Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.33 no.1 Bogotá Jan./Mar. 2018
https://doi.org/10.22516/25007440.227
Review articles
Clinical and Epidemiology of Hepatitis E Virus Infection
1 Grupo de gastrohepatología, Facultad de Medicina. Universidad de Antioquia. Medellín, Colombia
The hepatitis E virus, a hepatotropic pathogen transmitted by water and contaminated food, is one of the main etiological agents on the planet of enteral transmission of acute viral hepatitis.
Hepatitis E infections are usually self-limiting, but cases of chronic infection have been described in immunocompromised patients. While self-limiting infections do not require treatment, chronic infections should be treated because of risk of progression to cirrhosis and/or extra-hepatic manifestations.
In Colombia, hepatitis E infections are not included in the routine diagnosis of viral hepatitis, despite evidence of its presence in the country.
The objective of this review is to provide a general description of the hepatitis E virus and the natural history of infections and to highlight studies carried out in Colombia showing its presence in the country. The review was carried out through a search in the PUBMED, SCIELO and ScienceDirect databases for of original papers and subject reviews published between 1983 and 2017.
Keywords: Hepatitis E virus; infection; revision; Colombia
El virus de la hepatitis E es un patógeno hepatotrópico que se transmite por el agua y alimentos contaminados, y uno de los principales agentes etiológicos de hepatitis viral aguda de transmisión enteral en el mundo.
La infección por el virus de la hepatitis E usualmente es autolimitada; sin embargo, se han descrito casos de infección crónica en pacientes inmunocomprometidos. La infección autolimitada no requiere tratamiento; por el contrario, la infección crónica debe tratarse debido al riesgo de progresión a cirrosis o a alguna de las manifestaciones extrahepáticas reportadas.
En Colombia, la infección por el virus de la hepatitis E no hace parte del diagnóstico rutinario de hepatitis virales, a pesar de que existe evidencia de la circulación del virus en el país.
El presente artículo de revisión tiene como objetivo describir las generalidades del virus de la hepatitis E, así como la historia natural de la infección y los estudios realizados en Colombia que evidencian su presencia en el país. La revisión se realizó mediante una búsqueda de literatura en la base de datos PubMed, SciELO y ScienceDirect, de trabajos originales y revisiones de tema publicados entre el período 1983-2017.
Palabras clave: Virus de la hepatitis E; infección; revisión; Colombia
Introduction
Hepatitis E virus (HEV) was discovered in 1983 when a human volunteer ingested a stool sample from a patient. 1 The volunteer developed a acute viral hepatitis that could not be identified as hepatitis A, B, or C. In the 1990s, the viral sequence was described. 2
HEV infections are a public health problem, and, according to the World Health Organization (WHO), there are approximately 20 million cases of acute infections every year, mainly in Asia and Africa. 3,4 The most important transmission route is the fecal-oral route from consumption of contaminated water. HEV is common in areas where the drinking water supply and wastewater treatment are both inadequate.3,5 Zoonotic transmission can also occur, and pigs are the most important reservoir. Transmission occurs as a result of occupational exposure, consumption of poorly cooked pork and water contaminated with fecal material from pigs. 5,6
In Colombia, HEV infections have not been recorded because HEV is not included in the viral hepatitis diagnosis guidelines. Nevertheless, there is evidence that the virus circulates in the human population, in the pig population and in the water supply and in sewage. 3,5-8
Overview and hev genome
HEV is part of the Hepeviridae, Orthohepevirus genus, and Orthohepevirus A species. 9 It is an unwrapped virus of 27 to 34 nm in diameter, but viral particles with lipid envelopes have also been found circulating in blood. 10 This form allows the virus to evade the humoral immune response. 10,11 Viral particles released from hepatocytes present a transient lipid bilayer that is slowly lost, first during passage through the bile duct because of the action of deoxycholic acid, and then in the duodenum due to the action of proteases. In the feces, these particles are present in their naked unwrapped form. 12
The naked viral particle is highly resistant to environmental conditions, so a viral particle isolated from fecal matter can remain stable at temperature conditions below 56° C. However, its infectivity is lost when temperature rises above 60° C, 13 and at 71° C viral particles become inactive in a pig’s liver. 14 The HEV particle is resistant to acidic and alkaline pH and to freezing and thawing processes. 6
The HEV genome consists of a linear single strand of ribonucleic acid (RNA) of approximately 7.2 kb.6 It has with a positive polarity and contains three open reading frames (ORF): ORF1, ORF2 and ORF3.
