Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.33 no.2 Bogotá abr./jun. 2018
https://doi.org/10.22516/25007440.190
Original articles
Levels of intraoperative glycemia and their relations to short and long term morbidity and mortality in liver transplant patients at a highly complex hospital
1Médica de planta, Hospital San Vicente Fundación Rionegro. Rionegro, Colombia.
2Medicina interna y endocrinología, Hospital San Vicente Fundación Rionegro. Rionegro, Colombia.
3Dirección de investigaciones, Hospital Universitario San Vicente Fundación Rionegro. Rionegro, Colombia.
4Cirugía de trasplantes de órganos abdominales, Hospital San Vicente Fundación Rionegro. Rionegro, Colombia.
5Medicina interna y hepatología, Hospital San Vicente Fundación Rionegro. Rionegro, Colombia.
6Cirugía de trasplantes de órganos abdominales, Hospital San Vicente Fundación Rionegro. Grupo de investigación en trasplantes de órganos (INTRO). Rionegro, Colombia.
Objective:
This study was of patients who underwent liver transplantation has the objective of determining glycemia values in each phase of liver transplant surgery and their relationships with post-operative morbidity and mortality.
Materials and Methods:
Liver transplant patients were identified in institutional records from 2013 to 2015. The information was taken from operative notes, laboratory records and clinical histories. We searched for differences in blood glucose levels during the three phases of transplantation and compared the incidences of infections and rejections for diabetics and non-diabetics.
Results:
A total of 73 transplant patients were studied: 54.8% (n = 40) were male, the median age was 59 years (RIQ = 52-53), and 32.9% (n = 24) had histories of Diabetes Mellitus. Differences were found between initial and final serum glucose levels of diabetics (127 mg/dl vs. 212 mg/dl, p = 0.001) as well as in non-diabetics (105 mg/dl vs. 190 mg/dl, p < 0.000). The proportion of rejection was highest among diabetics (14.3%, n = 7). No significant differences were found in the proportions of diabetic and non-diabetic patients who developed infections. Diagnosis of post-transplant diabetes was confirmed in 15.1% of the sample.
Conclusions:
Adequate monitoring of blood glucose levels during all trans-operative periods of liver transplantation can equalize the rate of infectious complications in diabetic and non-diabetic patients. Rejection continues to be more frequent among diabetic patients. An active search for post-transplant diabetes is necessary for every patient.
Keywords: Transplant; liver; glycemia; diabetes mellitus; complications of diabetes; graft rejection
Objetivo:
se realizó un estudio en pacientes sometidos a trasplante de hígado (TH) con el objetivo de determinar los valores de glucemia en cada una de las fases de la cirugía del TH y su relación con la morbimortalidad postoperatoria.
Materiales y métodos:
se identificaron los trasplantes hepáticos entre 2013 y 2015 en los registros institucionales. La información se tomó de la nota operatoria, registros de laboratorio y evoluciones de historia clínica. Se buscaron diferencias en la glucemia en las 3 fases del trasplante entre diabéticos y no diabéticos, la presencia de infección y rechazo.
Resultados:
en total, se estudiaron 73 pacientes trasplantados, 54,8% (n = 40) de sexo masculino, con una mediana en la edad de 59 años (rango intercuartílico [RIQ] = 52-53). El 32,9% (n = 24) tenía antecedente de diabetes mellitus (DM). Se encontraron diferencias en la glucemia inicial y final (127 mg/dL frente a 212 mg/dL) en diabéticos (p = 0,001), así como en los no diabéticos (glucemia inicial: 105 mg/dL frente a la final: 190 mg/dL) (p <0,000). La proporción de rechazo fue mayor en diabéticos (14,3%, n = 7). No se encontraron diferencias significativas en la presencia de infecciones entre diabéticos y no diabéticos. Se confirmó el diagnóstico de diabetes postrasplante en el 15,1%.
