Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.33 no.2 Bogotá Apr./June 2018
https://doi.org/10.22516/25007440.254
Review articles
A review of metastatic cancer with unknown primary cancer
1Médico, Universidad Nacional de Colombia. Bogotá D. C., Colombia
2Profesor de Medicina, Unidad de Gastroenterología, Universidad Nacional de Colombia, Hospital Universitario Nacional de Colombia. Bogotá D. C., Colombia.
3Internista oncólogo, Instituto Nacional de Cancerología, Universidad El Bosque, Centro Nacional de Oncología. Bogotá D. C., Colombia.
Metastatic tumors do not always have obvious origins: in one third of these cases, the primary tumor is never found. This article is a guide to the most recent advances in diagnostic approaches and patient management of these fatal and frequent tumors. An additional objective of this article is to help avoid common and serious errors. One of the most important errors is not taking the fundamental role of histological confirmation into account since it can avoid unnecessary investigations.
The article also details the components of a standard evaluation, classification according to prognosis and indications for a secondary evaluation. These include indications for upper and lower endoscopy, tumor markers, positron emission tomography, and the roles of genetic profiling, epigenetics and viral DNA. It also indicates the moment at which an investigation should be stopped. Recently, treatment has changed, and these changes seems to have changed the history of these patients and their counterparts with known primary tumors.
Keywords: Metastatic tumor; unknown primary; cancer
El tumor metastásico no siempre tiene un origen evidente, hasta en un tercio de los casos nunca se encuentra el tumor primario. Este artículo es una guía de los avances más recientes para mejorar el enfoque diagnóstico y el manejo del paciente con este tumor fatal y frecuente. El objetivo de este artículo, además de ser una guía, es ayudar a evitar errores comunes y graves. Uno de los errores más importantes es no tener en cuenta el papel fundamental de la confirmación histológica, pues esta puede evitar investigaciones innecesarias.
En el artículo también se detallan los componentes de la evaluación estándar, la clasificación según su pronóstico y las indicaciones de la evaluación secundaria, que incluye las indicaciones de la endoscopia alta y baja, los marcadores tumorales, la tomografía por emisión de positrones (TEP), el papel que ocupa el perfil genético, la epigenética y el ácido desoxirribonucleico (ADN) viral. Adicionalmente, se indica el momento en que se debe detener la investigación. Recientemente, el tratamiento se ha modificado, lo que parece cambiar la historia de estos pacientes y de sus contrapartes con primario conocido.
Palabras clave: Tumor metastásico; primario desconocido; cáncer
Introduction
Cancer of unknown primary origin (CUP) is a heterogeneous group of malignant tumors identified by histological confirmation of one of the metastatic lesions when the primary lesion cannot be identified despite a standardized diagnostic approach. 1,2 It is the eighth most common cancer in the world, 1 and it is a neoplasm with poor prognosis: average survival time after diagnosis is three months. 3 In 10% to 30% of cases, it is not possible to find the tumor from which metastasis originates even after an exhaustive search with the most advanced techniques such as the molecular profiling and even after an autopsy. 4 Due to the great difficulty of finding the primary site and offering specific treatment, new ways of acting against this tumor have recently been studied. They include molecular, imaging, immunohistochemical and genetic studies that improve patients’ abilities to survive these tumors. 2,4,5,6,7
Taking into account the importance of this topic in daily clinical practice, especially in gastroenterology, we decided to perform the following review to guide clinicians in their approach and management of patients with this type of oncological presentation.
Methodology
Search terms were based on combinations of “cancer of unknown primary”; “neoplasms” and “unknown primary” [MeSH] with each point of interest the terms “MeSH” and “not MeSH” in Spanish and English. The search strategies used were “neoplasms, unknown primary AND epigenetic”: “neoplasms, unknown primary AND immunohistochemical diagnosis”; “neoplasms unknown primary AND molecular diagnosis”; “neoplasms, unknown primary AND colonoscopy”; “neoplasms, unknown primary AND diagnosis”; “neoplasms, unknown primary AND endoscopy”; and “neoplasms, unknown primary AND treatment”. We searched the scientific literature in the Pubmed, Embase, Cochrane, Science Direct and Lilacs databases. The limits used were Spanish or English, human species and publication date 2012 to 2017. Articles corresponding to clinical practice guidelines, observational studies, controlled and randomized clinical trials, reviews, systematic reviews and metaanalyses were included. Of these, those that the authors considered pertinent were selected. In addition, some of the articles mentioned in the references of the publications selected in the initial search were added to the review by the authors.