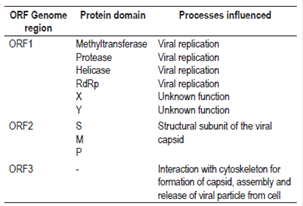
ORF1 encodes a polyprotein of approximately 1,690 amino acids and is essential for the replication of the viral genome. 6,15. The polyprotein is composed of a domain with a methyltransferase (MeT) function, a domain with a protease function, a domain with helicase function (Hel) and a domain with a RNA-dependent polymerase function (RdRp). In addition, it has an X domain and a Y domain with unknown functions (Figure 1).6 It is not clear whether this polyprotein is processed into individual proteins or if the activity of the domains is conserved in the polyprotein (Table 1).16
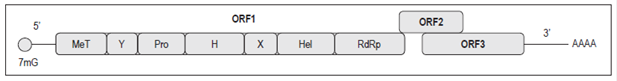
Figure 1 HEV genomic organization. 17 H: domain H; Pro: cysteine-papain protease; X: X domain; Y: Y domain; 7mG: Cap 7-methylguanine. Modified from Panda SK et al. Rev Med Virol. 2007; 17 3: 151-80.
ORF2 encodes the preORF2 structural subunit whose glycosylated form self-assembles to become a subunit of the viral capsid.6,15 This protein has three domains, S, M and P, which are involved in the assembly of the viral particle and in the interaction of the virus with the host cell (Table 1).6,16
ORF3 partially overlaps with ORF2 and encodes a small protein whose function may be to interact with the cytoskeleton for processes of assemblying the capsid and the viral particle (Figure 1, Table 1). 6,18
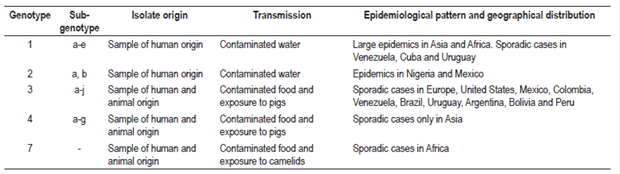
Various strains have been identified in patients as well as in domestic and wild animals such as pigs, wild boars, deer, rabbits, mongooses and camels. Importantly, they exhibit great genetic diversity.19,20 Four genotypes characterized in patients have a nucleotide divergence of less than 20% in isolates of the ORF2 region (Table 2).15
Genotype one has been isolated exclusively from human samples obtained during epidemics in Asia and Africa while genotype two has been isolated exclusively from human samples in Mexico and Nigeria.6,15,21 In Latin America, sporadic cases of HEV genotype 1 infections have been reported in Venezuela, Cuba and Uruguay. 22-24 In addition, this genotype has been associated with fulminant hepatitis, abortions and death in pregnant women in countries such as India and Angola. 25 Genotypes three and four have been isolated in sporadic cases of hepatitis in humans, as well as in domestic and wild animals which indicates their zoonotic potential. 15,20,21 On the other hand, genotype three has the greatest global distribution and is found in Asia, Europe, Oceania and America while the only documented evidence of genotype four infections is from Asia.15,21 Recently, genotype seven has been identified in a report of zoonotic transmission from consumption of meat and camel milk in a a liver transplant patient from the United Arab Emirates (Table 2). 20
Genotype one is subdivided into five subgenotypes which are designated with the letters from a to e. Genotype two has subgenotypes 2a and 2b, genotype three has ten subgenotypes designated with the letters a to j, and genotype four has seven subgenotypes designated with the letters a to g (Table 2). 15
Viral replication
HEV replication begins with entry of a viral particle into a target cell by endocytosis mediated by a still-to-be-identified receptor (Figure 2). Heparan sulfate proteoglycans (HSPG) and 70 kilodalton heat shock proteins (HSP70) have been proposed as receptors. 26
Once the viral particle is in the endosome, the activity of the lysosomal acid lipase (LAL) causes lipid degradation of the particle’s membrane. 10
After entry and decapsulation, the first two-thirds of the viral genome are translated to produce pORF1. 17 Once pORF1 is synthesized, the RdRp domain synthesizes a complementary polarity RNA chain (antigenomic RNA) that serves as a template for the synthesis of 2.2 kb subgenomic RNA strands and genomic RNA strands. 17 Subsequently, pORF2 and pORF3 proteins are translated from this subgenomic RNA. Then dimers of pORF2 interact which allow self-assembly of the capsid. Subsequently, the genome is packaged and new viral particles are generated (Figure 2). 17,27
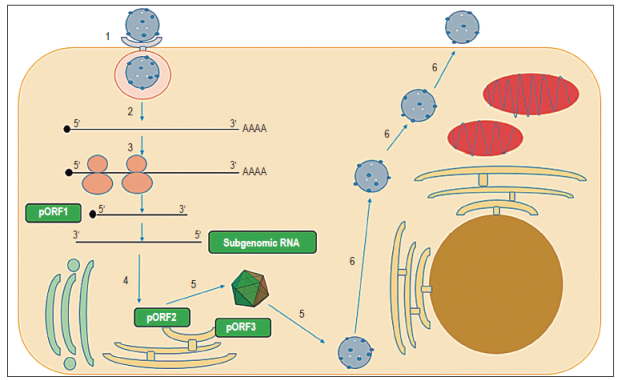
Figure 2 Scheme of HEV replication. 1. Endocytosis occurs with entry of HEV particle into target cell mediated by a receptor 2. Decapsidation and release of RNA 3. translation of nonstructural polyprotein from ORF1 (pORF1) and generation of negative polarity template and subgenomic RNA 4. Translation of pORF2 and pORF3 5. Assembly of the capsid, packaging of the genome and generation of new viral particles 6. Viral particles exit the cell. pORF1: preORF1, pORF2: preORF2, pORF3: preORF3.
