Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.33 no.1 Bogotá Jan./Mar. 2018
https://doi.org/10.22516/25007440.233
Case report
Case Report of Conversion Therapy in Stage IV Gastric Cancer
1Gastrointestinal Surgery and Digestive Endoscopy Group of the National Cancer Institute in Bogotá, Colombia
2Clinical Oncology Group of the National Cancer Institute in Bogotá, Colombia
3Clinical Research Group of the National Cancer Institute in Bogotá, Colombia
We report the case of a 47-year-old patient initially diagnosed with a Krukenberg tumor, an adnexal lesion 10 cm in diameter and a 3 cm lesion in the gastric corpus. A biopsy showed a poorly differentiated adenocarcinoma with signet ring cells. Initial laparoscopy showed an index of peritoneal carcinomatosis of 24 which indicated chemotherapy with palliative intent (cisplatin and capecitabine). The patient improved significantly and underwent a total hysterectomy with salpingo-oophorectomy. Chemotherapy continued with excellent clinical response as evidenced in CT scans. Together with the patient’s family, it was decided that she should undergo surgery. A total gastrectomy with D2 lymphadenectomy with curative intent was performed. The patient continued to receive capecitabine and cisplatin for three more months until para-aortic lymph node involvement was demonstrated and it became necessary to restart chemotherapy with a new scheme using iriniotecan. The patient has completed 22 months after the initial diagnosis in very good and clinical condition without symptoms.
Keywords: Stage IV gastric cancer; conversion surgery
Se reporta el caso de una paciente de 47 años, con diagnóstico inicial de un tumor de Krukenberg por una lesión anexial de 10 cm de diámetro y una lesión corporal gástrica de 3 cm. La biopsia mostró un adenocarcinoma pobremente diferenciado con células en anillo de sello. Una laparoscopia inicial mostró un índice de carcinomatosis peritoneal (ICP) de 24, por lo cual se le indica a la paciente quimioterapia con intención paliativa (cisplatino y capecitabina). Con mejoría clínica importante, respuesta adecuada y favorable a la quimioterapia, la paciente se remitió a una salpingo-ooforectomía bilateral. Continúa con quimioterapia evidenciándose mejoría de las imágenes tomográficas y una excelente respuesta clínica. Por este motivo, se decide en conjunto con la familia llevarla a cirugía. Se le practica una gastrectomía total con linfadenectomía D2 con intención curativa. La paciente recibe quimioterapia con capecitabina y cisplatino por 3 meses más, hasta cuando se evidencia compromiso ganglionar paraaórtico, por lo que es necesario reiniciar la quimioterapia con un nuevo esquema, entonces se le formula irinotecán. La paciente completa 22 meses desde el diagnóstico inicial, la condición clínica es muy buena y está asintomática.
Palabras clave: Cáncer gástrico; estadio IV; cirugía de conversión
Introduction
Gastric cancer (GC) remains a prevalent disease throughout the world and has a very poor prognosis. In 2012, approximately one million new cases of GC were diagnosed, and nearly 700,000 patients died from this disease. GC is the fourth most common cancer and the third cause of cancer mortality in the world. 1 In Colombia, it is the leading cause of death in men and the third in women. 2
GC is usually diagnosed in advanced stages, and less than 30% of those diagnosed survive more than 5 years. Patients with non-resectable GC are generally candidates for systemic chemotherapy before any surgical management except for those patients with palliative indications for surgery such as obstructions or bleeding. 3. However, there are alternatives to surgical palliation including radiological embolization for bleeding and endoscopic stenting of obstructions.
Several combinations of new chemotherapy regimens have made conversion of unresectable GC into resectable GC possible. This additional surgery may allow longer survival for selected patients. 4
Conversion surgery is radical resection performed for unresectable lesions, stage IV cancer and metastatic cancers that, after the administration of chemotherapy with adequate response and regression, become technically and oncologically resectable. 5
The purpose of this study is to present the clinical case of a patient with stage IV GC with several non-curative factors who underwent surgery twice after receiving a combined chemotherapy regimen. The patient’s clinical evolution, CT scans, laparoscopic and surgical findings, and final pathology results have been reviewed to evaluate the prognostic value of conversion surgery. The last surgery was classified as R0 (complete resection without residual tumor). Clinical monitoring used Computed tomography and tumor markers (carcinoembryonic antigen [ACE], carbohydrate antigen [CA] 19-9) at least every 4 or 5 weeks.