Conclusiones:
un adecuado control glucémico en los diferentes períodos del transoperatorio en el TH logra igualar la tasa de complicaciones a nivel infeccioso en pacientes diabéticos y no diabéticos; el rechazo continúa siendo más frecuente en pacientes diabéticos. Es necesaria una búsqueda activa de la diabetes postrasplante en cada uno de nuestros pacientes.
Palabras clave: Trasplante; hígado; glucemia; diabetes mellitus; complicaciones de la diabetes; rechazo de injerto
Introduction
Liver transplantation (LT) is the current treatment of choice for patients with chronic hepatic insufficiency, acute liver failure with indicators of poor prognosis, selected primary liver tumors and for some patients with inborn errors of metabolism. 1,2.
Patients with chronic hepatic dysfunction have altered blood glucose regulation secondary to hyperinsulinemia and insulin resistance, mechanisms which are responsible for hepatogenic diabetes. 3 Patients with acutely decompensated liver failure frequently develop hypoglycemia and hyperglucagonemia. 4,5
In the 2012 (Organ Procurement and Transplantation Network/Scientific Registry of Transplant Recipients [OPTN/SRTR]) report, about 25% of liver transplant patients had preexisting diabetes mellitus (DM), 6 and 80% of the patients on the liver transplant waiting list were suffering from insulin resistance and impaired glucose metabolism. 7
Hyperglycemia is associated with increased morbidity and mortality, higher rates of infection, renal dysfunction, graft dysfunction, ischemic-reperfusion liver damage and vascular complications in transplant patients. In spite of this, there is still no conclusive evidence for establishing a glucose level control guide. 8
Current recommendations regarding optimal blood glucose level propose a maximum glucose level of 180 mg/dL and acceptance of glucose levels up to 150 mg/dL. 9,10 The American Association of Clinical Endocrinologists and the American Diabetes Association (ADA) recommend initiating insulin infusion in critically ill patients with the goal of maintaining blood glucose levels below 180 mg/dL. 11
In addition, an incidence of post liver transplant diabetes from 7% to 30% has been documented. This has been associated with various risk factors. They include pretransplant body mass index (BMI) over 25 kg/m2; ages over 40 years; African-American and Hispanic ethnicity; genetic polymorphisms in IRS-1, HNF-4, TCF7L2, KCNJ11-Kir6.2, inflammatory inducers such as hepatitis B (HBV); incompatibility of the major histocompatibility complex (HLA); episodes of rejection; cytomegalovirus infections; and the use of immunosuppressants especially inhibitors of calcineurin, glucocorticoids and mTOR inhibitors. 12
Once the patient has been discharged and has prescriptions for immunosuppressors at maintenance doses, diagnosis of post-transplant diabetes can be established. According to previous publications, any of the criteria of the ADA or the World Health Organization (WHO) for diagnosis of DM can be used (Table 1). 13 Nevertheless, glycosylated hemoglobin (HbA1c) should not be used as the sole criterion in the first year after transplantation given its lack of diagnostic accuracy in this period.
Table 1 Diagnostic criteria for diabetes in post-transplant patients

Taken from: Shivaswamy V. et al. Endocrine Reviews. 2016; 37 (1): 37-61
The main objective of this investigation was to determine glucose levels in each phase of LT surgery and then determine their relationships with pretransplant diabetes, the appearance of infections, transplant rejection and postsurgical mortality.
Materials and methods
Study Design
This is a retrospective cohort study of 73 adult patients who underwent LT using cadaveric donors in the Hospital San Vicente Fundación Rionegro between October 2013 and December 2015. Patients who died during induction of anesthetic were excluded (n = 3). The research protocol was approved by the Investigation Unit and the Research Ethics Committee of the Hospital San Vicente Fundación.
LT Protocol
The Hospital San Vicente Fundación is a national and regional referral center for transplantation. On average, 26 LTs from cadaveric donors are performed every year.
