-SCAN endoscopes. Excess secretions were removed by aspiration or washing with water through the working channel in the segments examined. For the purposes of the study, the following segments were examined: the fornix, upper corpus, lower corpus and antrum.
Visualization of the mucosa of each segment examined was quantified according to a version of Kuo’s scale modified by Chang (Figures 1, 2, 3 and 4). 8,13 Each segment observed was quantified from 1 to 4 and the sum of the scores of the four locations was defined as the total gastric mucosal visualization score (TGMV). The best score of four was considered excellent while sixteen was the worst possible score. In addition, the volume of water required to wash the gastric cavity was measured as more or less than 50 cc.
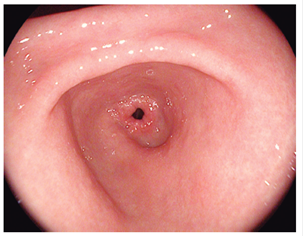
Figure 1 Kuo scale modified by Chang. Score: 1 point. Non-adherent mucus and clear visualization of the gastric mucosa. 8,13
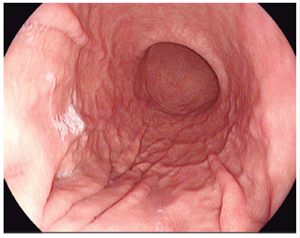
Figure 2 Kuo scale modified by Chang. Score: 2 points. A thin layer of mucus that does not obstruct gastric view. 8,13
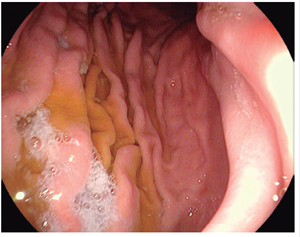
Figure 3 Kuo scale modified by Chang. Score: 3 points. A large amount of mucus on the gastric mucosa that required <50 cc of water for clearance. 8,13
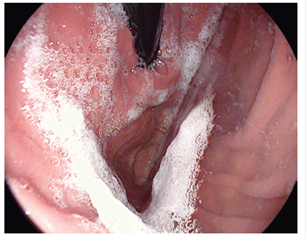
Figure 4 Kuo scale modified by Chang. Score: 4 points A large amount of mucus on the gastric mucosa that required > 50 cc of water for its clearance. 8,13
The data was tabulated in Excel. Then frequencies and percentages were calculated using Epi Info CDC (version 7.2 for Windows, United States). For all results, p <0.05 was considered to be statistically significant.
Results
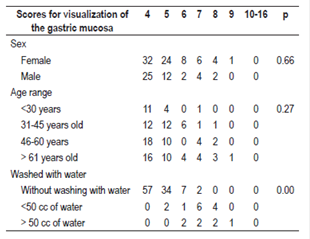
Between October and December 2016, 120 patients were included (Table 1). O these, 75 (62.50%) were women and 45 (37.50%) were men. With respect to the TGMV, there were no statistically significant differences between men and women (p = 0.66). The average age was 52.5 +/- 16.95 (range 15 to 89). There were no significant differences for TGMV between and among age ranges (p = 0.27) (Table 1). The total number of patients with each visibility score of the gastric mucosa were as follows: a score of four (excellent): 57 patients (47.50%), five points: 36 (30%), six and seven points: 10 (8.33%), eight points: 6 (5%), and nine points: 1 (0.83%). There were no cases with scores of 10 to 16 (Figure 5). One hundred of the patients (83.3%) who were premedicated did not need additional water washes to visualize the gastric mucosa, thirteen (10.83%) required less than 50 cc of water, and seven (5.83%) t needed more than 50 cc of water (p = 0.00) (Table 1, Figure 6). No overt complications such as aspiration or allergic reactions were observed during the study.
