Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.33 no.1 Bogotá ene./mar. 2018
https://doi.org/10.22516/25007440.236
Original articles
Prevalence of Inappropriate Prescription of Acid Suppression Therapy among Adults Hospitalized at a General Hospital in Bogotá
1Médico de la Universidad de los Andes, asistente de investigación PediAFe. Bogotá D. C., Colombia
2Estudiante de la Facultad de Medicina, Universidad de los Andes, PediAFe. Bogotá D. C., Colombia
3Gastroenterólogo, nutriólogo y epidemiólogo pediatra de la Fundación Santa Fe de Bogotá. Universidad de los Andes. PediAFe. Bogotá D. C., Colombia
Objective:
This study’s objective was to determine the prevalence of prescriptions of acid suppression therapy consisting of proton pump inhibitors (PPIs) or H2 receptor antagonists (H2RA) in adult patients hospitalized in the Hospital Universitario - Fundación Santa Fe de Bogotá (HU-FSFB - Santa Fe de Bogotá Foundation University Hospital) that are not in accordance with clinical practice guidelines (CPG).
Methods:
This is a cross-sectional descriptive observational study that included adult patients hospitalized in the HU-FSFB who were treated with acid suppressors for the first time. We determined the indications for prescriptions used and compared them with validated indications. We excluded patients who had previously taken acid suppressants for two weeks, patients admitted to the intensive care unit, and patients who stayed in the hospital for less than one day.
Results:
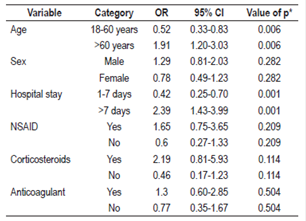
Between January and July 2015, 306 patients with an average age of 56.6 years were included in this study. The prevalence of acid suppression prescriptions without indications based on the evidence was 59.5%. The most common indications were prophylaxis of bleeding due to gastrointestinal ulcers in low risk patients (64.9%) and cause not established (13.7%). Statistically significant associations were found between inappropriate prescription of acid suppression and hospitalization times of less than seven days (OR: 2.39 95% CI 1.4-3.9) and ages of less than 60 years (OR: 1.9 95% CI 1.2-3.03).
Conclusion:
The prevalence of inappropriate prescriptions of acid suppression for adult patients hospitalized in the HU-FSFB was (59.5%). There were positive associations with ages under 60 years and short hospital stays.
Keywords: Proton pump inhibitors; hospitalized; clinical practice guidelines; H2 receptor antagonists
Objetivo:
determinar la prevalencia de prescripción de terapia supresora de ácido (TSA) conformada por los inhibidores de la bomba de protones (IBP) y los antagonistas de los receptores H2 (AR-H2) en pacientes adultos hospitalizados en el Hospital Universitario Fundación Santa Fe de Bogotá (HU-FSFB) que no corresponda con las indicaciones de las guías de práctica clínica (GPC).
Métodos:
estudio observacional descriptivo de corte transversal que incluyó pacientes adultos hospitalizados en el HU-FSFB con prescripción de TSA de novo, determinando las indicaciones de la TSA y comparándolas con las indicaciones validadas. Se excluyeron los pacientes con prescripción o consumo previo de TSA durante 2 semanas y pacientes internados en la unidad de cuidados intensivos (UCI) o con hospitalización menor de un día.
Resultados:
entre enero y julio de 2015 se incluyeron 306 pacientes con un promedio de edad de 56,6 (+38) años, con una prevalencia de prescripción de TSA sin indicación basada en la evidencia del 59,5%. Las indicaciones no basadas en la evidencia más comunes fueron profilaxis de sangrado por úlcera gastrointestinal en pacientes de bajo riesgo (64,9%) y causa no establecida (13,7%). Se encontró una asociación estadísticamente significativa entre el tiempo de hospitalización menor de 7 días (odds ratio [OR]: 2,39; intervalo de confianza [IC] 95%: 1,4-3,9) y edad menor de 60 años (OR: 1,9; IC 95%: 1,2-3,03) con prescripción inapropiada de TSA.
