Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.33 no.2 Bogotá abr./jun. 2018
https://doi.org/10.22516/25007440.192
Review articles
Approach to diarrhea in HIV patients
1Médico. internista, gastroenterólogo, servicio de gastroenterología, Instituto Gastroclínico. Medellín, Colombia
2Médico, servicio de urgencias, Hospital de Urgencia Asistencia Pública. Santiago de Chile, Chile
3Médico, servicio de medicina interna, Hospital Universitario San Ignacio. Bogotá D. C., Colombia
Diarrhea is the most common gastrointestinal symptom in people with human immunodeficiency virus infections. Diarrhea can appear to be a consequence of infection by an opportunistic germ or the side effect of antiretroviral treatment. It can be acute or chronic, but the latter leads to greater morbidity and alteration in patients’ quality of life. Stages of the diagnostic approach range from taking a complete clinical history, to microbiological, endoscopic and imaging studies. Finally, if infectious or organic causes have been ruled out (idiopathic enteropathy), management provided to the patient should seek symptomatic relief and optimization of adherence to antiretroviral treatment.
Keywords: Diarrhea; human immunodeficiency virus; HIV antiretrovirals HIV enteropathy
La diarrea es el síntoma gastrointestinal más frecuente en las personas infectadas por el virus de la inmunodeficiencia humana (VIH). La diarrea puede aparecer como consecuencia de infección por un germen oportunista, así como ser un efecto secundario del tratamiento antirretroviral. Esta, a su vez, puede ser aguda o crónica, esta última es la que causa mayor morbilidad y alteración en la calidad de vida del paciente. El enfoque diagnóstico se realiza por etapas que van desde una historia clínica completa hasta estudios microbiológicos, endoscópicos e imagenológicos. Finalmente, si se han descartado causas infecciosas u orgánicas (enteropatía idiopática), se debe brindar manejo al paciente en busca de aliviar los síntomas y optimizar la adherencia al tratamiento antirretroviral.
Palabras clave: Diarrea; virus de inmunodeficiencia humana; VIH; antirretrovirales; enteropatía por VIH
Introduction
Diarrhea is the most frequent gastrointestinal symptom in people infected with the human immunodeficiency virus (HIV). 1 Up to 40% to 80% of patients with untreated HIV get diarrhea, 2,3 and it can appear due to the use of antimicrobials or as a side effect of antiretroviral therapy (ART). 1 Diarrhea of more than one month duration combined with weight loss is a condition included in the definition of acquired immunodeficiency syndrome (AIDS). (1
To date, only a few studies have evaluated diarrhea in patients with HIV in Colombia. A study in Medellin of 159 hospitalized patients found that gastrointestinal symptoms occurred in 50.3%, and AIDS as defined by chronic diarrhea was found in 4.7% of cases. A total of 33% of opportunistic infections were diagnosed. They included tuberculosis (37%), histoplasmosis (17%) and cryptococcosis (9.7%). 4 Another Colombian study of 115 patients with diarrhea found Cryptosporidium infections in 10.4%. and modified chromotrope staining found that 29% were positive for microsporidia. The prevalence of parasites was 59.1% (Blastocystis hominis: 25.2% and Entamoeba histolytica: 13%). 5 A study carried out in India found parasites more common that bacterial and fungal infections in patients with diarrhea (58.3% vs. 29.17% and 12.50%, respectively). The most common parasite was Isospora (25.9%) and the most common bacterium was enterotoxigenic Escherichia coli (18.5%) with some cases of Shigella and Mycobacterium tuberculosis (3.7% each). 6
Pathophysiology
Gut-associated lymphoid tissue (GALT) is part of the immune system of the mucous membranes of the digestive tract (GALT) which fulfills functions of a protective barrier. It discriminates among allergens (pathogenic antigens) and promotes their elimination or tolerance to them. The intraepithelial lymphocytes, which are located in the basal membrane of the intestinal epithelium between the enterocytes, are part of the GALT. The majority are T lymphocytes of the CD3, CD4 and CD8 phenotypes. In conjunction with B lymphocytes, they regulate tolerance or elimination of antigens. 2 Once HIV penetrates through or between the epithelial cells of the digestive system, it is deposited through the CCR5 receptors of the M cells into the basal pocket where it comes into contact with lymphocytes of the lamina propria. These lymphocytes are the virus’s targets. HIV causes apoptosis and subsequent decrease in the number of lymphocytes initially within the epithelium and then throughout the lymphatic system. 1,2,3
HIV ribonucleic acid (RNA) has been identified in the intestines of 66% of patients with diarrhea, compared to in the intestines of only 45% of patients without diarrhea. 7 This type of study has raised the possibility of a direct cytopathic damage by HIV to the enterocyte, apparently mediated directly by glycoprotein 120 (gp120) and able to generate the so-called idiopathic enteropathy associated with HIV which has been ruled out as an infection by opportunistic germs.