LTs were performed with complete donor livers using a piggyback technique without venovenous bypass. An end-to-end portoportal anastomosis was created, then after organ reperfusion performed an arterial anastomosis was created. Histidine-tryptophan-ketoglutarate (HTK) was normally used as the preservation solution, but in isolated cases the solution from the University of Wisconsin was used.
Central blood glucose was monitored in all patients before they entered operating rooms, anesthetic management was performed according to hospital protocols, intraoperative transfusions were performed based on hematocrit levels, and vasoactive agents were used according to the hemodynamic parameters of the patients.
Intraoperatively, capillary glucose levels were measured with glucose meter strips during the hepatectomy, the anhepatic phase and the post-reperfusion phase of the organ. With the data obtained, a decision was made to either administer 10% dextrose in distilled water or administer regular insulin with a goal of maintaining intraoperative glucose level below 80 mg/dL. During the anhepatic phase, an infusion of 500 mg of methylprednisolone was administered for immunosuppression as per protocol.
Study Variables and Sources of Information
Data were obtained from the SAP electronic history system which stores information from hospital patients’ medical records. Information was extracted from clinical histories by researchers using a pre-designed standardized form.
Demographic variables of age, gender and year of transplantation were recorded. Clinical variables recorded included any background of pretransplant diabetes, glucose levels during the initial phase, during the anhepatic phase and at the end of the transplant surgery, model for end-stage liver disease (MELD) classifications and Child-Pugh classifications. The pretransplant MELD scale was calculated with the following formula: R = 9.57 X loge [creatinine in mg/dL] + 3.78 x loge [bilirubin in mg/dL] + 11.20 x loge [international normalized ratio -INR -] + 6.43, as recommended by Kamath et al. 14
Additional data regarding infections in the postoperative period, organs affected and microorganisms identified by culturing were taken from the medical records. We evaluated whether criteria for diagnosis of post-transplant diabetes were met, whether the graft was rejected, and overall mortality from any cause.
Statistical Analysis
Data were processed using SPSS (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0, Armonk, New York: IBM Corp.). Categorical variables for univariate descriptive analysis were expressed in absolute and relative frequencies. Quantitative variables were evaluated for normal distributions, and for normally distributed variables measures of central tendency and dispersion such as the arithmetic mean and standard deviation were used. Variables that were not normally distributed variables were described by means of medians and interquartile ranges (IQR).
We looked for differences between patients who had diabetes before transplantation and those who did not have diabetes before transplantation in early infection, transplant rejection and mortality in the first 30 days after the procedure. Pearson’s chi-squared test (χ2) of was used for comparisons of qualitative variables, and the Mann-Whitney U test of independent samples was used for quantitative variables. In cases of repeated measures, the Friedman test was used. Differences were considered to be significant when p was less than 0.05.
Results
A total of 73 patients who had undergone LT between October 2013 and December 2015 were studied. Twelve transplants (16.4%) were performed in 2013, 31 (42.5%) in 2014 and 30 (42, 1%) in 2015. Male patients accounted for 54.8% (n = 40) of the study population, the median age was 59 years (IQR: 52-63.5. The youngest patient was 33 years old, while the oldest was 71 years old. LTs accounted for 90.4% of all procedures while the rest were combined liver and kidney transplantations.
The median MELD score was 22 (IQR: 16-24), and the largest group of patients was Child-Pugh category C (41.1%, n = 30). This was followed by category B which accounted for 28.8% (n = 21).
Of the total number of patients studied, 24 (32.9%) had histories of DM prior to LT. No differences between diabetic and non-diabetic patients before transplantation were found in MELD scores (p = 0.104, Mann-Whitney U from independent samples test or in Child-Pugh scores (Table 2).
Table 2 Demographic characteristics and severity of liver damage
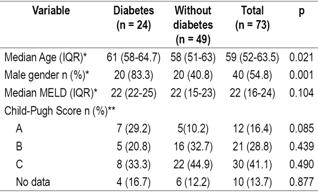
* Mann-Whitney U test of independent samples. ** Pearson χ2 test, Significance: p <0.05.