Although hepatocytes are the main white cells involved in HEV replication, extrahepatic replication has also been demonstrated. Studies in animals have found the HEV genome in organs such as the small intestine, colon, spleen and lymph nodes of pigs and organs such as the kidney, small intestine, spleen and stomach in rats. 28,29 In addition, genomic and antigenomic RNA has been reported in the central nervous system (CNS) viral replication has been demonstrated in the brain and spinal cord in rodents concomitant with necrosis of neurons, lymphocytic infiltration, perineural invasion and damaged myelin. 30
Epidemiology
There are 2 epidemiological patterns for HEV infections: epidemic and non-epidemic. 6 The epidemic pattern has been observed mainly in India, China, North and West Africa. In these cases, contaminated bodies of water are the main sources of infection. Usually, the population affected consists of young adults between 15 and 30 years of age. 6,21,31 In Latin America, the only outbreaks that have been reported occurred in Mexico in 1986 and 1987. 6,21
The non-epidemic pattern occurs in industrialized countries where sporadic cases can be related to the zoonotic character of genotypes three and four. 6,21
Prevalence rates are generally higher in developing countries than in developed countries because parts of these populations do not have access to clean drinking water. 6,21 Areas reported to have high levels of seroprevalence in the general adult population include rural areas of Malaysia (45%), China (20% -30%), Egypt (26%), India (20%) and Saudi Arabia (17%). 6,21,32 In developed countries lower seroprevalence rates have been reported in the general adult population. Some examples are rates of the United States (1% -3%), Germany (2.1%) and Spain (2% -7%). 6,21,32,33
It is important to note that there are differences in seroprevalence of anti-HEV antibodies associated with occupational risk since veterinary care staff and farmers have higher prevalences than does the general population. 34,35,36 A study in the United States has shown that 27% of veterinarians in eight states with occupational risk have anti-HEV immunoglobulin G (IgG) antibodies which is a much greater percentage than that found in blood donors in the same country. 37 In Moldova, a prevalence of 51% has been found for anti-HEV IgG antibodies in workers at pig farms compared to a 25% seroprevalence in a control group without occupational exposure to pigs. 36 In Colombia it has been shown that the prevalence of anti-HEV IgG antibodies for pig farm workers varies between 11.25% and 15.7%. 35,38 In contrast, a prevalence of 2.5% has been reported for anti-HEV immunoglobulin M (IgM) type antibodies in the nearby population. 38 On the other hand, there is serologic evidence that 5.9% of the people who live with pig farms workers in this region have anti-HEV IgG antibodies (Table 3). 38
Table 3 Studies of HEV infections in Colombia
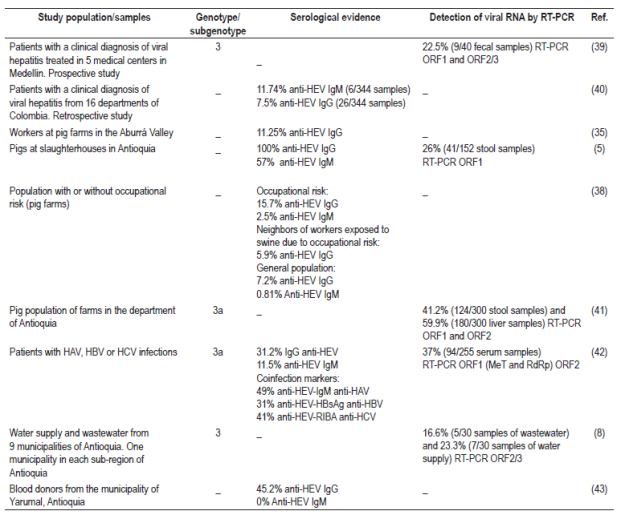
HBsAg: hepatitis B surface antigen; RIBA: Recombinant ImmunoBlot Assay; RT-PCR: Reverse transcription polymerase chain reaction; HAV: hepatitis A virus; HBV: hepatitis B virus; HCV: hepatitis C virus
Studies conducted in Colombia have reported porcine HEV seroprevalence of 100% for IgG antibodies and 57% for anti-HEV IgM type antibodies. In addition, there is molecular evidence that shows the prevalence of the viral genome in fecal samples from the porcine population ranges between 26% and 41%, and is 60% in liver samples of the same population.15,44 Amplified and isolated sequences from the porcine population are genotype 3 subgenotype 3a (Table 3). 15
Serological and molecular evidence of human HEV infections includes 22.5% presence of the viral genome in stool samples. Amplification has established that this is also genotype 3. 39 Serological evidence shows a prevalence range of 7.5% to 31.2% for anti-HEV IgG antibodies and a range of 1.74% to 11.5% for anti-HEV IgM type antibodies (Table 3). 40,42
In addition, 45.2% of the blood samples analyzed from rural blood donors in Yarumal, Antioquia were positive for IgG anti-HEV antibodies (Table 3). 43