Alterations in the cytoskeleton at weak intercellular junctions between intestinal epithelial cells have been described. These changes cause greater permeability and consequent loss of fluids and electrolytes. Villous atrophy, crypt hyperplasia and decreased amounts of disaccharidases have also been described. Together with some ileal dysfunction, they promote malabsorption of carbohydrates, bile salts and vitamins. The Tat I protein is also involved in direct damage due to HIV. It induces the secretion of chlorine and inhibits proliferation of enterocytes. Another is the R protein which promotes the formation of free radicals. 1,2,3
Another rarely studied phenomenon in idiopathic HIV enteropathy is exocrine pancreatic insufficiency which is unrelated to didanosine. This too can worsen nutrient malabsorption. Treated coinfection with hepatitis C (HCV) and alcohol use seem to be factors associated with this pancreatic alteration. This might be explained by generation of autoantibodies against the gland.1,2,3 Autonomic neuropathy for direct nerve damage by HIV has also been described. 1,2,3 In particular, cytopathic changes in the small intestine tend to improve with ART, confirming direct damage to the enterocyte and the immune system of the digestive tract.
Infectious causes
It is important to bear in mind that pathogenic germs are not isolated in up to 50% of patients with HIV infections and diarrhea. In any case, studies should always be conducted to search for microbiological agents because the prognosis and outcome of the patient is significantly modified by timely diagnosis. An extensive list of potential germs associated with diarrhea in HIV has been described. The most relevant ones in our environment are briefly reviewed below.
Bacterial Infections
Patients with HIV have risks of developing diarrhea due to bacteria similar to those of immunocompetent patients, although for HUV patients these infections result in greater systemic compromise. 2,8 Campylobacter infections occur with diarrhea, abdominal pain, fever and even bacteremia. 9 The diagnosis is made with a stool culture. Infection with non-typhoid Salmonella causes gastroenteritis, bacteremia, and local or disseminated infections in patients with HIV. 10 The diagnosis can be made by stool or blood cultures.
Management of these infections is based on ciprofloxacin which is recommended for 14 days. In patients with proctocolitis and HIV, chlamydia trachomatis infections should be suspected. The diagnosis is made with a stool culture and a blood culture given the high prevalence of bacteremia in immunosuppressed patients. 11 Treatment with 100 mg doxycycline every 12 hours for 7 days or with a single 1g dose of azithromycin is recommended.
Although mycobacterial infections occur infrequently, they can compromise the digestive system. The mycobacterium avium complex (MAC) is the most common. 12,13 The disease disseminated by MAC was the most common opportunistic bacterial infection in patients with AIDS. With the advent of ART, its incidence has declined, but it still occurs in patients with poor immunovirological responses. Its clinical presentation varies and can include fever, weight loss, nocturnal diaphoresis, watery diarrhea, malabsorption, lymphadenopathy and unusual enlargement of organs. 14 Usually, it compromises the duodenum and should be suspected when mucous nodules or yellowish patches can be seen in esophagogastroduodenoscopy. 15 In a biopsy, the infection is indicated by macrophages with acid-fast bacilli inclusions similar to those presented in Whipple’s disease. It can also be diagnosed in blood cultures and stool cultures. 14 Management with oral administration of 500 mg of clarithromycin every 12 hours and 15 mg/kg of ethambutol once a day VO with or without 300 mg of rifabutin daily is suggested. 16
Clostridium difficile bacteria can also be associated with diarrhea in patients with HIV. The diagnosis can be established by immunoassay techniques that detect toxins A and B. Their sensitivity is between 70% and 78%. Testing for the common antigen provides greater sensitivity, but it does not discriminate between pathogenic and non-pathogenic strains. (17
Polymerase chain reactions (PCR) amplify genes for toxins A and B and offer greater sensitivity and specificity than do other techniques, 18 but the gold standard of diagnostic testing is culturing, but it has the limitation that it can take up to 72 hours. Standard management consists of oral administration of 500 mg of metronidazole every 8 hours for 10 to 14 days or oral administration of 125-250 mg of vancomycin every 6 hours for 10 to 14 days.