Glucose levels were evaluated in the initial phase of the transplant, the anhepatic phase, and at the end of the transplant surgery. Significantly increased blood glucose levels were found in all patients including those with diabetes prior to transplantation and those without diabetes prior to transplantation (p < 0.000, Friedman test). The glucose level of diabetic patients was 127 mg/dL (IQR: 111-167) in the initial phase and 212 mg/dL in the final (IQR: 169-264) (p = 0.001, Friedman test) whereas for non-diabetic patients the initial blood glucose level was 105 mg/dL (IQR: 88-122), and the final glucose level was 190 mg/dL (IQR: 149-236) (p <0.000, Friedman test) (Table 3).
Table 3 Pretransplant diabetes and blood glucose levels before, during and after transplantation
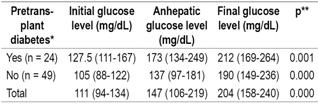
* Median (IQR). ** Friedman test, significance: p <0.05.
Glucose level variations during the three transplantation phases were greater in non-diabetic patients than in non-diabetic patients, but comparisons of final phase glucose levels to initial phase levels showed no statistically significant differences was found (p = 0.690).
The comparison of stratified analyses of glucose levels in the three LT phases of LT for diabetic patients with those of non-diabetics showed that in the initial phase the highest proportion of patients presented glucose levels between 71 and 140 mg/dL (82.2%) while in the final phase the glucose levels of the largest proportion were over 180 mg/dL (61.6%). No statistically significant differences were found between the groups (Table 4).
Table 4 Relation of pretransplant diabetes to stratification of blood glucose levels according to transplantation stage
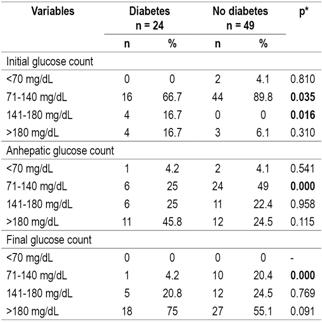
* Pearson’s χ2, significance: p <0.05.
Evaluation of infections after transplantation found that diabetic patients suffered infections more frequently (50%) than did non-diabetics (44.9%). Evaluation of the relation between post-transplant infections and blood glucose ranges in the diabetic and non-diabetic patient groups shows no significant differences between the two groups in the initial phase (Table 5). Similarly, there were no significant differencesin the other two phases between the two groups. in any of the three phases nor in relation to the blood glucose levels in each of the phases of the transplant, both in diabetic and non-diabetic patients (Table 6).
Table 5 Relation of infections to diabetes and glucose level levels in the initial phase of transplantation
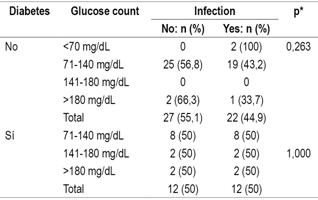
* Pearson’s χ2, significance: p <0.05.
Table 6 Relationship of blood glucose levels and infections in LT patients
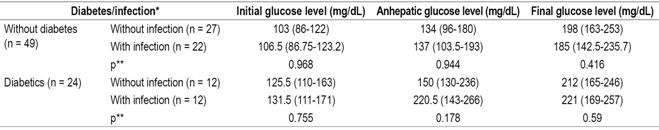
* Median (IQR). ** Mann-Whitney U test, significance: p <0.05.
Most types of infections were more frequent among diabetic patients, but urinary tract infections were strikingly more frequent in non-diabetic patients (16.3%) than in diabetics (8.3%). (Table 7).
Table 7 Relation of postsurgical infections and pretransplant diabetes
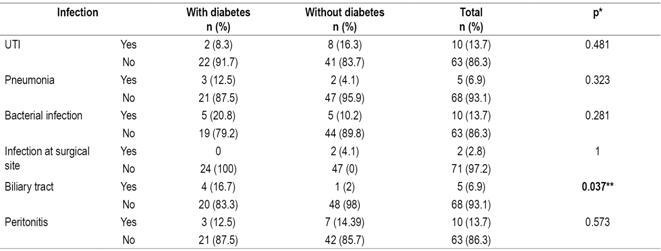
* Fisher’s statistic. ** Significance: p <0.05. UTI: urinary tract infection.