CUP is a very aggressive tumor with poor prognosis. Despite being the eighth cause of cancer, it is the fourth cause of cancer death in the world.1,2,8 Survival after diagnosis varies from 11 weeks to 11 months with an average of three months. 3,9 It accounts for 3% to 5% of all malignant tumors and has an incidence of 7 to 12 cases/100 000 inhabitants/year.1,2,8,10 Each year 30,000 new cases are diagnosed in the United States. 9 As the population ages, cases within the 60-65 year old age group also increase. 1 In addition to its aggressiveness, it has early metastatic capacity in unpredictable locations. 1,8 These metastases affect three or more organs in a third of the patients at diagnosis. 8 The most frequent sites of metastases include the abdomen and liver, followed by the thorax, neck and bones. 11 From the histological point of view, it can be classified into five subtypes: the most frequent subtype is well and moderately differentiated adenocarcinoma (60%), followed by adenocarcinoma or poorly differentiated carcinoma (29%), squamous cell carcinoma (5%) and poorly differentiated malignant neoplasms (5%). 1
Initial approach
Histological confirmation and immunohistochemical study of the metastatic tumor is the fundamental, defining, first step to consideration of a diagnosis of CUP. 2 Omission of this step is a serious error that will substantially impact a patient’s outcome, time taken by diagnosis and the performance of unnecessary examinations and/or interventions which often occur. Advancing testing and treatment before histological confirmation is invalid: it is not justified to perform upper and lower endoscopies or positron emission tomography (PET) while awaiting the result of the biopsy of a metastasis. Similarly, it is necessary to perform the standard evaluation in order to define CUP. This has been studied in depth and the evaluation includes a detailed clinical history in which family and personal background of cancer are evaluated together with any symptoms that point towards a probable primary tumor as well as also risk factors such as smoking. 1,2,12
The physical examination should be complete including a search for masses and adenopathies. The examination should include examinations of the breasts, skin, pelvis and rectum. 1,9 However, due importance is often not given to clinical history and thorough physical examination for diagnosing this particular disease. If, after these steps have been taken the origin of the primary tumor remains unknown, the next part of the standard evaluation includes the following laboratory tests: a complete blood count; blood biochemistry, 2 blood glucose, electrolytes, calcium, liver profile, creatinine, urea and lactic dehydrogenase, 13 urine analysis, occult blood in fecal matter, and computed tomography (CT) with contrast of the thorax, abdomen and pelvis 2,14,15,16. The exception is CUP of the head and neck at nodal levels 1 to 3 for which it is suggested that the CT scan include the area from the base of the skull to the pelvis. 17
Even after the initial steps supplemented with more advanced examinations, the original tumors that caused the metastasis of 20% to 50% cannot be found. 18 Under these circumstances, one is faced with a diagnosis of CUP by its strictest definition. 19,20
The lungs and the pancreas are the most frequent sites of primary tumors found in autopsies with percentages of 27% and 24%, respectively. 4,5 Other sites that have been found frequently are the kidneys and adrenal glands (8%), liver and bile ducts (8%), colon (7%), genital system (7%) and stomach (6%). 4,5
Pathophysiology
The biological events that allow a primary tumor to remain hidden after the development of metastases have yet to be defined. 16 Even after an autopsy, the primary tumor may not be detected. For these cases, various theories have been proposed including regression or involution of the primary and development of the CUP in stem cells with capacity to differentiate into multiple cell lines to the liver, muscles, skin or even the cells of the gastrointestinal tract. They may be located in the connective tissue after birth. 7 There is no evidence that CUP is a different biological entity with exclusive genetic or phenotypic characteristics that differentiate it from other tumors. Various studies have shown chromosomal abnormalities, aneuploidies, and overexpression of several genes that are not specific to CUPs and which, to the contrary, occur in other malignancies. 10,16,20 The mutations and genetic alterations found have been divided into 6 groups (Table 1).
Table 1 Genetic mutations found in the CUP
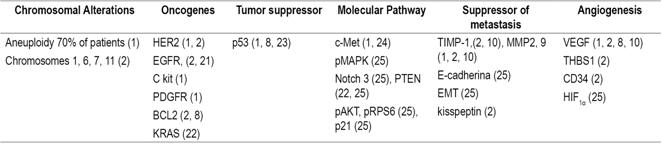
BCL2: B-cell CLL/lymphoma 2; C-kit: tyrosine-protein kinase receptor; CD34: cluster of differentiation 34; c-Met: hepatocyte growth factor receptor protein; EGFR: epidermal growth factor receptor; EMT: epithelial-mesenchymal transition (EMT); HER2: human epidermal growth factor receptor 2; HIF1α: hypoxia-inducible factor 1-alpha; KRAS: Kirsten rat sarcoma 2 viral oncogene homolog; MMP: matrix metalloproteinases; Notch 3: Neurogenic locus notch homolog protein 3; p21CIP1: cyclin-dependent kinase inhibitor 1A; p53: tumor suppressor protein p53; PKB/Akt: phosphorylated protein kinase B; PDGFR: platelet-derived growth factor receptor; pMAPK: phosphorylated mitogen-activated protein kinase; pRPS6: plastid ribosomal protein S6; PTEN: phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase; TIMP: tissue inhibitor of metalloproteinases; THBS1: thrombospondin 1; VEGF: vascular endothelial growth factor. Modified from: Pavlidis N et al. J Adv Res. 2015; 6 3: 375-82.
Classification
CUP is divided into two groups according to prognosis: favorable (20% of cases) and unfavorable (80% of cases). 2 Average survival time of patients in the favorable group is 12 to 36 months but is only 6 to 7 months for the unfavorable group. 19) Patients in the unfavorable group usually receive empirical chemotherapy with palliative intent, but they still have poor prognoses. Favorable subgroups are the most important, and all efforts are directed toward them. Identification of the subgroup is necessary for treatment specific to the type of cancer which improves prognosis. Some patients may survive for the long-term and even have the possibility of a cure. (25- 33).