HEV has also been detected in sources of water supply and wastewater in the department of Antioquia. Of 60 samples analyzed, 20% (12/60) were positive for the HEV genome in RT-PCR tests. Genotype 3 was found in water samples from the municipalities of San Pedro de los Milagros, Venecia and Cisneros (Table 3). 8
Clinical profile
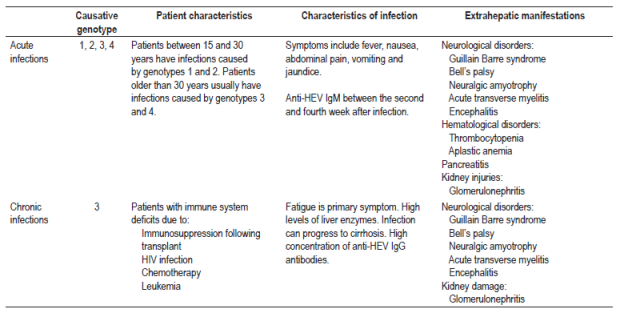
In the majority of patients, HEV causes a self-limiting and usually asymptomatic infection. 6,31 The incubation period lasts for 15 to 60 days, with an average of 40 days. During this time, signs and symptoms develop. These include fever, nausea, abdominal pain, vomiting, malaise and, in some cases, hepatomegaly. 6,31,45
Seventy-five percent of patients with acute infections develop jaundice in the second to fourth weeks after infection (Table 4). 6,31 HEV can be detected in feces before the onset of symptoms and for up to five weeks later while viral RNA in blood serum is detectable for up to three weeks after the onset of symptoms. 46,47 Anti-HEV IgM antibodies can be detected during the acute phase of the disease from the fourth day after the onset of jaundice and for up to 5 months after infection. 48 Anti-HEV IgG antibodies may appear simultaneously with the IgM antibody response, but this response increases throughout the acute phase and remains for years after infection. Nevertheless, the exact duration of IgG antibodies is unknown. 48 The appearance of anti-HEV antibodies in serum coincides with the period in which serum transaminases are elevated (Figure 3). 46
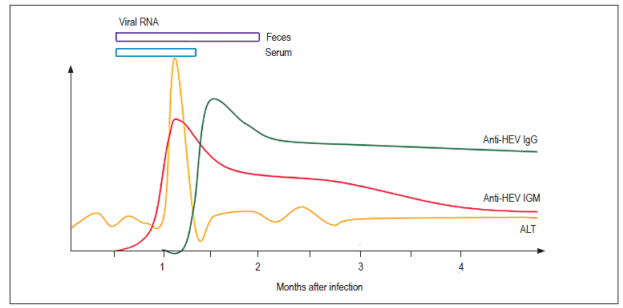
Figure 3 Serological and molecular markers of HEV infections. 49 ALT: alanine aminotransferase. Modified from Aggarwal R. Semin Liver Dis. 2013; 33 1: 30-40.
Chronic infections have been associated with genotype three in patients receiving immunosuppressive therapy for organ transplantation, patients infected with HIV, and patients undergoing chemotherapy (Table 4). 50,51,52 It should be borne in mind that the epidemiological weight is still unknown, but it is suggested that patients receiving immunosuppressive therapy for organ transplantation and HEV infections may rapidly progress to hepatic fibrosis and then to cirrhosis. 52,53
It has been proposed that immunosuppressive therapy be reduced in the first line of treatment since many immunosuppressants including cyclosporine A, tacrolimus and everolimus favor replication of HEV while mycophenolic acid blocks antiviral activity. 53,54 If a patient cannot resolve an infection with this strategy, antiviral treatment with ribavirin for three months has had good results. Nevertheless, antiviral resistance may be associated with mutations such as G1634R. 53,55 Therapy with pegylated interferon type I has been proposed and has demonstrated moderate in vitro antiviral activity against HEV. 53 Finally, the drug sofosbuvir has been shown to inhibit virus replication, and antiviral activity increases when it is combined with ribavirin. 56
Since hepatitis E is clinically indistinguishable from other types of viral hepatitis, diagnosis requires serological and molecular tests. 6 Serological tests are based on detection of anti-HEV antibodies type IgG and IgM by immunoassays which use recombinant proteins or HEV peptides corresponding to epitopes of pORF2 as targets. 6,47,48 It is noteworthy that some studies have shown discordant results in sensitivity, and some have even failed to detect IgM antibodies in patients infected with HEV, so false negative results have been generated. Differences between available serological tests could be explained by genotypic diversity, by the antigens used since the antigens used in commercial kits only come from genotypes one and three, or by the methodology used. 47,49 Since available serological tests vary in sensitivity and specificity, interpretation of results is complicated. 47,49 For this reason it is recommended that diagnosis of HEV use both serological and molecular techniques to ensure that there are no false negative results. 47
Extrahepatic manifestations
Recently, HEV has been associated with extrahepatic manifestations such as neurological and hematological disorders, renal lesions and pancreatitis (Table 4).