The overall percentage of patients who rejected grafts was 11%, but it was 14.3% in diabetic patients and only 4.2% in non-diabetic patients (p = 0.258, Fisher’s statistic). Overall mortality was 34.2%, but it was in 45.8% in diabetic patients, but only 28.6% in non-diabetic patients (p = 0.191, Fisher’s statistic).
During outpatient follow-up of patients, 11 (15.1%) met the criteria for post-transplant diabetes. However, it is important for the analysis that no record of any follow-up of glucose levels was found in 16.4% of the medical records reviewed.
Discussion
Insulin resistance and hyperglycemia are strongly associated with increased perioperative morbidity and mortality in major abdominal surgery including LT. 15,16,17. However, there are factors that theoretically suggest greater perioperative metabolic impacts in patients with liver disease who have hyperinsulinemia and hyperglucagonemia, 18 in patients whose own liver or whose donor liver has suffered metabolic changes, and in patients who have had infusions of high doses of glucocorticoids together with surgical stress. 19
Findings from analysis of MELD and Child-Pugh scores of our patients were similar to those reported in other studies .20 In our institution, transplant patients were most seriously ill according to the Child-Pugh scale. Previous studies have reported a relationship between severity as indicated by these scores and blood glucose levels prior to surgery. 21
Hepatogenic diabetes occurs in 30% to 60% of patients with cirrhosis. 3 In our study, patients with diabetes before transplantation had higher glucose levels than did non-diabetics. Similarly, blood glucose levels increased during the surgical procedure which is expected due to surgical trauma and glucocorticoid boluses. It should be noted that in the postoperative period glucose levels decreased progressively until appropriate controlled levels were reached in both diabetics and non-diabetics. This finding is explained by the intraoperative and postoperative treatment by the anesthesiologist.
Previous studies have shown that high blood glucose levels are associated with a higher incidence of postoperative complications including surgical site infection, delayed healing and increased mortality.22,23 In our analysis, no association was found between high glucose levels during transplantation and the development of infections which is similar other authors’ findings. 24 These results may be due to the small sample size.
Before transplantation, patients with diabetes had higher glucose levels, but during the procedure and at the end of the procedure similar glucose levels were achieved. This could explain why the complication rates found at the end of the study were not statistically different for the two groups.
Hepatogenic diabetes stimulate a number intracellular signaling pathways including the Janus kinase/signal transducer and activator of transcription (JAK/STAT), nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase), mitogen activated protein kinase (MAPK), extracellular signal regulated kinase [ERK] and c-Jun N-terminal kinase [JNK]. Together with the activation of transcription factors they have proinflammatory effects that allow increased expression of cytokines and growth factors and enhancement of the immune response to foreign bodies. 25 Our analysis found that this response was more frequent in non-diabetic patients before transplantation.
In addition, no relationship was found between the glucose levels in the three phases and graft rejections, and no modifications of the immunosuppressive management (recommended by several authors) was necessary to avoid rejection. 26
One very interesting finding was that patients who had diabetes prior to transplantation had a higher mortality rate due to cardiovascular events than did patients without histories of diabetes. This has also been demonstrated in multiple previous studies which considered diabetes to be a risk factor for microvascular and macrovascular compromise which can be tested in a state of metabolic stress as high as that which occurs during LT. (27
Although several problems were found in the collection of data during analysis of post-transplant diabetes, the 15.1% proportion of patients diagnosed with post-transplant diabetes matches proportions reported in the literature of the world which follows the recommendations of the ADA and the WHO. 28,29
Although this study, as do other retrospective studies, has significant limitations on data acquisition, striking information was found that should induce other LT groups to analyze their own blood glucose level and post-transplant diabetes statistics.