Favorable Subtypes
NUT carcinoma (formerly NUT midline carcinoma) of germ cells predominantly affects men. In most cases it presents as mediastinal or retroperitoneal adenopathy. 25,34
Serous peritoneal papillary adenocarcinoma predominates in women and may clinically present as pain, intestinal obstruction, a mass or ascites. 35
Metastatic squamous cell carcinoma of the neck that frequently manifests with cervical adenopathy that is unique and is not painful in most cases. 1 It is more frequent in men (80%). 25
Poorly differentiated neuroendocrine carcinomas which are usually located in the lymph nodes, liver or bones. 1,2,8,25
Adenocarcinomas affecting the axillary lymph nodes in women which behave like breast cancer. 1,8 Seventy percent of these hidden tumors are detected after mastectomies. 25
Isolated inguinal adenopathy whose pathology shows squamous cell carcinoma in which the primary tumor must be found in genital organs. 1,2
Men who have blastic bone lesions with elevated prostate antigen and whose pathology reports shows adenocarcinoma. 25
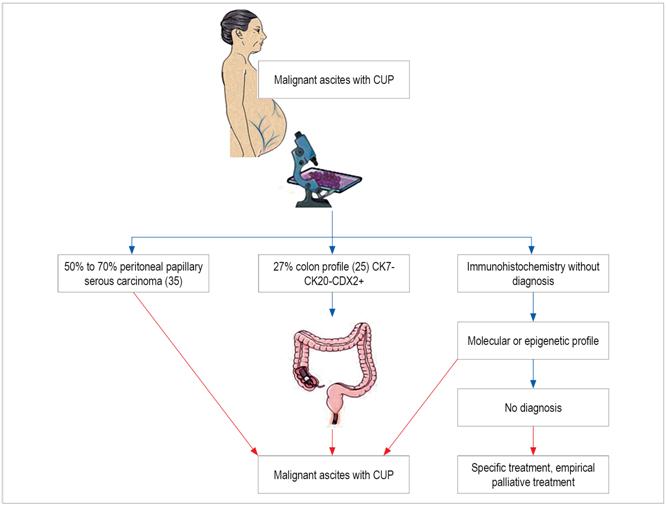
Adenocarcinoma with colon differentiation may present as hepatic metastasis (30%), abdominal adenopathies (51%), peritoneal surface metastasis (50%) or ascites (27%). 25
Unfavorable forecast subgroups
Metastatic adenocarcinoma in the liver or other organs.
Multiple brain metastases with adenocarcinoma or squamous cell differentiation.
Multiple pleural or pulmonary metastases with differentiation of adenocarcinoma.
Poorly differentiated carcinoma.
Squamous cell carcinoma of the abdominal cavity. 25
Despite their poor prognoses, researchers have not lost interest in these types of CUPs. To the contrary, they are more enthusiastic every day and strive to investigate and develop multiple tests, including endoscopic studies, functional diagnostic imaging, immunohistochemistry tests, genetic profiles and epigenetic analysis. 6,7,36
When the group of treating specialists has not made the correct assessment of the patient or when there is a possibility of additional investigation, the provisional diagnosis should be CUP. 13 If the patient has not been evaluated before, s/he must be referred to the oncology department. Indications for diagnostic studies are described below.
Tumor markers
Tumor markers have been studied extensively and are currently considered to have low sensitivity, low specificity, 13 and low positive predictive values (PPV). 25 They are not considered diagnostic and are not recommended, except in the following situations:
Verification of germline differentiation for cases of NUT carcinoma through tests for human chorionic gonadotropin β subunit (BHCG) 2 and alpha fetoprotein (AFP). 1
Test for AFP when hepatocellular carcinoma is suspected. 2 At high titers, this test is specific for this type of tumor, although AFP does not occur in all cases. 9
Prostate-specific antigen (PSA) in men with predominantly metastatic bone disease with blastic lesions. 13,16
Carbohydrate antigens (CA) 125 and 15-3 should be interpreted with caution given their limited specificity.2,13.
Diagnostic procedures
Colonoscopy
Colonoscopies are not routinely performed because they are not cost-effective while upper gastrointestinal endoscopy has low precision, sensitivity and specificity. 25,37 Performance of either of these examinations is recommended only for patients with significant symptoms suggesting pathologies in those sites of the digestive tract, in patients who test positive for occult blood in fecal matter, and for patients whose findings from imaging or histopathology are suggestive of colon adenocarcinoma (Figures 1 and 2). 10,14
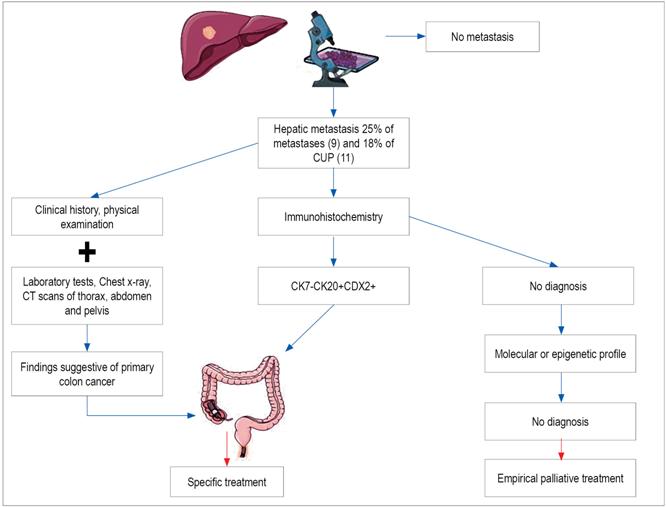
Figure 1 Diagnostic diagram of hepatic metastasis with colon adenocarcinoma profile. CDX2: caudal type homeobox 2; CK: cytokeratin.