Neurological manifestations observed in patients with acute and chronic infection caused by HEV include Guillain-Barré syndrome which is an autoimmune disorder that mainly affects myelin, 57,58 Bell’s palsy which results from damage or trauma of the facial nerves and which causes facial paralysis, 59 neuralgic amyotrophy and bilateral brachial neuritis that mainly affect the shoulders, 57,60 and acute transverse myelitis which is caused by an inflammatory process in the medulla oblongata. 61
A retrospective study that included 126 patients with acute and chronic HEV infections found various neurological manifestations in 5.5% (7/126) of the patients. They included Guillain-Barré syndrome, bilateral brachial neuritis and encephalitis. 62 Three of the cases were patients with acute HEV infections without any type of immunosuppression, but the remaining four cases were immunocompromised patients with chronic HEV infections. 62 In the same study, viral RNA was found in the cerebrospinal fluid of four patients with chronic HEV infections which suggested replication and possible passage of the blood-brain barrier. 62
With respect to hematological disorders, thrombocytopenia and aplastic anemia have been documented in cases of acute HEV infections. 63,64
Damage to renal functioning has been described in patients with acute or chronic HEV infections, mainly in liver transplants patients and patients who have taken medications that compromise renal functioning to cause diseases such as glomerulonephritis. 65
Finally, cases of pancreatitis have been reported in patients with acute infections due to HEV genotype one. 66.
Conclusion
It is necessary to alert medical personnel about the importance of including HEV in the diagnosis of viral hepatitis in Colombia given the evidence of this virus in patients, blood donors, pig farms workers and neighbors, pig populations themselves and in drinking and residual water. Although this infection is self-limiting in most cases, it can also progress to chronic infections and to cirrhosis. In addition, it is important to describe the epidemiology of an infection that is emerging in any population in order to control the virus.
Acknowledgements
REFERENCES
1. Balayan MS, Andjaparidze AG, Savinskaya SS, et al. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology. 1983;20(1):23-31. https://doi.org/10.1159/000149370. [ Links ]
2. Reyes GR, Purdy MA, Kim JP, et al. Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science. 1990;247(4948):1335-9. https://doi.org/10.1126/science.2107574. [ Links ]
3. Echevarría JM, González JE, Lewis-Ximenez LL, et al. Hepatitis E virus infection in Latin America: a review. J Med Virol. 2013;85(6):1037-45. https://doi.org/10.1002/jmv.23526. [ Links ]
4. Organización Mundial de la Salud. Hepatitis E. OMS [Internet] 2016 [acceso el 4 de febrero de 2016]. Disponible en: Disponible en: http://www.who.int/mediacentre/factsheets/fs280/es/ . [ Links ]
5. Gutiérrez CC, Ospina DA, Forero JE, et al. Detección serológica y molecular del virus de la Hepatitis E en cerdos de granjas antioqueñas. CES Med Vet Zootec. 2014;9(2):158-68. [ Links ]
6. Rodríguez F, Jardi R, Buti M. Hepatitis E: virología molecular, epidemiología y patogénesis. Enferm Infecc Microbiol Clin. 2012;30(10):624-34. https://doi.org/10.1016/j.eimc.2012.01.014. [ Links ]
7. Forero J, Gutiérrez C, Parra J, et al. Evidencia serológica de infección por el virus de hepatitis E en cerdos faenados en Antioquia, Colombia. Rev MVZ Córdoba. 2015;20(2):4602-13. https://doi.org/10.21897/rmvz.63. [ Links ]
8. Báez P, Jaramillo CM, Arismendi L, et al. Evidencia molecular de virus de la hepatitis E en fuentes de agua en Antioquia. Biomédica. 2015;35(Supl 1):40-1. [ Links ]
9. International Committee on Taxonomy of Viruses. ICTV taxonomy history: Orthohepevirus A. ICTV [Internet] 2016 [acceso el 10 de febrero de 2016]. Disponible en: Disponible en: http://www.ictvonline.org/taxonomyHistory.asp?taxnode_id=20142432&taxa_name=Orthohepevirus%20A . [ Links ]