For severe infections by virulent strains and for patients with HIV, vancomycin seems to be superior to metronidazole. Another alternative is 200 mg of fidaxomicin every 12 hours for 10 days.
Fidaxomicin is an antibiotic that is not absorbed in the gastrointestinal tract whose efficacy is close to that of vancomycin. 19
Viral Infections
Cytomegalovirus (CMV) generates high rates of morbidity and mortality in HIV patients. It can affect any part of the gastrointestinal tract and manifests with fever, weight loss, anorexia, abdominal pain and bloody diarrhea. A colonoscopy, the diagnostic method of choice, will show evidence of patchy erythema mucosa, erosions and ulcers. 20,21
The most effective antiviral treatment is intravenous administration of 5 mg/kg of ganciclovir every 12 hours for at least 3 weeks. Alternatively, 900 mg of valganciclovir can be administered orally every 12 hours for at least 3 weeks, or 90 mg of foscarnet can be administered intravenously every 12 hours for 3 to 6 weeks.
Parasitical Infections
Parasites that cause diarrhea in patients with HIV include those that can also generate infections in immunocompetent people as well as opportunistic parasites that do not generate diseases in the healthy population. The first group includes giardia lamblia, entamoeba histolytica and strongyloides stercoralis.
Cryptosporidium infections compromises the small intestine which causes severe diarrhea in patients with HIV. It also has the ability to infect the epithelium of the respiratory tract and the bile duct.22,23. With the advent of ART, the morbidity attributed to cryptosporidium has decreased.24,25 Diagnosis is made by modified Ziehl-Neelsen stains of fecal matter, or by identification of oocytes through PCR of biopsies of the small intestine or rectum. 8 Treatment is administration of ART to increase the CD4 T lymphocyte count. 23 There is some evidence that suggests management with 500 mg of nitazoxanide every 12 hours until the symptoms are resolved and eradication in fecal matter is achieved. 8,24
Isospora belli can cause diarrhea, vomiting, abdominal pain and weight loss. 25 Therapeutic options in this case are trimethoprim/sulfamethoxazole (160 mg/800 mg) every 6 hours for 10 days or 500 mg of ciprofloxacin every 12 hours for 7 days in cases of allergies to sulfas. 8
The spectrum of symptoms of strongyloides stercoralis infections in patients with HIV varies from chronic diarrhea and anemia, to digestive bleeding, intestinal obstruction and even hyperinfection syndrome. 26,27
Hyperinfection syndrome occurs most frequently in adult male patients and is characterized by uncontrolled larval multiplication including significant increases of the number of infective larvae outside the digestive system through the vascular and alveolar spaces. This causes pulmonary edema, pneumonia and alveolar hemorrhaging which can continue until respiratory failure and multiorgan failure with a mortality rate as high as 80%. The diagnosis is made by observing the larvae in the lungs.
Another system involved in hyperinfection is the central nervous system (CNS) where it can affect patients with meningitis with various cutaneous lesions ranging from Larva currens to periumbilical maculopapular eruptions, purpura and petechiae. The liver can also be affected through biliary obstructions and granulomatous portal inflammation. Less commonly, the heart, thyroid, pancreas and bladder can be affected.26,27
The diagnosis is usually made by direct study of fecal matter, although the most sensitive method is a duodenal biopsy. Recommended treatment is oral administration of 200 μg/kg of ivermectin once a day for one or two days. If necessary, the treatment can be repeated two to three weeks after the first dose. 27 In cases of hyperinfection syndrome, ivermectin should be administered daily until the symptoms resolve and fecal tests are negative for two weeks. 27
Fungal Infections
Gastrointestinal histoplasmosis due to histoplasma capsulatum occurs infrequently, usually in patients with low CD4 levels and usually in the ileocecal region. Diagnosis can be made with cultures of fecal material, blood cultures, identification of urinary antigens and analysis of biopsy specimens.28,29 It should be treated with amphotericin B for one to two weeks followed by itraconazole for 12 months. Patients with HIV and a CD4 count of less than 150 cells/mm3 who live in endemic areas should receive prophylaxis with 200 mg/day of itraconazole. 29
Manifestations of microsporidial infections include non-inflammatory chronic diarrhea, weight loss, abdominal pain, nausea, vomiting and fever. (30 Diagnosis is a challenge given the germ’s small size of 1 to 2 μm. The gold standard for diagnosis remains modified Ziehl-Neelsen staining, but, there are other methods such as PCR and enzyme-linked immunosorbent assays (ELISA). 30 Treatment is based on 400 mg of albendazole every 12 hours for three weeks, but ART with an increase in CD4 tends to resolve this infection. 11
Non-infectious causes
Idiopathic HIV enteropathy is diagnosed once infection by pathogenic germs has been ruled out. It occurs in 50% of patients and is characterized by rather watery diarrhea and worsens with the consumption of food but improves with defecation. 31 Because its symptoms are similar to those described for irritable bowel syndrome (IBS), it is possible to fall into the error of diagnosing IBS early in patients with HIV and chronic diarrhea. Given the multiple pathophysiological mechanisms with organic alterations of the digestive tract, some of which are irreversible despite treatment, this diagnosis practically reduces into a subgroup of patients with HIV. It is suggested that the diagnosis of IBS be reserved only for HIV patients who managed to maintain adequate immune-virological control and for whom an exhaustive study has ruled out an opportunistic infection or structural alteration of the digestive system (including exocrine pancreatic insufficiency).