Conclusión:
existe una alta prevalencia (59,5%) de prescripción inapropiada de TSA en pacientes adultos hospitalizados con asociación positiva en menores de 60 años y corta hospitalización en el HU-FSFB.
Palabras clave: Inhibidores de la bomba de protones; hospitalizados; guías de práctica clínica; antagonistas de los receptores H2
Introduction
Acid suppression therapy (AST) using either proton pump inhibitors (PPI) or H2 receptor antagonists (H2RA) is one of the most-widely prescribed drug sets in the world. Its use has been considered safe due to the relative paucity of adverse effects which include headaches, abdominal pain, nausea, diarrhea, vomiting and flatulence. This has favored indiscriminate use in recent years. 1 PPIs’ mechanism of action consists in irreversible blockage of the H +/K + ATPase (adenosine-triphosphatase) pump in the parietal cells while H2RA blocks H2 receptors resulting in the reduction of acid secretion. 2
Recent studies motivated by interest in evaluating repercussions of increasing numbers of AST prescriptions have shown that administration of PPIs and H2RA is associated with decreased absorption of nutrients such as iron, vitamin B12, calcium and magnesium. 3,4,5 On the other hand, the chronic use of PPIs has been associated with increased risk of fractures, 6 and there is a positive association of AST with increased risk of gastroenteritis, clostridium difficile infections, community-acquired pneumonia and chronic kidney disease. 7,8,9,10,11,12
The literature has reported a prevalence of AST prescriptions that does not coincide with clinical practice guidelines (GPC) or prescription indications from the Food and Drug Administration (FDA). It ranges from 50% to 70% demonstrating that these drugs are excessively prescribed and that the potential risk of serious adverse for patients receiving AST without proper indications. 13-23
Because there have been few reports evaluating the use of AST in Latin America and Colombia, this study seeks to evaluate the prevalence of prescriptions to hospitalized patients that are not in accordance with prescription indications in a fourth level general hospital.
Methods
This is an observational, descriptive, prevalence and cross-sectional study of patients at the University Hospital Universitario Fundación Santa Fe de Bogotá (HU-FSFB). Patients included were hospitalized adult patients who had been prescribed AST for the first time and who received at least one dose of PPI (omeprazole, esomeprazole, pantoprazole or lansoprazole) or H2RA (ranitidine) during their hospital stay. AST indications were determined and compared with the validated indications, most frequent reasons and associations among clinical variables to establish the prevalence of inappropriate AST prescriptions. Patients were excluded if they had previously been treated with or prescribed any of these medications for at least 2 weeks, and/or if they had been admitted to the intensive care unit (ICU) or stayed in the hospital less than 1 day.
To determine sample size, Harold A. Kahn and Christopher T. Sempos’ formula (1989) was used for point prevalence, and a minimum theoretical population of 271 patients was established with a type I error of 0.05 and 90% accuracy.
Data were collected from medical records of the HIS-ISIS® electronic information system of the HU-FSFB and included both sociodemographic and clinical variables such as history of acid-peptic disease, medications used, main reason for hospitalization, time of hospitalization, type of AST prescribed, dose, duration of treatment, reason for using AST and prescription for AST following discharge.
For this study, a list of prescription indications for AST in hospitalized patients was used that was based on the recommendations of the FDA, the American Gastroenterology Association (AGA), the American College of Gastroenterology (ACG) and the American Society of Health-System Pharmacists (ASHP). The list had previously been validated and has been used in other studies (Table 1). 19,22,24
Table 1 Indications for AST prescriptions for hospitalized patients according to the evidence 19,22,24 *
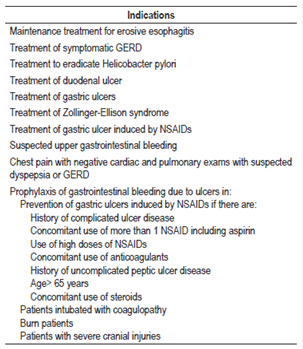
* FDA, AGA, ACG and ASHP. GERD: gastroesophageal reflux disease.