Bronchoscopy
A bronchoscopy is performed when a patient tests positive for thyroid transcription factor 1 and/or CK 7 which indicate the possibility of pulmonary origin. 1,15,16,25 For patients with cervical adenopathy whose histology shows squamous cells, panendoscopy consisting of indirect and direct laryngoscopy, bronchoscopy and upper digestive endoscopy should be performed. 17,38
Imaging studies
CT scan of the chest, abdomen and pelvis
In the absence of contraindications, a contrast CT scan of the chest, abdomen and pelvis should be performed as the standard for all patients. 16
Testicular ultrasound
Testicular ultrasound is indicated for patients with metastatic tumors who have either germinal differentiation or NUT carcinoma. 1,13
Mammography
It is a mistake to routinely perform mammographies. 13 They are only indicated when there are symptoms, positive findings from a physical examination, or positive findings from histopathology. They are especially important for patients with axillary adenopathy. 16
Magnetic resonance imaging (MRI) of the breast
A breast MRI is indicated for CUP with axillary adenopathy when a mammography is normal. A breast MRI can detect up to 70% of hidden tumors. 39
PET scan with 5-fluorodeoxyglucose
The use of PET scans with 5-fluorodeoxyglucoseis is currently limited to patients who have CUP with squamous cells in the neck.40,41 For these patients, the scan can help guide the biopsy, determine the extent of the disease, facilitate planning of radiation therapy and help in follow-up. It has been found that PET scans can detect the primary tumor in 30% to 45% of cases even when other imaging studies have not been conclusive. Other studies favor PET scans over panendoscopy for this type of patients. 40,42 Apart from this indication, the role of PET scans is not clear. 16
PET scan with Gallium
Another scenario in which a PET scan is useful is a tumor of neuroendocrine differentiation. The best diagnostic image is made by PET/CT DOTA NOC (gallium (68) Ga-labeled [1, 4, 7, 10-tetraazacyclododecane-1, 4, 7, 10-tetraacetic acid] -1-NaI (3) -octreotide) which is more accurate than Octreoscan, CT scans, and MRI 1,16,43,44. While Octreoscan has a detection rate of 39% for CUP with neuroendocrine differentiation, 45 PET/CT DOTA has sensitivity of 94%, specificity of 86%, PPV of 91%, negative predictive value (NVP) of 92% and accuracy of 91% NOC for CUP with neuroendocrine differentiation. 43
Immunohistochemistry
Immunohistochemistry is a procedure used by pathologists that is based on the use of antibodies directed against keratins (family of proteins that make up the intermediate filaments expressed in carcinomas), transcription factors, membrane markers, nuclear markers and cytoplasmic markers which are used to define cell differentiation. 46
It is essential that the pathologist have an adequate sample of tissue and clinical information. Immunohistochemistry finds the primary tumor is found in 25% to 30% of cases, 16 but a recent metaanalysis found that it can detect the primary tumor in up to 65.6% of cases. 47. Despite being the most accepted algorithm, further studies are required to establish whether the identification of the primary tumor in groups without good prognoses or of certain types of tumor for which there are no specific treatments improves patient outcomes.1,2,48
Classically, it has been suggested that the pathologist follow a diagnostic algorithm for use of immunohistochemistry, and the Pavlidis algorithm is used most frequently. 1 This algorithm has three steps. The first step differentiates lymphoma, sarcoma and melanoma which are managed differently than are carcinomas. The second step differentiates among adenocarcinoma, squamous cell, neuroendocrine, thyroid, renal, hepatocellular and germinal carcinomas, and the third step differentiates among types of adenocarcinomas. This is very important, since adenocarcinomas account for 80% of metastatic CUPs. 49 In the first step (Table 2), lymphoma is differentiated by protein tyrosine phosphatase receptor type C (PTPRC) since lymphomas can be positive for cytokeratins. 2 If it is positive for carcinoma, continue with the second step. 2,9,25
Table 2 First immunohistochemistry step

AE1-AE3: acidic and base subfamilies of cytokeratin; HMB-45: human melanoma black 45; S100: multigenic family of non-ubiquitous Ca (2+) - modulated proteins. Modified from: Pavlidis N et al. Lancet. 2012; 379 (9824): 1428-35.
The second and third steps for differentiation of carcinomas and adenocarcinomas (1, 25) are shown in Tables 3 and 4, respectively.