HIV enteropathy has a negative impact on the quality of life of patients as evidenced by multiple demographic studies. It has been found that up to 40% of these patients have their social lives affected which is why all therapeutic options should be used in the search for symptomatic improvement.
Diarrhea has also been described as a side effect of ART. This is important because of the high rates of treatment discontinuation. 32 Protease inhibitors are the agents with the greatest association. 33 Ritonavir in combination with lopinavir and fosamprenavir is one of the most often reported with up to 10% to 15% of patients using it affected. There are other drug combinations with lower rates of diarrhea such as atazanavir-ritonavir, darunavir-ritonavir and saquinavir-ritonavir. 2,8
Several pathophysiological mechanisms have been proposed that explain the diarrhea associated with ART. For example, it has been found that nelfinavir can stimulate signaling pathways in epithelial cells which causes loss of chlorine through the epithelial membranes. Lopinavir has been associated with cellular apoptosis and disruption of the intestinal barrier which causes erosions of the mucosa in the duodenum and ileum. 2,33 The functional and structural alterations of enterocytes in patients taking protease inhibitors produce increased concentrations of electrolytes and fecal pH with a change in the osmotic gap and, consequently, cause secretory diarrhea.
Diagnostic approach
First stage: evaluation of infections by germs34
The first step is to take a complete medical history that focuses on the evolution of the HIV infection, treatment received, adherence to treatment and risk factors for infections such as travel to endemic areas (important for amebae and giardia), anal sex (C. trachomatis or herpes), and recent use of antibiotics (C. difficile). Whether diarrhea is acute or chronic must be defined, and it should be categorized into one of four grades. 35
Grade 1. Mild: transient or intermittent diarrhea with less than 3 stools/day in the normal pattern.
Grade 2. Moderate: persistent liquid diarrhea or 4 to 6 bowel movements.
Grade 3. Severe: bloody diarrhea or more than 7 bowel movements/day that require handling with IV fluids.
Grade 4. Potentially incompatible with life with shock or organ dysfunction.
This classification allows establishment of whether the patient should be hospitalized or an outpatient, priority of tests, probability of diagnosing opportunist infections, and whether empirical treatment should be initiated.
In the physical examination, the patient’s nutritional profile and signs of dehydration should be evaluated. If there are eye symptoms, the fundus of the eye should be checked for CMV retinitis or microsporidium retinitis. Whether or not hepatosplenomegaly is present must be determined. If the patient reports perianal pain, it is necessary to perform a digital rectal examination to rule out sexually transmitted infections (Figure 1).
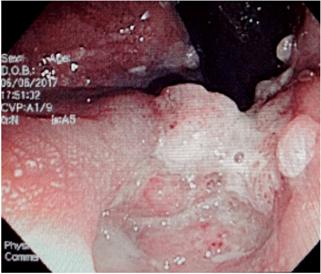
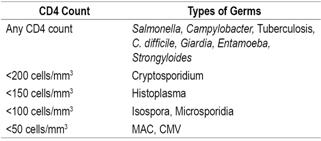
Figure 1 Sigmoidoscopy of a patient with HIV. Multiple verrucous lesions in the rectum that extend into the anal canal are suggestive of perianal condylomata.