A statistical analysis was performed with measures of descriptive statistics of central tendency and dispersion. A chi-squared test and calculation of odds ratios (OR) were used to establish associations among age, sex, time of hospitalization and use of ulcerogenic drugs (non-steroidal anti-inflammatory drugs [NSAIDs], steroids and anticoagulants) with prescription of AST not based on evidence. P less than 0.05 was taken as statistically significant. STATA 12.0 was used to analyze the data.
This study was approved by the ethics committee of the HU-FSFB at its meeting of December 14, 2015 and meets the criteria of good clinical practice and the 2013 Helsinki Declaration.
Results
We included 306 patients who met the inclusion and exclusion criteria, between January and July 2015. Average patient age was 56.6 (± 38) years, 48% of the patients were women, 54% had completed at least some university level education, and 70.4% had prepaid medical plans in the contributory health care system.
Only 42 (13.7%) of the patients had previous histories of gastrointestinal disease: twenty (6.5%) had had acute or chronic gastritis, ten (3.3%) had had gastroesophageal reflux disease, and nine (3%) had had gastric or duodenal ulcers.
Anticoagulants had been prescribed for twenty-eight patients (9.1%), NSAIDs or aspirin had been prescribed for 27 patients (8.8%), corticosteroids had been prescribed for 17 patients (5.5%).
The mean hospital stay was 7.4 days, with a minimum of one day and a maximum of 55 days. Prescriptions for AST included 266 prescriptions (86.9%) for PPIs, 28 (10.5%) for H2RA and 12 (4%) for both types of medications.
The most commonly used medications, dosage and route of administration were 40 mg of IV omeprazole every 24 hours (32.9% of total prescriptions), 20 mg of oral omeprazole every 24 hours (17% of total prescriptions), and 20 mg of oral esomeprazole every 24 hours (13% of total prescriptions). AST was most commonly administered intravenously.
The prevalence of inappropriate prescriptions of AST among patients hospitalized at HU-FSFB in the period from January to July 2015 was 59.5% (Figure 1).
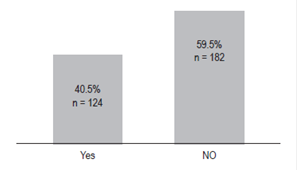
Figure 1 Prescriptions of AST according to the evidence (n = 306) 19,22,26. Yes = based on the evidence; No = not based on evidence. FDA, AGA, ACG and ASHP.
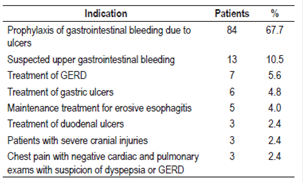
Based on the evidence, the most common reason for prescription of AST for these 124 patients was prophylaxis of gastrointestinal bleeding due to ulcers (67.7%) followed by the suspicion of upper gastrointestinal bleeding (10.5%) (Table 2).
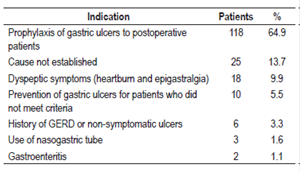
Among the reasons for prescriptions given to the 182 patients that were not based on the evidence, the most common were prophylaxis of gastrointestinal bleeding in patients following low-risk surgery who did not meet the established criteria for prophylaxis (64.9%), cause not established or justified in the patients’ clinical histories (13.7%), dyspeptic symptoms of pyrosis and epigastralgia (10%) and prevention of gastrointestinal bleeding due to ulcers in patients who did not meet criteria (5.5%) (Table 3).
Of the total sample, 47 patients (15.4%) were discharged from the hospital with prescriptions for AST that were not based on the evidence.
Statistically significant associations were found between inappropriate prescriptions of AST and patient age under 60 years (OR: 1.9, 95% CI: 1.2-3.03) and hospital stays of less than 7 days (OR: 2.39, 95% CI: 1.4-3.9). No associations were found between inappropriate prescriptions of AST and sex of the patients or use of ulcerogenic medications (NSAID, anticoagulant or corticoid) using a chi-squared test with a significant p value of less than 0.05 (Table 4).