10. Yin X, Ambardekar C, Lu Y, et al. Distinct entry mechanisms for nonenveloped and quasi-enveloped hepatitis E viruses. J Virol. 2016;90(8):4232-42. https://doi.org/10.1128/JVI.02804-15. [ Links ]
11. Takahashi M, Tanaka T, Takahashi H, et al. Hepatitis E Virus (HEV) strains in serum samples can replicate efficiently in cultured cells despite the coexistence of HEV antibodies: characterization of HEV virions in blood circulation. J Clin Microbiol. 2010;48(4):1112-25. https://doi.org/10.1128/JCM.02002-09. [ Links ]
12. Okamoto H. Culture systems for hepatitis E virus. J Gastroenterol. 2013;48(2):147-58. https://doi.org/10.1007/s00535-012-0682-0. [ Links ]
13. Emerson SU, Arankalle VA, Purcell RH. Thermal stability of hepatitis E virus. J Infect Dis. 2005;192(5):930-3. https://doi.org/10.1086/432488. [ Links ]
14. Barnaud E, Rogée S, Garry P, et al. Thermal inactivation of infectious hepatitis E virus in experimentally contaminated food. Appl Environ Microbiol. 2012;78(15):5153-9. https://doi.org/10.1128/AEM.00436-12. [ Links ]
15. Lu L, Li C, Hagedorn CH. Phylogenetic analysis of global hepatitis E virus sequences: genetic diversity, subtypes and zoonosis. Rev Med Virol. 2006;16(1):5-36. https://doi.org/10.1002/rmv.482. [ Links ]
16. Holla RP, Ahmad I, Ahmad Z, et al. Molecular virology of hepatitis E virus. Semin Liver Dis. 2013;33(1):3-14. https://doi.org/10.1055/s-0033-1338110. [ Links ]
17. Panda SK, Thakral D, Rehman S. Hepatitis E virus. Rev Med Virol. 2007;17(3):151-80. https://doi.org/10.1002/rmv.522. [ Links ]
18. Zafrullah M, Ozdener MH, Panda SK, et al. The ORF3 protein of hepatitis E virus is a phosphoprotein that associates with the cytoskeleton. J Virol. 1997;71(12):9045-53. [ Links ]
19. Schlauder GG, Mushahwar IK. Genetic heterogeneity of hepatitis E virus. J Med Virol. 2001;65(2):282-92. https://doi.org/10.1002/jmv.2031. [ Links ]
20. Lee GH, Tan BH, Teo EC, et al. chronic infection with camelid hepatitis E virus in a liver transplant recipient who regularly consumes camel meat and milk. Gastroenterology. 2016;150(2):355-7.e3. https://doi.org/10.1016/S0016-5085(16)31244-6 https://doi.org/10.1053/j.gastro.2015.10.048. [ Links ]
21. Aggarwal R, Naik S. Epidemiology of hepatitis E: current status. J Gastroenterol Hepatol. 2009;24(9):1484-93. https://doi.org/10.1111/j.1440-1746.2009.05933.x. [ Links ]
22. García CG, Sánchez D, Villalba MC, et al. Molecular characterization of hepatitis E virus in patients with acute hepatitis in Venezuela. J Med Virol. 2012;84(7):1025-9. https://doi.org/10.1002/jmv.23277. [ Links ]
23. Mirazo S, Mainardi V, Ramos N, et al. Indigenous hepatitis E virus genotype 1 infection, Uruguay. Emerg Infect Dis. 2014;20(1):171-3. https://doi.org/10.3201/eid2001.131471. [ Links ]
24. Villalba Mde L, Lay Lde L, Chandra V, et al. Hepatitis E virus genotype 1, Cuba. Emerg Infect Dis. 2008;14(8):1320-2. https://doi.org/10.3201/eid1408.080049. [ Links ]
25. Navaneethan U, Al Mohajer M, Shata MT. Hepatitis E and pregnancy: understanding the pathogenesis. Liver Int. 2008;28(9):1190-9. https://doi.org/10.1111/j.1478-3231.2008.01840.x. [ Links ]
26. Kalia M, Chandra V, Rahman SA, et al. Heparan sulfate proteoglycans are required for cellular binding of the hepatitis E virus ORF2 capsid protein and for viral infection. J Virol. 2009;83(24):12714-24. https://doi.org/10.1128/JVI.00717-09. [ Links ]
27. Li TC, Takeda N, Miyamura T, et al. Essential elements of the capsid protein for self-assembly into empty virus-like particles of hepatitis E virus. J Virol. 2005;79(20):12999-3006. https://doi.org/10.1128/JVI.79.20.12999-13006.2005. [ Links ]
28. Williams TP, Kasorndorkbua C, Halbur PG, et al. Evidence of extrahepatic sites of replication of the hepatitis E virus in a swine model. J Clin Microbiol. 2001;39(9):3040-6. https://doi.org/10.1128/JCM.39.9.3040-3046.2001. [ Links ]