To improve detection of germs, at least three coloproctological samples should be taken in a period of 10 days. 36 Whenever there is a risk for infection by microsporidium, cryptosporidium or Isospora, modified Ziehl-Neelsen staining should be performed. If there is suspicion of CMV infection, a blood test should be performed to measure the antigen or immunoglobulin M (IgM) and, if the suspicion persists, direct methods such as PCR of blood and fecal matter should be used. The samples of Shigella and Salmonella for culturing must be transported immediately to the laboratory since any change of pH without refrigeration alters performance. For initial detection of C. difficile, test for toxin A and B in fecal matter. The probability of culturing mycobacteria from fecal matter is low, so it is not recommended. Determining the level of CD4 is essential for finding causes of diarrhea in patients with HIV (Table 1, Figure 2). 1,3
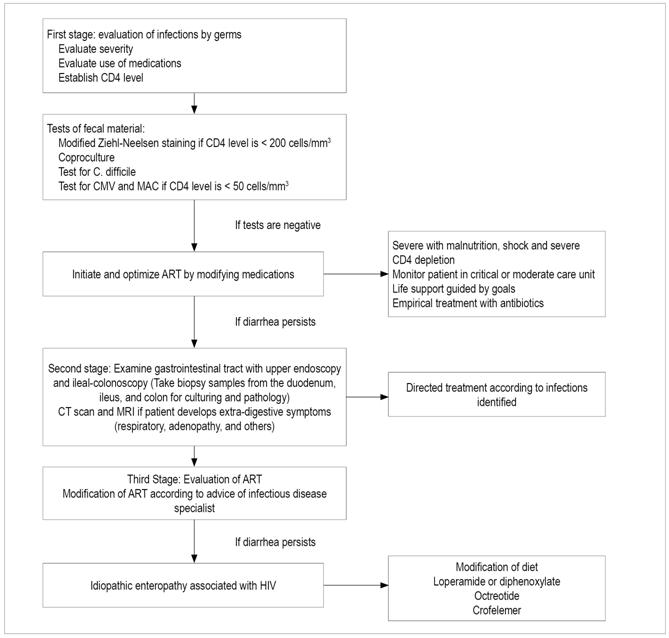
Figure 2 Flow diagram of approach to diarrhea in patients with HIV. EVDA: upper digestive tract endoscopy; NMR: nuclear magnetic resonance; CT scan: computerized axial tomography.
Second Stage: Gastrointestinal Tract Examination34
If a pathogen is not diagnosed in the first phase, and diarrhea persists and is severe, endoscopic or radiological studies should be performed.34,37 The guidelines for endoscopic study of diarrhea from the American Society for Gastrointestinal Endoscopy (ASGE) 37 recommend initially performance of sigmoidoscopy in patients with HIV, but they clarify that if the sigmoidoscopy is not positive and the probability of opportunistic infection is high, a colonoscopy should be performed with ileum and colon biopsies, and an esophagogastroduodenoscopy should be performed with duodenal biopsies (Figure 3).
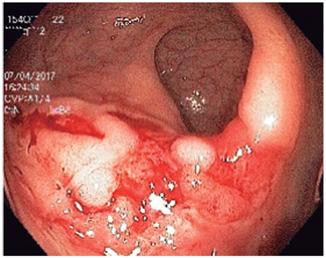
Figure 3 Colonoscopy of a patient with HIV and anal intercourse. Large rectal ulcer due to herpes virus.
Several studies have demonstrated the usefulness of endoscopic procedures, but it should be clarified that there is no typical endoscopic pattern of opportunist infection, so biopsies should always be taken. It has been suggested that Salmonella infections predominates in the right colon and ranges from erythema to ulcerations. Amoeba infections usually affect the cecum and rectosigmoid with ulcerations and areas of necrosis (Figure 4). CMV can generate ulcerations that predominate in the left colon (Figure 5). The yield of colonoscopy ranges from 27% to 39%, and CMV is the most common germ found. 38
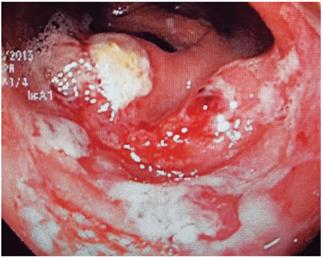
Figure 4 Colonoscopy in a patient with HIV and bloody diarrhea. There are multiple, poorly defined ulcerations with necrotic material on the surface and an active inflammatory background in the cecum and right colon. Structural alterations with hemophagocytosis and trophozoites of E. histolytica were observed in biopsies.