Discussion
PPIs accounted for the majority of AST prescriptions in these patients which coincides with reports in the literature that prescriptions of PPIs account for 76% of AST with H2RA accounting for the rest. 13
The prevalence of AST prescription not based on the evidence in the Santa Fe Foundation of Bogotá is 59.5%, which is significantly high. However, this prevalence is in agreement with other studies such as those of Reidd et al. and Gupta et al. in the United States. The former found that 50% of the patients at a hospital in Colorado in the United States did not have any valid indication for the prescription of AST while the latter reported a 73% prevalence of inadequate indication at the Medical Center of the University of Florida. 16,19 Other reports include one from a hospital in Singapore which report that 55% of AST use was inappropriate. 20 In Peru, one study reported that the 54.57% of the prescriptions of PPIs were not based on any CPG. 22 A study conducted at the San Ignacio Hospital in Bogotá has reported a rate of inappropriate use of 59.7% which is very similar to that found in this study. 23 Another descriptive study that analyzed more than 100,000 prescriptions of PPIs in 2010 in Colombia through a review of data from the general system of social security in health found that the annual unjustified cost amounts to US $2,202,590. 25 The prevalence of inappropriate prescriptions of AST in our hospital is in accordance with other reports in the literature that range between 50% and 70%. This situation is related to high unjustified costs. 13-23
Among the reasons for prescription of AST based on the evidence in this study, the most important was prophylaxis of gastrointestinal bleeding in patients with risk factors such as the use of NSAIDs. This is consistent with other studies such as that by Bustamante and Scagliarini. 22,26
The most frequent indication not based on evidence was prophylaxis of gastrointestinal bleeding in low risk patients, especially following surgery. These patients did not meet the criteria for prophylaxis such as the use of NSAIDs, cranial injuries, intubation and burns. This agrees with findings of other studies whose frequencies range between 20% and 30% and which show that the foremost reasons is prophylaxis of stress ulcers in low-risk patients. 19,22,24,26 Similarly, a study of patients who had received PPIs in the general surgery service of a hospital in Lausanne, Switzerland has shown that 79% had no risk factors for prophylaxis of gastrointestinal bleeding which is similar to the findings of our study. 21
A review of the literature on the incidence of gastrointestinal bleeding in low-risk patients found one important study of more than 78,000 patients who were not in the ICU. It found that the incidence of evident bleeding was 0.26% in patients who received antacid medications and was 0.18% in patients without antacid medication. This translates into a number needed to treat (NNT) of 834 patients in order to prevent one episode of significant gastrointestinal bleeding. This is not cost-effective. 14,27
In this study, no significant association was found between prescriptions of AST and the use of ulcerogenic drugs (NSAIDs, anticoagulants and steroids). This differs from studies such as that by Gupta which found that the use of ulcerogenic drugs accounts for 15% of the total. 19 Similarly, Bustamante found that polypharmacy was the most important indication of inappropriate AST prescription in an internal medicine the hospital, and Chia et al. found this same association in 35% of the patients in their study. 20,22
It is noteworthy that up to 15% of patients were given AST prescriptions without evidence. Nevertheless, this rate is lower than some found in other studies such as that by Ahrens in Germany which found that 58% of 506 patients followed in 36 primary care centers had been given AST prescriptions without evidence-based indications after hospital discharge. This could expose patients to chronic use of a drug with adverse effects and unnecessary costs. 28
On the one hand, this study is subject to limitations such as the information bias found in the review of medical records, since not all the antecedents or reasons for a course of action related to a given patient are reported including prescriptions of AST. On the other hand, this study is limited to inpatients at the hospital and excludes outpatient consultations within which a significant excess of these medications has also been reported.