Clinical case
The patient was a 47-year-old woman who had suffered abdominal pain in the right iliac fossa for four months before being examined in June 2015. An abdominal ultrasound found a 10 cm in diameter right adnexal mass. Upper digestive endoscopy showed an ulcerated lesion with raised borders 3 cm in diameter located in the posterior wall of the gastric corpus. Pathology showed a poorly differentiated adenocarcinoma with signet ring cells and non-atrophic chronic gastritis without intestinal metaplasia or dysplasia. She tested negative for Helicobacter pylori, and a total colonoscopy was normal. Blood tests showed CA-125 at 1,396 U/mL, ACE at 24.9 ng/mL, and a creatinine level of 0.7 mg/dL.
Computerized axial tomography (CT) with contrast of the abdomen on April 24, 2015 showed a slight increase in the number of retroperitoneal nodes. The uterus was enlarged with the presence of myomas and a well-defined solid heterogeneous right adnexal mass with smooth contours that measured 110 x 79 x 71 mm. There was a moderate amount of free liquid in the cavity, the gastric wall of the corpus was thickened, and the peritoneum was nodular (Figure 1).
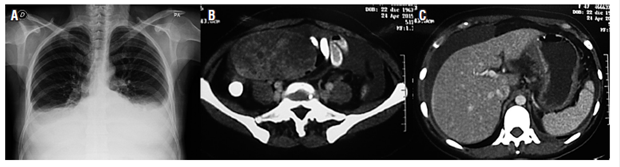
Figure 1 A. Chest x-ray showing plural effusion. B. CT scan showing right adnexal mass. C. CT scan showing peritoneal fluid and thickening of stomach walls.
The patient was assessed by the gastroenterology and gynecology departments and diagnosed with a Krukenberg tumor. A laparoscopy performed on June 12, 2015 found 400 mL of ascitic fluid, multiple peritoneal implants in the pelvic peritoneum, diaphragmatic domes, omentum, a right ovarian mass of 10 cm in diameter and a gastric tumor in region of the corpus. The peritoneal carcinomatosis index (PCI) was 24. The cytology of ascitic fluid was negative for malignancy.
The oncology department evaluated these results and chemotherapy was started with 1.5 g of capecitabine administered orally every 12 hours from July 7, 2015 for 14 days plus a single 125 mg dose of cisplatin administered intravenously. She completed 8 cycles. Significant clinical relief was detected at periodic follow-up appointments. The patient recovered her functional and nutritional status and numbers for biochemical markers such as ACE, CA 19-9 and CA-125 improved. Ascites disappeared and the ovarian mass stopped growing. Another laparoscopy on November 12, 2015 found an ICP of 8, with no peritoneal implants in diaphragmatic domes, a mobile stomach without adhesions and metastatic compromise of the right ovary. There was no free fluid in the abdominal cavity (Figure 2).
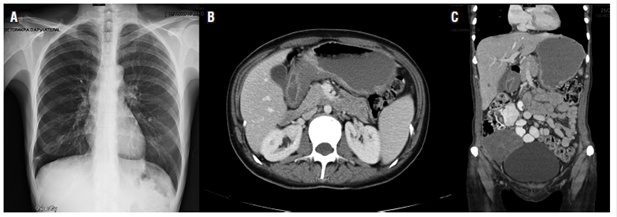
Figure 2 A. Normal chest radiograph. B. Tomography with thickening of the stomach-body walls. C. Right adnexal mass.