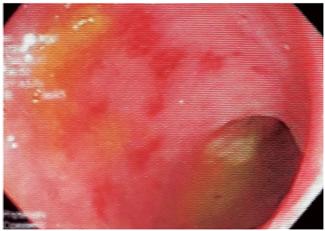
Figure 5 Sigmoidoscopy in a patient with HIV and bloody diarrhea. Multiple patchy round erosions can be seen. Immunohistochemistry (IHC) suggests a CMV infection.
One study that compared microbiological examinations of fecal matter with endoscopic biopsies has found that the latter have better diagnostic yields in patients with CD4 <200 cells/mm3. 39 Another study diagnosed opportunistic infections by endoscopic methods in 21/48 patients (44%; 95% confidence interval: 30% to 58%). Colonoscopy found the diagnosis in 13 patients, including nine cases of CMV. In the majority of patients, the diagnosis was made by biopsies of the rectosigmoid. Esophagogastroduodenoscopy diagnosed seven cases of microsporidium infections and two cases of cryptosporidium infections. 40
Another prospective study of 79 patients found diagnoses in 22 cases with biopsies from the left colon contributed the most diagnoses (17/22 patients with sensitivity of 77%) including all 15 patients diagnosed with CMV infections (sensitivity of 100%). The combination of left and right colon biopsies had a sensitivity of 82%. Duodenal biopsies taken during esophagogastroduodenoscopy did not contribute to diagnosis unlike those taken during colonoscopy. 41 A study of 40 patients found diagnoses by colonoscopy in 65% of the patients. Amoebic colitis and CMV were the main causes. 42
Whenever endoscopic procedures are performed, regardless of the findings, biopsies of both the colon and small intestine should be taken. Although the diagnostic yield of healthy mucosal biopsies is lower, opportunistic infections can sometimes be diagnosed 43). Diagnoses of isolated CMV infections in the right colon occur in as many as 29% to 39% of patients, so a total colonoscopy is preferable to a sigmoidoscopy. 38,44 If the suspicion is high, testing by immunohistochemistry and PCR should be performed on biopsies. Whenever biopsies are taken for microbiological study, they should be sent in a dry tube or saline solution, never in formaldehyde.
When an esophagogastroduodenoscopy is performed, duodenal biopsies should be taken as distal as possible, from the third or fourth duodenal portion, in order to increase detection of microsporidium. Examination of duodenal aspirate has not been shown to increase the detection of pathogens and should not be performed. 38 The use of endoscopic capsules in HIV patients has been studied, and abnormalities in the small intestine were found in 89% of the cases. 45 For now, its cost-effectiveness has not been evaluated, and it is not considered to be a routine examination.
Several useful radiological patterns have been described. One example is that tuberculosis tends to affect the ileocecal region with thickening of the ileum and cecum which simulates Crohn’s disease. 46 MAC infections compromise the jejunum and cause thickening of the folds. 3 In addition to ulcers in the colon, CMV infections result in thrombosis due to vasculitis, and ischemia and perforations of the viscera can be found. 3 Kaposi’s sarcoma can affect any part of the digestive system and is seen as long, flat, or submucosal lesions associated with thickening of the folds. 47 Non-Hodgkin’s lymphoma (NHL) usually involves the terminal ileum and causes mass-type lesions and ulcers with tumor extensions to the mesentery and adjacent ganglia (Figure 2). 48
Third Stage: Evaluation of ART34
Diarrhea associated with ART has been described in 2% to 19% of patients. (49 If it is considered that there is a relationship between ART medication and diarrhea, this third stage may precede the second. You should always have a clear idea about infectious disease before considering suspension or change of ART medications (Figure 2).
Treatment of diarrhea associated with hiv enteropathy
Achieving control of diarrhea in this group of patients is important. It helps improve adherence to ART, nutritional status, stability in weight and quality of life. 50,51 Initial attempts should aim to improve symptoms through lifestyle modifications. Depending on the response, medications should be added. In patients with low CD4 counts, the intervention with greatest effectiveness will be initiation of ART.