Table 3 Second immunohistochemistry step
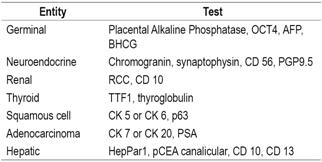
HepPar1: hepatocyte paraffin 1 antibody; OCT4: octamer-binding transcription factor 4; p63: oncogene belonging to the p53 gene family; pCEA: canalicular polyclonal carcinoembryonic antigen; PGP9.5: protein gene product 9.5; RCC: renal cell carcinoma; TTF1: thyroid transcription factor 1. Modified from: Pavlidis N et al. Lancet. 2012; 379 (9824): 1428-35.
Table 4 Third immunohistochemistry step
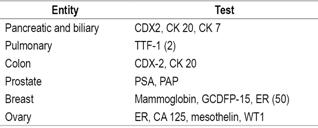
ER: estrogen receptor; GCDFP-15: gross cystic disease fluid protein-15; PAP: prostatic acid phosphatase; TTF-1: thyroid transcription factor 1; WT1: Wilms tumor protein. Modified from: Pavlidis N et al. Lancet. 2012; 379 (9824): 1428-35.
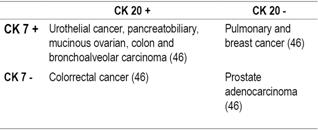
Tumors can also be classified according to CK 7 and 20. Four groups of tumors have been created which may suggest a tumor’s origin (Table 5). 9,50 CKs are not completely specific, so they should not be used to invoke a primary site in the absence of morphological or immunohistochemical support. 46
Molecular profiling
Several gene expression studies available today have had their effectiveness validated through identification of primary tumors in patients with known primary tumors. Their precision range is 85% to 90%. In the case of patients with CUP, a probable primary is identified in 70% to 75% of cases 28 by real-time polymerase chain reaction (PCR) of messenger RNA (mRNA), micro-RNA (miRNA) or microarrays. 2,9 Nevertheless, their impact on directing treatment according to the outcome of the possible primary remains questionable, and they have not yet been tested in randomized trials. 27,28 A prospective non-randomized phase II study of 252 patients suggests that survival may be improved with these studies, particularly for patients with tumors sensitive to chemotherapy whose outcomes have been better than those of historical cohorts. Additional caution should be exercised since these studies are susceptible to biases and confounding variables given the great heterogeneity of unknown primaries. 16
Currently, a phase III clinical trial is being conducted in Europe to compare the benefit of targeted therapy by molecular profile study against empirical treatment (NCT01540058). 27
Molecular profiling may be indicated when immunohistochemistry and routine examinations have failed to establish a primary tumor even though these studies should not be performed routinely in all patients according to international guidelines. (28,48,51
Treatment
The treatment of choice for patients in subgroups with unfavorable prognoses or whose primary tumor has not been established is palliative chemotherapy based on platinum and taxane. 2 Other chemotherapy schemes have been studied, but a review conducted in 2000 found no evidence of superiority of any chemotherapy regimen which includes platinum salts, taxanes or new generation cytotoxic agents (gemcitabine, vinca alkaloids or irinotecan).27,52,53 Response rates are around 20% with average survival times of 6 to 7 months with or without chemotherapy.1,25 However, other therapeutic objectives are valued in oncology. These include quality of life related to health, control of symptoms, indirect results, safety and results perceived by patients. 54 Modest prolongation of survival and palliation of symptoms with preservation of quality of life is the real goal in these patients although remission has been reported in rare cases. 27
On the other hand, favorable subgroups primarily receive regional treatment with surgery, radiation therapy and/or chemotherapy. 1 Survival is similar to that for patients with metastatic tumors of the same origin, 48 and treatments are also similar.
Poorly differentiated NUT carcinomas
NUT carcinomas receive platinum chemotherapy with schemes similar to those used in extra-gonadal germ cell tumors. Complete responses are achieved in 20% cases, partial responses are achieved in another 25%, of cases with average times survival of 12 months. Cure rates have been reported to be from 10% to 20%. 1,8,55
Adenocarcinomas in women with axillary lymph node involvement
Patients with axillary adenopathy are treated as breast cancer patients and may require complete axillary lymph node dissection, breast mastectomy(ies), radiation therapy, adjuvant chemotherapy or hormone therapy. When indicated, the use of trastuzumab (HER2 antibodies) is appropriate. The five year survival rate is 72%, and the ten year survival rate is 60%,1,18 but relapses occur in up to 55% of the patients who do not receive local therapy. 25
Squamous cell carcinomas in inguinal lymph nodes
Resection and radiation therapy are used to treat inguinal adenopathies with or without chemotherapy. 1 The 5-year survival rate has been estimated to be 20%. 55
Squamous cell carcinomas in cervical lymph nodes
Radical neck dissection with or without bilateral tonsillectomy and radiation therapy is performed to treat cervical adenopathy. 1 Cisplatin-based chemotherapy combined with radiation therapy is used in selected cases. 25 Local-regional control is achieved in 80% to 90% of these cases with 5 year survival rates over 65%. 25
Papillary adenocarcinoma of the peritoneal cavity
Like stage III and IV ovarian cancer, papillary serous peritoneal adenocarcinoma is managed with surgery and chemotherapy based on platinum and paclitaxel. Complete responses are obtained in 30% to 40% of patients, and 70% obtain partial responses with a median survival time of 36 months. Sixteen percent of patients survive long-term. 7,8,25.