The case and a proposal for Sugarbaker type surgery was presented to the surgical board. The proposal was based on the patient’s response to systemic chemotherapy. The board concluded that there would be no benefits from Sugarbaker-type cytoreductive surgery due to the histological type and the ICP. Instead, bilateral salpingo-oophorectomy was performed on January 13, 2016. Solid tumors were found in both ovaries: on the right a 15 cm tumor, and on the left a 10 cm tumor. There were no adhesions or implants in the peritoneum. The diaphragmatic domes were free, there was thickening of the gastric walls, and there was 500 mL of ascitic fluid. Pathology showed poorly differentiated adenocarcinoma with ring-cell components in the right ovary and fallopian tube plus lymphovascular invasion. Immunohistochemical tests for keratin 19, keratin 7 and keratin 20 were positive, but tests for human epidermal growth factor receptor 2 (HER2), CA-125, Wilms’ tumor 1 (WT1), CDX2 and TF1 were all negative. The left ovary had metastatic involvement due to adenocarcinoma and lymphovascular invasion.
Chemotherapy with capecitabine and cisplatin continued, and May 16 after a decision was made together with the family, a total gastrectomy with D2 lymphadenectomy was performed. Pathology for the product of total gastrectomy showed diffuse type gastric adenocarcinoma with signet ring cells; a 6 x 5 cm lesion; tumor invasion into subserosal fat; lymphovascular and neural invasion; tumor-free proximal and distal edges; 5 of 17 lymph nodes were compromised by tumors staged at pT3pN2; the omentum, left diaphragm, hepatic artery lymph node, and esophageal were tumor free and tested negative for HER2 (Figure 3).
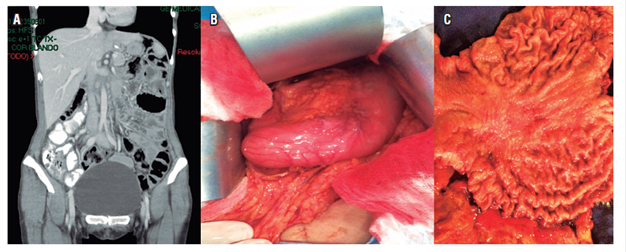
Figure 3 A. Normal abdominal tomography. B. Thickening of the walls near the gastric corpus. C. Surgical piece with corporal gastric lesion.
The patient was referred to the oncology department ad underwent three more cycles of chemotherapy with cisplatin and capecitabine. At her final check-up in November 2016, she was asymptomatic but with high levels of tumor markers and a Karnofsky index of 100%. A CT scan showed a compromised para-aortic lymph node. Chemotherapy was reinitiated, but this time with 250 mg/day of intravenously administered irinotecan for fifteen days.
Discussion
Several guidelines have established standards for GC treatment. For stage IV, the principal management strategy is palliative chemotherapy. The average survival time of these patients is only 13 to 16 months. 6
Stage IV GC represents more than 30% of cases seen at the time of diagnosis. 7 In addition, its clinical presentation is very complex. Its spectrum includes metastatic liver lesions of different sizes and numbers, metastases in various areas of the peritoneum, ovarian metastases which are usually bilateral and solid (Krukenberg tumor), compromised lymph nodes at a distance and positive ascitic fluid. 8 Sometimes the term “unresectable Gastric Cancer” is used with patients, but the clinical implications of this term vary greatly and do not represent the true extent of the disease. 5
The patient reported on in this case study had stage IV GC with a tumor in the corpus with bilateral metastatic involvement in the ovaries and peritoneum plus the presence of ascitic fluid.
At this point it is important to differentiate between GC that is not resectable because it is locally advanced; and stage IV GC which implies the presence of distant metastasis, as in the case reported. Locally advanced GC implies that the tumor is attached or joined to other organs or vascular structures. Stage IV GC due to interaortocaval adenopathies can be used to illustrate this difference by comparing them to M1 tumors. Interaortocaval adenopathies compromise non-regional while M1 is cancer that had disseminated to other parts of the body. Technically, a surgeon can resect these nodes in addition to the primary tumor, but a stage IV stage would be treated with initial surgery. In other words, there is oncological lack of resectability, although this is not technical.