Non-Pharmacological Management
A comparison of 75 patients divided into two groups, one with a conventional diet and one with a restricted diet, has found that diarrhea had improved in the restricted diet group by 24 weeks. 52 Their diet was low in fat, insoluble fiber and caffeine, lactose free, and high in soluble fiber. A clinical trial of 25 patients, has found that supplementation with L-glutamine reduced the severity of diarrhea more than did placebos. 53
The evidence for the use of probiotics is controversial. A clinical trial of administration of Lactobacillus GG to 17 patients for two weeks could not demonstrate positive outcomes. 54 Another two day clinical trial with Lactobacillus strains resolved diarrhea in 12/12 patients compared to 2/12 patients who received yogurt without probiotics. 55 A study of 69 patients with Lactobacillus strains for 25 weeks showed no improvement in symptoms related to diarrhea. 56 Based on the results of these small studies, the use of probiotics cannot be recommended.
Pharmacological Management
Antimotility Agents
Loperamide and diphenoxylate seek to slow intestinal transit and increase water and sodium absorption. A Cochrane review of these drugs did not show positive results for diarrhea associated with HIV. 57 A retrospective study found that 32% of patients receiving nelfinavir responded to the use of loperamide. 58 It should be remembered that chronic use of loperamide can cause adverse effects including interactions with protease inhibitors. In any case, the use of this drug in HIV seems to be effective and safe, and should be used as the first line of treatment. 50 The evidence for diphenoxylate is scarce and controversial. 58 This medicine crosses the blood-brain barrier and has risks of abuse and dependence. It is suggested that it be reserved for patients who are refractory to other pharmacological measures.
Antisecretory Agents
Subcutaneous use of octreotide has been studied, but most studies date from the pre-ART era. Some studies found a reduction in the volume and frequency of bowel movements, 59,60 but adverse effects such as hypoglycemia, biliary mud formation, nausea, abdominal pain and constipation should be taken into account. 50 Its use should be reserved for refractory patients, and a risk/benefit analysis should be made.
Crofelemer has been approved by the Food and Drug Administration (FDA) for symptomatic improvement of non-infectious diarrhea in patients with HIV. The recommended dose is 125 mg twice a day. It acts by simultaneously inhibiting two chloride channels: the CFTR (cystic fibrosis transmembrane conductance regulator) in the apical membranes and the CaCC (calcium-activated chloride channel) in the epithelial membranes. This decreases the secretion of sodium and water into the intestinal lumen. It has minimal systemic absorption and acts directly in the intestine. 61 Consequently, there is little interaction with other medications. It has clinical effect at four weeks. 62,63
The ADVENT study included 374 patients with noninfectious diarrhea associated with HIV which persisted despite management with loperamide. 64 Its first phase compared doses of 125 and 500 mg of Crofelemer with placebos and found that administration of Crofelemer improved patients’ symptoms (20.5%, 19.6%, and 2%, respectively. p <0.0024). Long-term safety was evaluated in a 48 week phase III study of 250 patients. The results showed that 9.2% of adverse events could be attributed to the drug but that there were no changes in the level of CD4 + or viral load 65. If patients who do not response after three months, the drug should be discontinued and other alternatives should be tried. This medicine has already been approved by the National Institute for the Surveillance of Drugs and Foods (INVIMA) here in Colombia.
The evidence is not strong, or the results have not been positive, for some other therapies including fiber supplements, bovine immunoglobulin, curcumin, bismuth salts and racecadotril. Consequently, they are not recommended.
Conclusions
Diarrhea is a common symptom HIV patients that can cause deterioration in quality of life, malnutrition and even systemic involvement. It requires an appropriate approach and treatment. Diagnosis should always consider infectious causes, including opportunistic germs. As demonstrated in our review, the immunovirological control of the patient should be established in order to make the approach to possible causes of diarrhea better targeted and more specific. Similarly, the impact of ART on diarrhea in patients with HIV should always be evaluated. Finally, if the patient is considered to have HIV enteropathy, management should focus on relief of symptoms, improvement of nutritional status and insurance of adequate adherence to ART. We now have new medications such as Crofelemer with which we hope to improve the quality of life of these patients.