Table 1 Frequencies of total gastric mucosal cleaning scores according to age, sex and water washings
Discussion
This is the first Colombian study on the application of a scale of visualization of the gastric mucosa in patients premedicated with NAC-SIM. A total visibility score of four, for which visualization of the gastric mucosa was considered to be optimal without the use of water to clear the gastric cavity, was the most frequent finding. It should be highlighted that the effect of premedication is best when visualization scores are lowest and the volume of water used in lavage is lowest. The first scale to measure visualization of the gastric mucosa was proposed by McNally in 1998. 14 That scale had three scores according to the amount of residual gastric bubbles and foam and difficulty of evaluation of the mucosa. It was first used in colonoscopy. Subsequently, in 2002 Kuo established a similar classification, 13 but specified measurement of the volume of water needed to clear the mucosa be either <30 cc or > 30 cc. Five years later, Chang 8 increased the amount of liquid to be measured to > 50 cc or <50 cc which has currently achieved great acceptance. This scale was recently validated in Chile by Mansilla who concluded that it is easy to use and should be considered a quality criterion for practicing upper endoscopy. 15
Due to its relevance in a country like Colombia with a high rate of gastric cancer and its valued use, we considered evaluation of this scale and the use of NAC-SIM premedication prior to upper endoscopy to be pertinent for study. Asian countries have provided the first references with the use of premedication to improve visualization of the gastric mucosa. A randomized prospective study conducted in Taiwan found that Pronase plus SIM presented significantly better visualization either SIM or Pronase alone (<0.005). It concluded that another drug should be added to the Pronase to improve its performance. 13 In Korea, Kim GH also compared Pronase combined with SIM to SIM alone and obtained similar findings (73% vs. 49%. p <0.05). 16 Lee added bicarbonate and warm water to the SIM-Pronase combination and found it to be superior to a SIM plus bicarbonate combination (p = 0.002). 17 Chang introduced the use of NAC combined with SIM for endoscopic premedication and compared it with NAC plus water and SIM plus Pronase and water. 8 There were no differences in the visibility scores of the gastric mucosa in those results (p = 0.14).
The author recommends that NAC combined with SIM be used whenever and wherever Pronase is not available, as is currently the case in Colombia. However, several publications have stated that SIM alone can be an effective intervention as shown by comparisons with placebos. 18,19 Two randomized double-blind studies have also compared SIM alone with the combination of NAC and SIM and have shown that the two strategies are equally effective. 20,21 SIM alone improves visualization of the mucosa during endoscopy and can be easily acquired and used in all endoscopy centers worldwide. In addition, to universal availability, its cost is low. In the United Kingdom, Neale has directed the first randomized and controlled study of the combination of SIM and NAC in a western population. That study determined that this combination significantly improves visualization of the gastric mucosa and reported that 61% (p <0.01) of the patients did not require additional washes to clear the mucosa. 22 Consequently, premedication reduced average procedure time to 8.5 minutes (range 5.0-12 min) whereas average procedure time in a control group was 10.5 minutes (range 7.5-13.5 min). In addition, this intervention is safe and inexpensive. Another study of the SIM and NAC combination included 1849 individuals. 23 Its purpose was to verify the effects of premedication in terms of dosage, volume and administration time prior to procedure. It established that the most effective dosage was 100 mg of SIM and 200 mg of NAC diluted in 100 cc of water. This decreased the probability of additional washes to clear the gastric mucosa (p <0.006). It also found that administration of premedication 30 minutes before endoscopy was effective for improving visualization of the gastric mucosa (p <0.005). These results have recently been legitimized by the NICEVIS study of the combination of NAC and SIM. 24 It is the first study to test visualization in the esophagus with a potential benefit for monitoring Barrett’s esophagus. Administration of NAC plus SIM could become part of routine pre-endoscopic preparation to improve detection of neoplastic lesions of the upper digestive tract.
This study used recommendations published in the medical literature for premedication using NAC plus SIM and its effect on visualization of the gastric mucosa. This investigation also proved that the drugs used are safe because they present no adverse effects. This analysis had a significant limitation: it was carried out by only one observer which could generate information and detection biases. Nevertheless, its main contribution and scope was to present experience in quantifying the visibility of the gastric mucosa with easy available premedication that can be routinely used in every endoscopy unit in the country.