The guidelines of the National Comprehensive Cancer Network (NCCN) for stage IV GC establish palliative management and, depending on the patient’s functional condition, palliative systemic treatment with chemotherapy or the best support care with symptomatic intention. Patients with a Karnofsky index of over 60% or an ECOG (Eastern Cooperative Oncology Group) score of less than two are candidates for management with palliative chemotherapy or can be included in controlled clinical studies. 9
When the guidelines of the European Society for Medical Oncology (ESMO) refer to unresectable or metastatic GC, they indicate palliative chemotherapy or the best supportive care if the patient’s functional condition is not adequate, but they also establish an alternative which indicates surgery for those patients who have received palliative chemotherapy. Within this guide, it mentions, “However, a small number of patients with initial locally advanced or unresectable disease can be taken to surgery after a good response to systemic therapy.” 10
In the Japanese guidelines, the main management strategy for stage IV GC is palliative chemotherapy. 6 For metastatic and recurrent GC, new chemotherapy regimens have been developed which aim to improve survival. Currently, the combination of 5-fluorouracil (5-FU) or capecitabine and a platinum analogue (cisplatin or oxaliplatin) are the most widely used first-line regimens for patients with non-resectable or recurrent GC outside Japan. 11 Docetaxel is recommended only after failure of first-line chemotherapy. 11
In patients with metastatic colorectal cancer, the use of new chemotherapy regimens and target therapy have increased average survival time from 6 to 30 months. Surgical management of these lesions subsequent to chemotherapy has played a crucial role in prolonging survival. 6 In the case of patients with metastatic or unresectable GC, a similar management approach can be used, but lit has not yet achieved any big changes in average survival time. 12
The introduction of new agents and the development of multiple regimens have allowed the conversion of a non-resectable stage IV GC into resectable GC. This type of surgery, known as a conversion gastrectomy with curative intent, differs from a palliative gastrectomy. 4 The REGATTA study showed that palliative gastrectomy is not indicated unless there is bleeding or obstruction due to the primary lesion. 6,8 In contrast, conversion surgery is indicated in patients with non-resectable GC who have responded well enough to chemotherapy for stage IV that they can undergo cancer surgery with curative intent. This illustrates the differences between neoadjuvant chemotherapy and conversion therapy. 3
Neoadjuvant chemotherapy has been evaluated in patients with locally advanced GC which has not metastasized. This type of treatment aims to downstage the primary lesion to achieve greater R0 resections and simultaneously treat micrometastases in their earliest forms. 3,13 This is prior to, and different from, conversion therapy.
Conversion therapy for stage IV GC is a different therapeutic concept in which the treatment strategy starts with a new chemotherapy scheme that allows conversion of tumors that are not originally resectable or are marginally resectable into conditions that are potentially curable by surgery. 6 This clinical situation is represented in the clinical case reported on in this study in which a stage IV cancer responded well to chemotherapy allowing the patient to undergo surgery with curative intent.
Among the various options for managing stage IV GC, systemic chemotherapy, a peritonectomy, intraperitoneal chemotherapy and hyperthermic intraperitoneal chemotherapy are mentioned. All of these can potentially improve the prognosis of these patients. 14