29. Liu P, Bu QN, Wang L, et al. Transmission of hepatitis E virus from rabbits to cynomolgus macaques. Emerg Infect Dis. 2013;19(4):559-65. https://doi.org/10.3201/eid1904.120827. [ Links ]
30. Shi R, Soomro MH, She R, et al. Evidence of hepatitis E virus breaking through the blood-brain barrier and replicating in the central nervous system. J Viral Hepat. 2016;23(11):930-9. https://doi.org/10.1111/jvh.12557. [ Links ]
31. Purcell RH, Emerson SU. Hepatitis E: an emerging awareness of an old disease. J Hepatol. 2008;48(3):494-503. https://doi.org/10.1016/j.jhep.2007.12.008. [ Links ]
32. Bihl F, Negro F. Hepatitis E virus: a zoonosis adapting to humans. J Antimicrob Chemother. 2010;65(5):817-21. https://doi.org/10.1093/jac/dkq085. [ Links ]
33. Fogeda M, Avellón A, Echevarría JM. Prevalence of specific antibody to hepatitis E virus in the general population of the community of Madrid, Spain. J Med Virol. 2012;84(1):71-4. https://doi.org/10.1002/jmv.22270. [ Links ]
34. Meng XJ. Hepatitis E virus: animal reservoirs and zoonotic risk. Vet Microbiol. 2010;140(3-4):256-65. https://doi.org/10.1016/j.vetmic.2009.03.017. [ Links ]
35. Betancur CA, Mejía MV, Portillo S. Seroprevalencia de hepatitis E en trabajadores de fincas porcícolas del Valle de Aburrá 2011-2012. Acta Médica Colomb. 2013;38(2):68-70. [ Links ]
36. Drobeniuc J, Favorov MO, Shapiro CN, et al. Hepatitis E virus antibody prevalence among persons who work with swine. J Infect Dis. 2001;184(12):1594-7. https://doi.org/10.1086/324566. [ Links ]
37. Meng XJ, Wiseman B, Elvinger F, et al. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J Clin Microbiol. 2002;40(1):117-22. https://doi.org/10.1128/JCM.40.1.117-122.2002. [ Links ]
38. Gutiérrez CC, Rodríguez B, Parra J, et al. Determinación de anticuerpos totales (IgG/IgM) y específicos (IgM) para el virus de la hepatitis E y detección molecular del virus en heces de humanos con o sin exposición ocupacional a porcinos en 10 municipios de Antioquia. Iatreia. 2015;28(3):248-58. [ Links ]
39. Rendon J, Hoyos MC, di Filippo D, et al. Hepatitis E Virus Genotype 3 in Colombia: Survey in Patients with Clinical Diagnosis of Viral Hepatitis. PLoS One. 2016;11(2):e0148417. https://doi.org/10.1371/journal.pone.0148417. [ Links ]
40. Peláez D, Martínez D, Escalante M, et al. Coinfección del virus de la hepatitis E con otras hepatitis virales en Colombia y su caracterización genotípica. Biomédica. 2016;36(Supl):2-30. [ Links ]
41. Forero JE, Gutiérrez-Vergara C, Parra Suescún J, et al. Phylogenetic analysis of Hepatitis E virus strains isolated from slaughter-age pigs in Colombia. Infect Genet Evol. 2017;49:138-45. https://doi.org/10.1016/j.meegid.2017.01.005. [ Links ]
42. Peláez D, Hoyos MC, Rendón JC, et al. Infección por el virus de la hepatitis E en pacientes con diagnóstico clínico de hepatitis viral en Colombia. Biomédica. 2014;34(3):354-65. https://doi.org/10.7705/biomedica.v34i3.2236. [ Links ]
43. Duque A, Restrepo LF, Mantilla C, et al. Frecuencia de anticuerpos contra el virus de la hepatitis E en donantes de sangre del municipio de Yarumal, Antioquia. Rev Colomb Gastroenterol. 2016;31(3):229-34. [ Links ]
44. Forero JE, Parra JE, López A. Detección del genoma del virus de la hepatitis e (VHE) en muestras de heces de cerdos en plantas de beneficio de Antioquia, Colombia. Rev Med Vet Zoot. 2014;61(3):221-7. https://doi.org/10.15446/rfmvz.v61n3.46868. [ Links ]
45. Teshale EH, Hu DJ, Holmberg SD. The two faces of hepatitis E virus. Clin Infect Dis. 2010;51(3):328-34. https://doi.org/10.1086/653943. [ Links ]
46. Krawczynski K, Meng XJ, Rybczynska J. Pathogenetic elements of hepatitis E and animal models of HEV infection. Virus Res. 2011;161(1):78-83. https://doi.org/10.1016/j.virusres.2011.03.007. [ Links ]