Future research areas
We propose to study whether the premedication analyzed has any impact on the detection of early neoplastic lesions of the stomach and whether the performance of SIM as a single premedication is similar to that of the SIM plus NAC mixture in Colombian patients.
Conclusions
Premedication with 400 mg (10 cc) of 4% NAC plus 133.3 mg (2 cc) of SIM diluted in 100 cc of lukewarm water administered twenty minutes prior to upper endoscopy results in optimum scores for visualization of the gastric mucosa. In addition to less need for washing with water to clean the gastric cavity of mucus and bubbles, this intervention is easily accessible in our environment. Moreover, it is also considered to be safe as no complications occurred due to its administration.
REFERENCES
1. Dinis-Ribeiro M, da Costa-Pereira A, Lopes C, et al. Magnification chromoendoscopy for the diagnosis of gastric intestinal metaplasia and dysplasia. Gastrointest Endosc. 2003;57(4):498-504. https://doi.org/10.1067/mge.2003.145. [ Links ]
2. McDonald GB, O’Leary R, Stratton C. Pre-endoscopic use of oral simethicone. Gastrointest Endosc . 1978;24(6):283. https://doi.org/10.1016/S0016-5107(78)73542-X. [ Links ]
3. Fujii T, Iishi H, Tatsuta M, et al. Effectiveness of premedication with pronase for improving visibility during gastroendoscopy: a randomized controlled trial. Gastrointest Endosc . 1998;47(5):382-7. https://doi.org/10.1016/S0016-5107(98)70223-8. [ Links ]
4. Kim GH, Cho YK, Cha JM, et al. Efforts to increase image quality during endoscopy: The role of pronase. World JGastrointest Endosc . 2016;8(5):267-72. https://doi.org/10.4253/wjge.v8.i5.267. [ Links ]
5. Ahsan M, Babaei L, Gholamrezaei A, Emami MH. Simethicone for the preparation before esophagogastroduodenoscopy. Diagn Ther Endosc. 2011;2011:484532. https://doi.org/10.1155/2011/484532. [ Links ]
6. Tse HN, Tseng CZ. Update on the pathological processes, molecular biology, and clinical utility of N-acetylcysteine in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:825-36. https://doi.org/10.2147/COPD.S51057. [ Links ]
7. Wu L, Cao Y, Liao C, et al. Systematic review and meta-analysis of randomized controlled trials of Simethicone for gastrointestinal endoscopic visibility. Scand J Gastroenterol. 2011;46(2):227-35. https://doi.org/10.3109/00365521.2010.525714. [ Links ]
8. Chang CC, Chen SH, Lin CP, et al. Premedication with pronase or N-acetylcysteine improves visibility during gastroendoscopy: an endoscopist-blinded, prospective, randomized study. World J Gastroenterol. 2007;13(3):444-7. https://doi.org/10.3748/wjg.v13.i3.444. [ Links ]
9. Gómez M, Riveros J, Ruiz O, et al. Guía de práctica clínica para la prevención, diagnóstico y tratamiento del cáncer gástrico temprano-2015. Rev Col Gastroenterol. 2015;30 supl 1:34-42. [ Links ]
10. Rollan A, Cortés D, Calvo A, et al. Diagnóstico precoz de cáncer gástrico. Propuesta de detección y seguimiento de lesiones premalignas gástricas: protocolo ACHED. Rev Med Chile. 2014;142(9):1181-92. https://doi.org/10.4067/S0034-98872014000900013. [ Links ]
11. Emura F, Mejía J, Mejía M, et al. Utilidad de la cromoendoscopia sistemática el diagnóstico del cáncer temprano y lesiones gástricas premalignas, resultado de dos campañas masivas consecutivas de tamizaje en Colombia (2006-2007). Rev Col Gastroenterol. 2010;25(1):19-30. [ Links ]