Conclusions
Retrospective analysis of data from our fourth-level hospital in Colombia showed good control of blood glucose levels throughout the different phases of LT in patients with pre-existing DM. This could be due to the institution’s treatment scheme which provides for appropriate adequate levels of glucose throughout all phases of surgery.
There was no evidence of a relationship between blood glucose levels and increased mortality or incidence of infections in our comparison of patients who had pre-existing diabetes with patients who did not have diabetes prior to LT. This is considered to be a consequence of adequate control of blood glucose levels in the patients analyzed.
In spite of this, the group of patients with DM had a higher overall mortality rate which is mostly attributable to cardiovascular disease.
With regard to the graft rejection rate, no statistically significant differences were found between the groups. Additional longer term studies with larger numbers of patients are required to evaluate this issue.
Finally, the incidence of post-transplant DM in our analysis was similar to those found in other cohorts. There may have even been underreporting due to lack of data in some of the clinical histories. This raises the need for active search for DM in patients.
Referencias
1. Santos O, Londoño M, Martín J, et al. An experience of liver transplantation in Latin America: a medical center in Colombia. Colomb Med (Cali). 2015;46(1):8-13. [ Links ]
2. Vera A, Contreras F, Guevara F. Incidence and risk factors for infections after liver transplant: single-center experience at the University Hospital Fundación Santa Fe de Bogotá, Colombia. Transpl Infect Dis. 2011;13:608-15. doi: 10.1111/j.1399-3062.2011.00640.x. [ Links ]
3. Garcia-Compean D, Jaquez-Quintana JO, Maldonado- Garza H. Hepatogenous diabetes. Current views of an ancient problem. Ann Hepatol. 2009;8(1):13-20. [ Links ]
4. Pfortmueller CA, Wiemann C, Funk GC, et al. Hypoglycemia is associated with increased mortality in patients with acute decompensated liver cirrhosis. J Crit Care. 2014;29(2):316.e7-12. doi: 10.1016/j.jcrc.2013.11.002. [ Links ]
5. Keller U, Sonnenberg GE, Burckhardt D, et al. Evidence for an augmented glucagon dependence of hepatic glucose production in cirrhosis of the liver. J Clin Endocrinol Metab. 1982;54(5):961-8. doi: 10.1210/jcem-54-5-961. [ Links ]
6. Kim WR, Smith JM, Skeans MA, et al. OPTN/SRTR 2012 Annual data report: liver. Am J Transplant 2014; 14: 69-96. doi: 10.1111/ajt.12581. [ Links ]
7. Zein NN, Abdulkarim AS, Wiesner RH, et al. Prevalence of diabetes mellitus in patients with end-stage liver cirrhosis due to hepatitis C, alcohol, or cholestatic disease. J Hepatol. 2000;32(2):209-17. doi: 10.1016/S0168-8278(00)80065-3. [ Links ]
8. Behrends M, Martinez-Palli G, Niemann CU, et al. Acute hyperglycemia worsens hepatic ischemia/reperfusion injury in rats. J Gastrointest Surg. 2010;14(3):528-35. doi: 10.1007/s11605-009-1112-3. [ Links ]
9. Park CS. Predictive roles of intraoperative blood glucose for post-transplant outcomes in liver transplantation. World J Gastroenterol. 2015;21(22):6835-41. doi: 10.3748/wjg.v21.i22.6835. [ Links ]
10. Ammori JB, Sigakis M, Enlesbe MJ, et al. Effect of intraoperative hyperglycemia during liver transplantation. J Surg Res. 2007;140(2):227-33. doi: 10.1016/j.jss.2007.02.019. [ Links ]
11. Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353-69. doi: 10.4158/EP09102.RA. [ Links ]