This study warns about the high prevalence of prescriptions of AST that is not based on the evidence in a general hospital in Colombia and shows the inadequate level of knowledge of the approved prescription indications for AST. It also makes evident the need to disseminate the approved indications to medical personnel in order to reduce the rate of inappropriate indications, the associated cost and exposure to unnecessary risks that may be serious for patients receiving these medications. Therefore, we suggest the creation of Clinical Practice Guidelines adapted to our environment that are applicable to all hospitals in the country.
Acknowledgements
We would like to thank HU-FSFB and the University of the Andes for their support and commitment to research.
REFERENCES
1. Madanick RD. Proton pump inhibitor side effects and drug interactions: much ado about nothing? Cleve Clin J Med. 2011;78(1):39-49. DOI: 10.3949/ccjm.77a.10087. [ Links ]
2. Wallace JL, Sharkey KA. Pharmacotherapy of gastric acidity, peptic ulcers, and gastroesophageal reflux disease. En: Brunton LL, Chabner BA, Knollmann BC (editores). Goodman & Gilman’s the pharmacological basis of therapeutics. 12.a edición. Nueva York: McGraw-Hill; 2011. pp. 1309-22. [ Links ]
3. Corleto VD, Festa S, Di Giulio E, et al. Proton pump inhibitor therapy and potential long-term harm. Curr Opin Endocrinol Diabetes Obes. 2014;21(1):3-8. DOI: 10.1097/MED.0000000000000031. [ Links ]
4. Hess MW, Hoenderop JG, Bindels RJ, et al. Systematic review: hypomagnesaemia induced by proton pump inhibition. Aliment Pharmacol Ther. 2012;36(5):405-13. DOI: 10.1111/j.1365-2036.2012.05201.x. [ Links ]
5. Zipursky J, Macdonald EM, Hollands S, et al. Proton pump inhibitors and hospitalization with hypomagnesemia: a population-based case-control study. PLoS Med. 2014;11(9):e1001736. DOI: 10.1371/journal.pmed.1001736. [ Links ]
6. Yu EW, Bauer SR, Bain PA, et al. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med. 2011;124(6):519-26. DOI: 10.1016/j.amjmed.2011.01.007. [ Links ]
7. Deshpande A, Pant C, Pasupuleti V, et al. Association between proton pump inhibitor therapy and Clostridium difficile infection in a meta-analysis. Clin Gastroenterol Hepatol. 2012;10(3):225-33. DOI: 10.1016/j.cgh.2011.09.030. [ Links ]
8. Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107(7):1011-9. DOI: 10.1038/ajg.2012.108. [ Links ]
9. Eom CS, Jeon CY, Lim JW, Cho EG, Park SM, Lee KS. Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis. CMAJ. 2011;183(3):310-9. DOI: 10.1503/cmaj.092129. [ Links ]
10. Azab M, Doo L, Doo DH, et al. Comparison of the hospital-acquired clostridium difficile infection risk of using proton pump inhibitors versus histamine-2 receptor antagonists for prophylaxis and treatment of stress ulcers: a systematic review and meta-analysis. Gut Liver. 2017;11(6):781-788. DOI: 10.5009/gnl16568. [ Links ]
11. Nochaiwong S, Ruengorn C, Awiphan R, et al. The association between proton pump inhibitor use and the risk of adverse kidney outcomes: a systematic review and meta-analysis. Nephrol Dial Transplant. 2018;33(2):331-342. DOI: 10.1093/ndt/gfw470. [ Links ]
12. Wise J. Proton pump inhibitors are associated with risk of chronic kidney disease, study finds. BMJ. 2016;352:i128. DOI: 10.1136/bmj.i128. [ Links ]
13. Barletta JF, Lat I, Micek ST, et al. Off-label use of gastrointestinal medications in the intensive care unit. J Intensive Care Med. 2015;30(4):217-25. DOI: 10.1177/0885066613516574. [ Links ]