12. Emura F, Mejía A, Donney A, Ricaurte O, Sabbagh L, Giraldo-Cadavid L, et al. Therapeutic outcomes of endoscopic submucosa dissection of differentiated early gastric cáncer in western endoscopy setting. Gastrointest Endosc . 2015; 82; 804-11. doi: 10.1016/j.gie.2015.03.1960. [ Links ]
13. Kuo CH, Sheu BS, Kao AW, et al. A defoaming agent should be used with pronase premedication to improve visibility in upper gastrointestinal endoscopy. Endoscopy. 2002;34(7):531-4. https://doi.org/10.1055/s-2002-33220. [ Links ]
14. McNally PR, Maydonovitch CL, Wong RK. The effectiveness of simethicone in improving visibility during colonoscopy: a double-blind randomized study. Gastrointest Endosc . 1988;34(3):255-8. https://doi.org/10.1016/S0016-5107(88)71324-3. [ Links ]
15. Mansilla R, Uslar T, Chahuán J, et al. Validez y confiabilidad de una escala de clasificación de limpieza gástrica en endoscopia digestiva alta en población chilena. Gastroentrol Latinoam. 2016;27(1):9-17. [ Links ]
16. Kim GH, Cho YK, Cha JM, et al. Effect of pronase as mucolytic agent on imaging quality of magnifying endoscopy. World J Gastroenterol. 2015;21(8):2483-9. https://doi.org/10.3748/wjg.v21.i8.2483. [ Links ]
17. Lee GJ, Park SJ, Kim SJ, et al. Effectiveness of Premedication with Pronase for Visualization of the Mucosa duringEndoscopy : A Randomized, Controlled Trial. Clin Endosc. 2012;45(2):161-4. https://doi.org/10.5946/ce.2012.45.2.161. [ Links ]
18. Wang C, Liu H, Wang X, et al. Benefit of a 360-degree horizontal turn following premedication with simethicone on image quality during gastroendoscopy: a randomized controlled trial. Int J Clin Exp Med. 2015;8(3):4281-6. [ Links ]
19. Song M, Kwek AB, Law NM, et al. Efficacy of small-volume simethicone given at least 30 min before gastroscopy. World J Gastrointest Pharmacol Ther. 2016;7(4):572-8. https://doi.org/10.4292/wjgpt.v7.i4.572. [ Links ]
20. Asl SM, Sivandzadeh GR. Efficacy of premedication with activated Dimethicone or N-acetylcysteine in improving visibility during upper endoscopy. World J Gastroenterol. 2011;17(37):4213-7. https://doi.org/10.3748/wjg.v17.i37.4213. [ Links ]
21. Elvas L, Areia M, Brito D, et al. Premedication with simethicone and N-acetylcysteine in improving visibility during upper endoscopy: a double-blind randomized trial. Endoscopy . 2017;49(2):139-45. [ Links ]
22. Neale JR, James S, Callaghan J, et al. Premedication with N-acetylcysteine and simethicone improves mucosal visualization during gastroscopy: a randomized, controlled, endoscopist-blinded study. Eur J Gastroenterol Hepatol. 2013;25(7):778-83. https://doi.org/10.1097/MEG.0b013e32836076b2. [ Links ]
23. Chang WK, Yeh MK, Hsu HC, et al. Efficacy of simethicone and N-acetylcysteine as premedication in improving visibility during upper endoscopy. J Gastroenterol Hepatol. 2014;29(4):769-74. https://doi.org/10.1111/jgh.12487. [ Links ]
24. Basford PJ, Brown J, Gadeke L, et al. A randomized controlled trial of pre-procedure simethicone and N-acetylcysteine to improve mucosal visibility during gastroscopy - NICEVIS. Endosc Int Open. 2016;4(11):E1197-E1202. https://doi.org/10.1055/s-0042-117631. [ Links ]
Received: June 12, 2017; Accepted: January 22, 2018











 text in
text in