12. Park C, Hsu C, Neelakanta G, et al. Severe intraoperative hyperglycemia is independently associated with surgical site infection after liver transplantation. Transplantation. 2009;87(7):1031-6. doi: 10.1097/TP.0b013e31819cc3e6. [ Links ]
13. Shivaswamy V, Boerner B, Larsen J. Post-transplant diabetes mellitus: Causes, treatment, and impact on outcomes. Endocrine Reviews. 2016;37(1):37-61. doi: 10.1210/er.2015-1084. [ Links ]
14. Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464-70. doi: 10.1053/jhep.2001.22172. [ Links ]
15. Sato H, Carvalho G, Sato T, et al. The association of preoperative glycemic control, intraoperative insulin sensitivity, and outcomes after cardiac surgery. J Clin Endocrinol Metab. 2010;95(9):4338-44. doi: 10.1210/jc.2010-0135. [ Links ]
16. Jackson RS, Amdur RL, White JC, et al. Hyperglycemia isassocia ted with increased risk of morbidity and mortality after colectomy for cancer. J Am Coll Surg. 2012;214(1):68-80. doi: 10.1016/j.jamcollsurg.2011.09.016. [ Links ]
17. Yoo HY, Thuluvath PJ. The effect of insulin-dependent diabetes mellitus on outcome of liver transplantation. Transplantation. 2002;74(7):1007-12. doi: 10.1097/01.TP.0000032436.89407.31. [ Links ]
18. Shangraw RE, Jahoor F. Mechanism of dichloroacetate-induced hypolactatemia in humans with or without cirrhosis. Metabolism. 2004;53(8):1087-94. doi: 10.1016/j.metabol.2004.02.020. [ Links ]
19. Nowak G, Ungerstedt J, Wernerman J, et al. Metabolic changes in the liver graft monitored continuously with microdialysis during liver transplantation in a pig model. Liver Transpl. 2002;8(5):424-32. doi: 10.1053/jlts.2002.32943. [ Links ]
20. Saab S, Landaverde C, Ibrahim AB, et al. The MELD score in advanced liver disease: association with clinical portal hypertension and mortality. Exp Clin Transplant. 2006;4(1):395-9. [ Links ]
21. Chung HS, Kim ES, Lee C, et al. Comparison of intraoperative changes in blood glucose according to model for end-stage liver disease score during living donor liver transplantation. Transplant Proc. 2015;47(6):1877-82. doi: 10.1016/j.transproceed.2015.03.052. [ Links ]
22. Furnary AP, Wu Y, Bookin SO. Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: The Portland Diabetic Project. Endocr Pract. 2004;10 suppl 2:21-33. doi: 10.4158/EP.10.S2.21. [ Links ]
23. Ramos M, Khalpey Z, Lipsitz S, et al. Relationship of perioperative hyperglycemia and postoperative infections in patients who undergo general and vascular surgery. Ann Surg. 2008;248(4):585-91. doi: 10.1097/SLA.0b013e31818990d1. [ Links ]
24. Van den Berghe G, Wouters PJ, Bouillon R, et al. Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control. Crit Care Med. 2003;31(2):359-66. doi: 10.1097/01.CCM.0000045568.12881.10. [ Links ]
25. Ott C, Jacobs K, Haucke E, et al. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411-29. doi: 10.1016/j.redox.2013.12.016. [ Links ]
26. Yue S, Zhou HM, Zhu JJ, et al. Hyperglycemia and liver ischemia reperfusion injury: a role for the advanced glycation endproduct and its receptor pathway. Am J Transplant. 2015;15(11):2877-87. doi: 10.1111/ajt.13360. [ Links ]
27. Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359-67. doi: 10.1056/NEJMoa011300. [ Links ]
28. Sharif A, Hecking M, Saemann MD. et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant. 2014;14(9):1992-2000. doi: 10.1111/ajt.12850. [ Links ]
29. American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care. 2015;38 Suppl:S8-S16. doi: 10.2337/dc15-S005. [ Links ]
Received: December 14, 2017; Accepted: April 13, 2018











 texto en
texto en 