Stage IV GC is a combination of technically resectable or unresectable metastases, hematologic metastases, peritoneal dissemination, and extra-regional lymph node metastasis. As there are different considerations with the spectrum of this stage, a new classification that would divide stage IV into several categories has been proposed. The objective of this proposal is to classify the biological tumor while allowing a clinical and surgical oncological approach. 6,8 This new classification includes:
Category 1: There is no peritoneal spread and metastases are potentially resectable. Patients are candidates for neoadjuvant chemotherapy. Resection of the primary tumor and metastases can now achieve conversion of a tumor that was not originally resectable. 6,8
Category 2: There is no peritoneal spread and metastases are marginally resectable. Treatment with intensive chemotherapy, if effective, can be followed with resection of the primary tumor and metastasis to achieve conversion of a tumor that was not originally resectable. 6,8
Category 3: Incurable or unresectable peritoneal dissemination which can be treated with systemic chemotherapy, intraperitoneal chemotherapy and targeted therapy followed by cytoreductive surgery. 6,8
Category 4: GC with peritoneal spread and unresectable metastases treated with chemotherapy, intraperitoneal chemotherapy and targeted therapy. If this is not effective, palliative chemotherapy should be used (Figure 4). 6,8
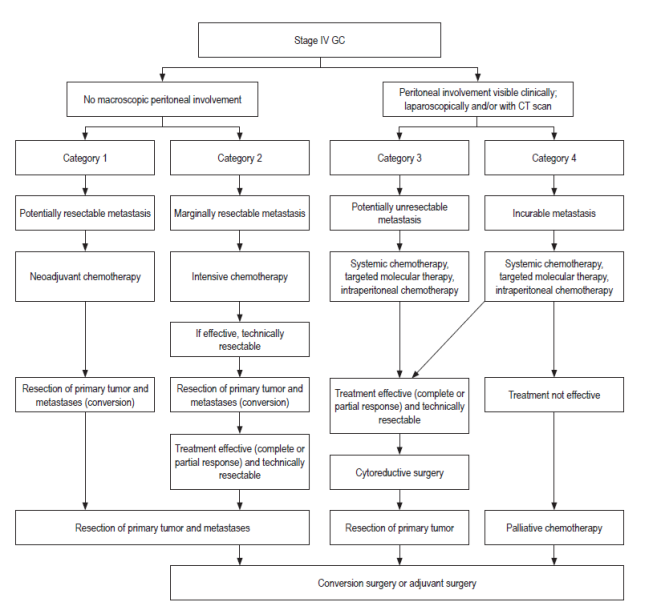
Figure 4 Treatment for new categories of GC Stage IV. 6 Modified from Yoshida K et al. Gastric Cancer. 2016; 19 2: 329-38.
This concept of conversion therapy or adjuvant surgery is aimed at patients with category 2, some patients with category 3 and very few with category 4. 6,8.
The clinical case reported is a patient who had stage IV GC category 4. Her response to chemotherapy and gastrectomy with curative intent was very good. She has been evaluated twice with laparoscopy to determine the extent of the disease and response to treatment.
When chemotherapy has produced an adequate response and curative surgery has been carried out, conversion surgery is associated with greater survival in selected patients. 4
There are few reports of conversion surgery in GC cases. At the INC in Tokyo, Nakajima was the first to report cases of conversion surgery. In 1977 he reported 30 cases with unresectable GC who received two cycles of chemotherapy with 5-FU, leucovorin, cisplatin and etoposide. Response to therapy was obtained in 50% of the patients, and 19 patients underwent surgery. Curative surgery was achieved in nine patients. 5
Patients treated with chemotherapy plus surgery had significantly longer survival times and significantly more of these patients were progression-free at 5 years than did patients treated with chemotherapy alone. Yoshida used the term adjuvant surgery for surgery performed after adequate response to chemotherapy 6.
Einama et al. reported ten stage IV GC patients who underwent radical surgery after receiving chemotherapy between 2009 and 2015. The average time between the start of chemotherapy and the time of surgery was 210 days. All patients survived more than one year, and five were without recurrence at the time of publication. The average survival time was 871 days after diagnosis. For this reason, curative resection after chemotherapy that is effective is associated with better prognoses. Similarly, the study showed that patients undergoing conversion surgery had better survival times than those who only received chemotherapy 15.