47. Cattoir L, Van Hoecke F, Van Maerken T, et al. Hepatitis E virus serology and PCR: does the methodology matter? Arch Virol. 2017;162(9):2625-32. https://doi.org/10.1007/s00705-017-3395-0. [ Links ]
48. Khudyakov Y, Kamili S. Serological diagnostics of hepatitis E virus infection. Virus Res. 2011;161(1):84-92. https://doi.org/10.1016/j.virusres.2011.06.006. [ Links ]
49. Aggarwal R. Hepatitis E: clinical presentation in disease-endemic areas and diagnosis. Semin Liver Dis. 2013;33(1):30-40. https://doi.org/10.1055/s-0033-1338112. [ Links ]
50. Kamar N, Mansuy JM, Cointault O, et al. Hepatitis E virus-related cirrhosis in kidney- and kidney-pancreas-transplant recipients. Am J Transplant. 2008;8(8):1744-8. https://doi.org/10.1111/j.1600-6143.2008.02286.x. [ Links ]
51. Gérolami R, Moal V, Picard C, et al. Hepatitis E virus as an emerging cause of chronic liver disease in organ transplant recipients. J Hepatol. 2009;50(3):622-4. https://doi.org/10.1016/j.jhep.2008.12.008. [ Links ]
52. Kamar N, Garrouste C, Haagsma EB, et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140(5):1481-9. https://doi.org/10.1053/j.gastro.2011.02.050. [ Links ]
53. Kamar N, Lhomme S, Abravanel F, et al. Treatment of HEV infection in patients with a solid-organ transplant and chronic hepatitis. Viruses. 2016;8(8). https://doi.org/10.3390/v8080222. [ Links ]
54. Wang Y, Zhou X, Debing Y, et al. Calcineurin inhibitors stimulate and mycophenolic acid inhibits replication of hepatitis E virus. Gastroenterology. 2014;146(7):1775-83. https://doi.org/10.1053/j.gastro.2014.02.036. [ Links ]
55. Debing Y, Ramière C, Dallmeier K, et al. Hepatitis E virus mutations associated with ribavirin treatment failure result in altered viral fitness and ribavirin sensitivity. J Hepatol. 2016;65(3):499-508. https://doi.org/10.1016/j.jhep.2016.05.002. [ Links ]
56. Dao Thi VL, Debing Y, Wu X, et al. Sofosbuvir inhibits hepatitis E virus replication in vitro and results in an additive effect when combined with ribavirin. Gastroenterology. 2016;150(1):82-85.e4. https://doi.org/10.1053/j.gastro.2015.09.011. [ Links ]
57. Cheung MC, Maguire J, Carey I, et al. Review of the neurological manifestations of hepatitis E infection. Ann Hepatol. 2012;11(5):618-22. [ Links ]
58. Chalupa P, Holub M. Jaundice complicated by an atypical form of Guillain-Barré syndrome. J Clin Virol. 2010;49(4):229-30. https://doi.org/10.1016/j.jcv.2010.07.017. [ Links ]
59. Jha AK, Nijhawan S, Nepalia S, et al. Association of Bell’s palsy with hepatitis E virus infection: a rare entity. J Clin Exp Hepatol. 2012;2(1):88-90. https://doi.org/10.1016/S0973-6883(12)60082-6. [ Links ]
60. Fong F, Illahi M. Neuralgic amyotrophy associated with hepatitis E virus. Clin Neurol Neurosurg. 2009;111(2):193-5. https://doi.org/10.1016/j.clineuro.2008.09.005. [ Links ]
61. Mandal K, Chopra N. Acute transverse myelitis following hepatitis E virus infection. Indian Pediatr. 2006;43(4):365-6. [ Links ]
62. Kamar N, Bendall RP, Peron JM, et al. Hepatitis E virus and neurologic disorders. Emerg Infect Dis. 2011;17(2):173-9. https://doi.org/10.3201/eid1702.100856. [ Links ]
63. Colson P, Payraudeau E, Leonnet C, et al. Severe thrombocytopenia associated with acute hepatitis E virus infection. J Clin Microbiol. 2008;46(7):2450-2. https://doi.org/10.1128/JCM.02295-07. [ Links ]
64. Shah SA, Lal A, Idrees M, et al. Hepatitis E virus-associated aplastic anaemia: the first case of its kind. J Clin Virol. 2012;54(1):96-7. https://doi.org/10.1016/j.jcv.2012.02.002. [ Links ]
65. Kamar N, Weclawiak H, Guilbeau-Frugier C, et al. Hepatitis E virus and the kidney in solid-organ transplant patients. Transplantation. 2012;93(6):617-23. https://doi.org/10.1097/TP.0b013e318245f14c. [ Links ]
66. Deniel C, Coton T, Brardjanian S, et al. Acute pancreatitis: a rare complication of acute hepatitis E. J Clin Virol. 2011;51(3):202-4. https://doi.org/10.1016/j.jcv.2011.04.009. [ Links ]
Received: July 10, 2017; Accepted: January 22, 2018











 text in
text in