14. Barletta JF, Sclar DA. Use of proton pump inhibitors for the provision of stress ulcer prophylaxis: clinical and economic consequences. Pharmacoeconomics. 2014;32(1):5-13. DOI: 10.1007/s40273-013-0119-5. [ Links ]
15. Farrell CP, Mercogliano G, Kuntz CL. Overuse of stress ulcer prophylaxis in the critical care setting and beyond. J Crit Care. 2010;25(2):214-20. DOI: 10.1016/j.jcrc.2009.05.014. [ Links ]
16. Reid M, Keniston A, Heller JC, et al. Inappropriate prescribing of proton pump inhibitors in hospitalized patients. J Hosp Med. 2012;7(5):421-5. DOI: 10.1002/jhm.1901. [ Links ]
17. Savarino V, Dulbecco P, de Bortoli N, et al. The appropriate use of proton pump inhibitors (PPIs): Need for a reappraisal. Eur J Intern Med. 2017;37:19-24. DOI: 10.1016/j.ejim.2016.10.007. [ Links ]
18. Thomas L, Culley EJ, Gladowski P, et al. Longitudinal analysis of the costs associated with inpatient initiation and subsequent outpatient continuation of proton pump inhibitor therapy for stress ulcer prophylaxis in a large managed care organization. J Manag Care Pharm. 2010;16(2):122-9. DOI: 10.18553/jmcp.2010.16.2.122. [ Links ]
19. Gupta R, Garg P, Kottoor R, et al. Overuse of acid suppression therapy in hospitalized patients. South Med J. 2010;103(3):207-11. DOI: 10.1097/SMJ.0b013e3181ce0e7a. [ Links ]
20. Chia CT, Lim WP, Vu CK. Inappropriate use of proton pump inhibitors in a local setting. Singapore Med J. 2014;55(7):363-6. DOI: 10.11622/smedj.2014087. [ Links ]
21. Bez C, Perrottet N, Zingg T, et al. Stress ulcer prophylaxis in non-critically ill patients: a prospective evaluation of current practice in a general surgery department. J Eval Clin Pract. 2013;19(2):374-8. DOI: 10.1111/j.1365-2753.2012.01838.x. [ Links ]
22. Bustamante Robles KY, Ticse Aguirre R, Cánepa Rondo IF, et al. Frequency of proton pump inhibitor prescription based in clinical practice guidelines in hospitalized patients in two academic hospitals in Lima, Peru. Rev Gastroenterol Peru. 2012;32(1):44-9. [ Links ]
23. Camacho R, Rodríguez A. Uso de los inhibidores de bomba de protones intravenosos en el Hospital Universitario de San Ignacio (HUSI). Universitas Médica. 2013;54(2):157-64. [ Links ]
24. Eid SM, Boueiz A, Paranji S, et al. Patterns and predictors of proton pump inhibitor overuse among academic and non-academic hospitalists. Intern Med. 2010;49(23):2561-8. DOI: 10.2169/internalmedicine.49.4064. [ Links ]
25. Machado-Alba J, Fernández A, Castrillón JD, et al. Prescribing patterns and economic costs of proton pump inhibitors in Colombia. Colomb Med (Cali). 2013;44(1):13-8. [ Links ]
26. Scagliarini R, Magnani E, Praticò A, et al. Inadequate use of acid-suppressive therapy in hospitalized patients and its implications for general practice. Dig Dis Sci. 2005;50(12):2307-11. DOI: 10.1007/s10620-005-3052-4. [ Links ]
27. Herzig SJ, Vaughn BP, Howell MD, et al. Acid-suppressive medication use and the risk for nosocomial gastrointestinal tract bleeding. Arch Intern Med. 2011;171(11):991-7. DOI: 10.1001/archinternmed.2011.14. [ Links ]
28. Ahrens D, Behrens G, Himmel W, et al. Appropriateness of proton pump inhibitor recommendations at hospital discharge and continuation in primary care. Int J Clin Pract. 2012;66(8):767-773. DOI: 10.1111/j.1742-1241.2012.02973.x. [ Links ]
Received: August 01, 2017; Accepted: January 22, 2018











 texto en
texto en