Neuroendocrine carcinomas
In tumors with neuroendocrine differentiation, chemotherapy based on platinum, taxanes, 5-fluorouracil or capecitabine, temozolomide or irinotecan is administered, with an average survival of 15 months, with complete responses up to 21% 7 and 13% of patients it presents long-term survival 18. In the case of well-differentiated neuroendocrine carcinomas, survival at 5 years can be> 50% 7.
Adenocarcinomas with colon cancer immune-profile
Metastasis with the immune-profile of colon cancer is treated as metastatic colon cancer. Average survival time rangers from 20 to 36 months, the complete response rate is 15%, and the partial response rate is 35%. 25.
Adenocarcinoma with bone metastases and elevation of prostate antigen
These cases should be managed with androgen deprivation therapy with or without docetaxel-based chemotherapy similar to cases of metastatic prostate cancer. 1,7,25 The survival time of these patients is usually over 120 months. (56
Conclusion
A metastatic tumor with an unknown primary tumor causes fear in the patient and in the doctor, but this review provides doctors with a guide for the initial approach, subsequent classification, and indications for complementary studies. In addition, it highlights recent scientific advances that focus on new methods of diagnosis and directed treatments.
Referencias
1. Pavlidis N, Pentheroudakis G. Cancer of unknown primary site. Lancet. 2012;379(9824):1428-35. doi: 10.1016/S0140-6736(11)61178-1. [ Links ]
2. Natoli C, Ramazzotti V, Nappi O, et al. Unknown primary tumors. Biochimica et biophysica acta. 2011;1816(1):13-24. doi: 10.1016/j.bbcan.2011.02.002. [ Links ]
3. Hemminki K, Bevier M, Hemminki A, et al. Survival in cancer of unknown primary site: population-based analysis by site and histology. Ann Oncol. 2012;23(7):1854-63. doi: 10.1093/annonc/mdr536. [ Links ]
4. Pentheroudakis G, Golfinopoulos V, Pavlidis N. Switching benchmarks in cancer of unknown primary: from autopsy to microarray. Eur J Cancer. 2007;43(14):2026-36. doi: 10.1016/j.ejca.2007.06.023. [ Links ]
5. Kim KW, Krajewski KM, Jagannathan JP, et al. Cancer of unknown primary sites: what radiologists need to know and what oncologists want to know. AJR Am J Roentgenol. 2013;200(3):484-92. doi: 10.2214/AJR.12.9363. [ Links ]
6. Moran S, Martinez-Cardus A, Boussios S, et al. Precision medicine based on epigenomics: the paradigm of carcinoma of unknown primary. Nat Rev Clin Oncol. 2017;14(11):682-694. doi: 10.1038/nrclinonc.2017.97. [ Links ]
7. Tomuleasa C, Zaharie F, Muresan MS, et al. How to diagnose and treat a cancer of unknown primary site. J Gastrointestin Liver Dis. 2017;26(1):69-79. doi: 10.15403/jgld.2014.1121.261.haz. [ Links ]
8. Pavlidis N, Briasoulis E, Hainsworth J, et al. Diagnostic and therapeutic management of cancer of an unknown primary. Eur J Cancer. 2003;39(14):1990-2005. doi: 10.1016/S0959-8049(03)00547-1. [ Links ]
9. Swaid F, Downs D, Rosemurgy AS. A practical approach to liver metastasis from unknown primary cancer: What surgeons need to know. Cancer Genet. 2016;209(12):559-566. doi: 10.1016/j.cancergen.2016.08.004. [ Links ]
10. Pavlidis N, Fizazi K. Carcinoma of unknown primary (CUP). Crit Rev Oncol Hematol. 2009;69(3):271-8. doi: 10.1016/j.critrevonc.2008.09.005. [ Links ]
11. Hemminki K, Pavlidis N, Tsilidis KK, et al. Age-dependent metastatic spread and survival: Cancer of unknown primary as a model. Sci Rep. 2016;6:23725. doi: 10.1038/srep23725. [ Links ]
12. Hemminki K, Chen B, Melander O, et al. Smoking and body mass index as risk factors for subtypes of cancer of unknown primary. Int J Cancer. 2015;136(1):246-7. doi: 10.1002/ijc.28969. [ Links ]
13. MacReady N. NICE issues guidance on cancer of unknown primary. Lancet Oncol. 2010;11(9):824. doi: 10.1016/S1470-2045(10)70215-1. [ Links ]
14. Collado Martin R, Garcia Palomo A, de la Cruz Merino L, et al. Clinical guideline SEOM: cancer of unknown primary site. Clin Transl Oncol. 2014;16(12):1091-7. doi: 10.1007/s12094-014-1244-0. [ Links ]
15. Greco FA. Cancer of unknown primary site: improved patient management with molecular and immunohistochemical diagnosis. Am Soc Clin Oncol Educ Book. 2013:175-81. doi: 10.1200/EdBook_AM.2013.33.175. [ Links ]
16. Varadhachary GR, Raber MN. Cancer of unknown primary site. N Engl J Med. 2014;371(8):757-65. doi: 10.1056/NEJMra1303917. [ Links ]