In conclusion, conversion therapy involves a series of questions that need answers:
What is the indication? Indications for conversion therapy include patients in category 2, some patients in category 3 and very few patients in category 4. 8
What is the best chemotherapy regimen? Chemotherapy regimens include the combination of S1, 5-FU or capecitabine with a platinum (cisplatin, oxaliplatin or carboplatin) or docetaxel. 12
What is the best time for surgery? The best time for surgery is when the tumor achieves its greatest response to chemotherapy, almost in a manner similar to the way the response is evaluated in patients receiving therapy for gastrointestinal stromal tumors (GIST). Generally, the complete or partial response is achieved after 4 to 6 cycles of combination chemotherapy. Some authors have reported benefits with only two cycles. 11
Is it necessary to use chemotherapy after surgery? Chemotherapy should be continued after surgery until the tumor acquires resistance or adverse effects of treatment develop. 6
The clinical value of multimodal therapy including chemotherapy and conversion surgery for stage IV GC remains controversial because these treatments involve patients with lesions that are initially unresectable due to the advanced state of disease and systemic spread. 4 This includes variable biological behavior and a spectrum of clinical conditions. However, the biological behavior of the tumor to chemotherapy can define a group of patients who have excellent responses that allow conversion with intention of palliation treatment to cure
Referencias
1. den Hoed CM, Kuipers EJ. Gastric Cancer: How Can We Reduce the Incidence of this Disease? Curr Gastroenterol Rep. 2016;18(7):34. https://doi.org/10.1007/s11894-016-0506-0. [ Links ]
2. International Agency for Research of Cancer, World Health Organization. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. IARC [internet] 2012. Disponible en: http://globocan.iarc.fr/default.aspx. [ Links ]
3. Fukuchi M, Ishiguro T, Ogata K, et al. Prognostic role of conversion surgery for unresectable gastric cancer. Ann Surg Oncol. 2015;22(11):3618-24. https://doi.org/10.1245/s10434-015-4422-6. [ Links ]
4. Kinoshita J, Fushida S, Tsukada T, et al. Efficacy of conversion gastrectomy following docetaxel, cisplatin, and S-1 therapy in potentially resectable stage IV gastric cancer. Eur J Surg Oncol. 2015;41(10):1354-60. https://doi.org/10.1016/j.ejso.2015.04.021. [ Links ]
5. Terashima M. Conversion therapy for gastric cancer: who can make conversion as successful as Goromaru? Gastric Cancer. 2016;19(3):685-6. https://doi.org/10.1007/s10120-016-0609-1. [ Links ]
6. Yoshida K, Yamaguchi K, Okumura N, et al. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer. 2016;19(2):329-38. https://doi.org/10.1007/s10120-015-0575-z. [ Links ]
7. Oliveros R, Navarrera LF. Diagnóstico, estadificación y tratamiento del cáncer gástrico en Colombia desde 2004 a 2008 (REGATE-Colombia). Rev Col Gastroenterol. 2012; 27(4):269-74. [ Links ]
8. Yamaguchi K, Yoshida K, Tanaka Y, et al. Conversion therapy for stage IV gastric cancer-the present and future. Transl Gastroenterol Hepatol. 2016;1:50. https://doi.org/10.21037/tgh.2016.05.12. [ Links ]
9. Ajani JA, D’Amico TA, Almhanna K, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14(10):1286-312. https://doi.org/10.6004/jnccn.2016.0137. [ Links ]
10. Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v38-v49. https://doi.org/10.1093/annonc/mdw350. [ Links ]
11. Takashima A, Yamada Y, Nakajima TE, et al. Standard first-line chemotherapy for metastatic gastric cancer in Japan has met the global standard: evidence from recent phase III trials. Gastrointest Cancer Res. 2009;3(6):239-44. [ Links ]
12. Wang S, Yuan L. Predictive biomarkers for targeted and cytotoxic agents in gastric cancer for personalized medicine. Biosci Trends. 2016;10(3):171-80. https://doi.org/10.5582/bst.2016.01078. [ Links ]
13. Sakamoto J. Neoadjuvant chemotherapy: a standard treatment for locally advanced gastric cancer in the near future? Gastric Cancer. 2003;6(3):131-3. https://doi.org/10.1007/s10120-003-0254-3. [ Links ]
14. Kim SW. The result of conversion surgery in gastric cancer patients with peritoneal seeding. J Gastric Cancer. 2014;14(4):266-70. https://doi.org/10.5230/jgc.2014.14.4.266. [ Links ]
15. Einama T, Abe H, Shichi S, et al. Long-term survival and prognosis associated with conversion surgery in patients with metastatic gastric cancer. Mol Clin Oncol. 2017;6(2):163-166. https://doi.org/10.3892/mco.2017.1128. [ Links ]
Received: May 08, 2017; Accepted: January 22, 2018











 text in
text in 