17. Muller von der Grun J, Tahtali A, Ghanaati S, et al. Diagnostic and treatment modalities for patients with cervical lymph node metastases of unknown primary site - current status and challenges. Radiat Oncol. 2017;12(1):82. doi: 10.1186/s13014-017-0817-9. [ Links ]
18. Massard C, Loriot Y, Fizazi K. Carcinomas of an unknown primary origin--diagnosis and treatment. Nat Rev Clin Oncol. 2011;8(12):701-10. doi: 10.1038/nrclinonc.2011.158. [ Links ]
19. Vajdic CM, Goldstein D. Cancer of unknown primary site. Aust Fam Physician. 2015;44(9):640-3. [ Links ]
20. Kamposioras K, Pentheroudakis G, Pavlidis N. Exploring the biology of cancer of unknown primary: breakthroughs and drawbacks. Eur J Clin Invest. 2013;43(5):491-500. doi: 10.1111/eci.12062. [ Links ]
21. Hainsworth JD, Spigel DR, Thompson DS, et al. Paclitaxel/carboplatin plus bevacizumab/erlotinib in the first-line treatment of patients with carcinoma of unknown primary site. Oncologist. 2009;14(12):1189-97. doi: 10.1634/theoncologist.2009-0112. [ Links ]
22. Ross JS, Wang K, Gay L, et al. Comprehensive genomic profiling of carcinoma of unknown primary site: new routes to targeted therapies. JAMA Oncol. 2015;1(1):40-9. doi: 10.1001/jamaoncol.2014.216. [ Links ]
23. Loffler H, Pfarr N, Kriegsmann M, et al. Molecular driver alterations and their clinical relevance in cancer of unknown primary site. Oncotarget. 2016;7(28):44322-9. doi: 10.18632/oncotarget.10035. [ Links ]
24. Stella GM, Senetta R, Cassenti A, et al. Cancers of unknown primary origin: current perspectives and future therapeutic strategies. J Transl Med. 2012;10:12. doi: 10.1186/1479-5876-10-12. [ Links ]
25. Pavlidis N, Khaled H, Gaafar R. A mini review on cancer of unknown primary site: A clinical puzzle for the oncologists. J Adv Res. 2015;6(3):375-82. doi: 10.1016/j.jare.2014.11.007. [ Links ]
26. Pavlidis N, Petrakis D, Golfinopoulos V, et al. Long-term survivors among patients with cancer of unknown primary. Critical reviews in oncology/hematology. 2012;84(1):85-92. doi: 10.1016/j.critrevonc.2012.02.002. [ Links ]
27. Fizazi K, Greco FA, Pavlidis N, et al. Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v133-8. doi: 10.1093/annonc/mdv305. [ Links ]
28. Ettinger DS, Handorf CR, Agulnik M, et al. Occult primary, version 3.2014. J Natl Compr Canc Netw. 2014;12(7):969-74. doi: 10.6004/jnccn.2014.0093. [ Links ]
29. Pentheroudakis G, Lazaridis G, Pavlidis N. Axillary nodal metastases from carcinoma of unknown primary (CUPAx): a systematic review of published evidence. Breast Cancer Res Treat. 2010;119(1):1-11. doi: 10.1007/s10549-009-0554-3.30. [ Links ]
30. Fayanju OM, Jeffe DB, Margenthaler JA. Occult primary breast cancer at a comprehensive cancer center. J Surg Res. 2013;185(2):684-9. doi: 10.1016/j.jss.2013.06.020. [ Links ]
31. Jentsch-Ullrich K, Kalinski T, Roessner A, et al. Long-term remission in a patient with carcinoma of unknown primary site. Chemotherapy. 2006;52(1):12-5. doi: 10.1159/000090235. [ Links ]
32. Lanzer M, Bachna-Rotter S, Graupp M, et al. Unknown primary of the head and neck: A long-term follow-up. J Craniomaxillofac Surg. 2015;43(4):574-9. doi: 10.1016/j.jcms.2015.03.004. [ Links ]
33. Eldeeb H, Hamed RH. Squamous cell carcinoma metastatic to cervical lymph nodes from unknown primary origin: the impact of chemoradiotherapy. Chin J Cancer. 2012;31(10):484-90. doi: 10.5732/cjc.012.10035. [ Links ]
34. Pentheroudakis G, Stoyianni A, Pavlidis N. Cancer of unknown primary patients with midline nodal distribution: midway between poor and favourable prognosis? Cancer Treat Rev. 2011;37(2):120-6. doi: 10.1016/j.ctrv.2010.06.003. [ Links ]
35. Pentheroudakis G, Pavlidis N. Serous papillary peritoneal carcinoma: unknown primary tumour, ovarian cancer counterpart or a distinct entity? A systematic review. Crit Rev Oncol Hematol. 2010;75(1):27-42. doi: 10.1016/j.critrevonc.2009.10.003. [ Links ]
36. Economopoulou P, Pentheroudakis G. Cancer of unknown primary: time to put the pieces of the puzzle together? Lancet Oncol. 2016;17(10):1339-1340. doi: 10.1016/S1470-2045(16)30377-1. [ Links ]
37. Saliminejad M, Bemanian S, Ho A, et al. The yield and cost of colonoscopy in patients with metastatic cancer of unknown primary. Aliment Pharmacol Ther. 2013;38(6):628-33. doi: 10.1111/apt.12429. [ Links ]
38. Mackenzie K, Watson M, Jankowska P, et al. Investigation and management of the unknown primary with metastatic neck disease: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130(S2):S170-S175. doi: 10.1017/S0022215116000591. [ Links ]
39. Tan S, David J, Lalonde L, et al. Breast magnetic resonance imaging: are those who need it getting it? Curr Oncol. 2017;24(3):e205-e213. doi: 10.3747/co.24.3441. [ Links ]
40. Yoo J, Henderson S, Walker-Dilks C. Evidence-based guideline recommendations on the use of positron emission tomography imaging in head and neck cancer. Clin Oncol (R Coll Radiol). 2013;25(4):e33-66. doi: 10.1016/j.clon.2012.08.007. [ Links ]
41. Rudmik L, Lau HY, Matthews TW, et al. Clinical utility of PET/CT in the evaluation of head and neck squamous cell carcinoma with an unknown primary: a prospective clinical trial. Head Neck. 2011;33(7):935-40. doi: 10.1002/hed.21566. [ Links ]
42. Karapolat I, Kumanlioglu K. Impact of FDG-PET/CT for the detection of unknown primary tumours in patients with cervical lymph node metastases. Mol Imaging Radionucl Ther. 2012;21(2):63-8. doi: 10.4274/Mirt.344. [ Links ]
43. Sharma P, Arora S, Mukherjee A, et al. Predictive value of 68Ga-DOTANOC PET/CT in patients with suspicion of neuroendocrine tumors: is its routine use justified? Clin Nucl Med. 2014;39(1):37-43. doi: 10.1097/RLU.0000000000000257. [ Links ]
44. Sadowski SM, Neychev V, Millo C, et al. Prospective Study of 68Ga-DOTATATE Positron Emission Tomography/Computed Tomography for Detecting Gastro-Entero-Pancreatic Neuroendocrine Tumors and Unknown Primary Sites. J Clin Oncol. 2016;34(6):588-96. doi: 10.1200/JCO.2015.64.0987. [ Links ]
45. Prasad V, Ambrosini V, Hommann M, et al. Detection of unknown primary neuroendocrine tumours (CUP-NET) using (68)Ga-DOTA-NOC receptor PET/CT. Eur J Nucl Med Mol Imaging. 2010;37(1):67-77. doi: 10.1007/s00259-009-1205-y. [ Links ]
46. Conner JR, Hornick JL. Metastatic carcinoma of unknown primary: diagnostic approach using immunohistochemistry. Adv Anat Pathol. 2015;22(3):149-67. doi: 10.1097/PAP.0000000000000069. [ Links ]
47. Anderson GG, Weiss LM. Determining tissue of origin for metastatic cancers: meta-analysis and literature review of immunohistochemistry performance. Appl Immunohistochem Mol Morphol. 2010;18(1):3-8. doi: 10.1097/PAI.0b013e3181a75e6d. [ Links ]
48. Greco FA. The impact of molecular testing on treatment of cancer of unknown primary origin. Oncology (Williston Park). 2013;27(8):815-7. [ Links ]
49. Dennis JL, Hvidsten TR, Wit EC, et al. Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clin Cancer Res. 2005;11(10):3766-72. doi: 10.1158/1078-0432.CCR-04-2236. [ Links ]
50. Kandalaft PL, Gown AM. Practical Applications in Immunohistochemistry: Carcinomas of Unknown Primary Site. Arch Pathol Lab Med. 2016;140(6):508-23. doi: 10.5858/arpa.2015-0173-CP. [ Links ]
51. Chiang WM, Kapadia M, Laver NV, Nystrom JS. Cancer of unknown primary: from immunohistochemistry to gene expression profiling. J Clin Oncol. 2012;30(29):e300-2. doi: 10.1200/JCO.2011.41.1827. [ Links ]
52. Golfinopoulos V, Pentheroudakis G, Salanti G, et al. Comparative survival with diverse chemotherapy regimens for cancer of unknown primary site: multiple-treatments meta-analysis. Cancer Treat Rev. 2009;35(7):570-3. doi: 10.1016/j.ctrv.2009.05.005. [ Links ]
53. Amela EY, Lauridant-Philippin G, Cousin S, et al. Management of “unfavourable” carcinoma of unknown primary site: synthesis of recent literature. Crit Rev Oncol Hematol. 2012;84(2):213-23. doi: 10.1016/j.critrevonc.2012.03.003. [ Links ]
54. Gaertner J, Weingartner V, Lange S, et al. The role of end-of-life issues in the design and reporting of cancer clinical trials: A structured literature review. PloS one. 2015;10(9):e0136640. doi: 10.1371/journal.pone.0136640. [ Links ]
55. Greco FA. Molecular diagnosis of the tissue of origin in cancer of unknown primary site: useful in patient management. Curr Treat Options Oncol. 2013;14(4):634-42. doi: 10.1007/s11864-013-0257-1. [ Links ]
56. Takagi T, Katagiri H, Kim Y, et al. Skeletal metastasis of unknown primary origin at the initial visit: a retrospective analysis of 286 cases. PLoS One. 2015;10(6):e0129428. doi: 10.1371/journal.pone.0129428. [ Links ]
Received: September 11, 2017; Accepted: April 13, 2018











 text in
text in