Referencias
1. Logan C, Beadsworth MB, Beeching NJ. HIV and diarrhoea: what is new? Curr Opin Infect Dis. 2016;29(5):486-94. doi: 10.1097/QCO.0000000000000305. [ Links ]
2. MacArthur RD, DuPont HL. Etiology and pharmacologic management of noninfectious diarrhea in HIV-infected individuals in the highly active antiretroviral therapy era. Clin Infect Dis. 2012;55(6):860-7. doi: 10.1093/cid/cis544. [ Links ]
3. Feasey NA, P. Healey P, Gordon MA. Review article: the aetiology, investigation and management of diarrhoea in the HIV-positive patient. Aliment Pharmacol Ther. 2011;34(6):587-603. doi: 10.1111/j.1365-2036.2011.04781.x. [ Links ]
4. Andrade FM, Quiroga A, Builes C, et al. Epidemiología de la infección por el virus de inmunodeficiencia humana en pacientes hospitalizados en una institución de alta complejidad y enseñanza universitaria en Medellín, Colombia. Infectio. 2016;20(1):9-16. doi: 10.1016/j.infect.2015.05.004. [ Links ]
5. Flórez AC, García DA, Moncada L, et al. Prevalencia de microsporidios y otros parásitos intestinales en pacientes con infección por VIH, Bogotá, 2001. Biomédica. 2003;23(3):274-82. doi: 10.7705/biomedica.v23i3.1221. [ Links ]
6. Shah S, Kongre V, Kumar V, et al. A study of parasitic and bacterial pathogens associated with diarrhea in HIV-positive patients. Cureus. 2016;8(9):e807. doi: 10.7759/cureus.807. [ Links ]
7. Oude Munnink BB, Canuti M, Deijs M, et al. Unexplained diarrhoea in HIV-1 infected individuals. BMC Infect Dis. 2014;14:22. doi: 10.1186/1471-2334-14-22. [ Links ]
8. Krones E, Högenauer Ch. Diarrhea in the immunocompromised patient. Gastroenterol Clin North Am. 2012;41(3):677-701. doi: 10.1016/j.gtc.2012.06.009. [ Links ]
9. Fernández-Cruz A, Muñoz P, Mohedano R, et al. Campylobacter bacteremia: clinical characteristics, incidence, and outcome over 23 years. Medicine (Baltimore). 2010;89(5):319-30. doi: 10.1097/MD.0b013e3181f2638d. [ Links ]
10. Hohmann EL. Nontyphoidal salmonellosis. Clin Infect Dis. 2001;32(2):263-9. doi: 10.1086/318457. [ Links ]
11. Wilcox CM. Gastrointestinal consequences of infection with human immunodeficiency virus. En: Feldman M, Friedman LS, Brandt LJ (editores). Sleisenger and Fordtrans’s gastrointestinal and liver disease. Filadelfia: Elsevier Saunders; 2010. pp. 526-30. [ Links ]
12. Burgers WA, Riou C, Mlotshwa M, et al. Association of HIV-specific and total CD8+ T memory phenotypes in subtype C HIV-1 infection with viral set point. J Immunol. 2009;182(8):4751-61. doi: 10.4049/jimmunol.0803801. [ Links ]
13. Wallace JM, Hannah JB. Mycobacterium avium complex infection in patients with the acquired immunodeficiency syndrome. A clinicopathologic study. Chest. 1988;93(5):926-32. doi: 10.1378/chest.93.5.926. [ Links ]
14. Gordin FM, Cohn DL, Sullam PM, et al. Early manifestations of disseminated Mycobacterium avium complex disease: a prospective evaluation. J Infect Dis. 1997;176(1):126-32. doi: 10.1086/514014. [ Links ]
15. Bhaijee F, Subramony C, Tang SJ, et al. Human immunodeficiency virus associated gastrointestinal disease: common endoscopic biopsy diagnoses. Patholog Res Int. 2011;2011:247923. doi: 10.4061/2011/247923. [ Links ]
16. Gordon SN, Cervasi B, Odorizzi P, et al. Disruption of intestinal CD4 1 T cell homeostasis is a key marker of systemic CD4 1 T cell activation in HIVinfected individuals. J Immunol 2010;185(9):5169-79. doi: 10.4049/jimmunol.1001801. [ Links ]
17. Loo VG, Bourgault AM, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365(18):1693-703. doi: 10.1056/NEJMoa1012413. [ Links ]
18. Deshpande A, Pasupuleti V, Rolston DD, et al. Diagnostic accuracy of real-time polymerase chain reaction in detection of Clostridium difficile in the stool samples of patients with suspected Clostridium difficile infection: a meta-analysis. Clin Infect Dis. 2011;53(7):e81-90. doi: 10.1093/cid/cir505. [ Links ]
19. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422-31. doi: 10.1056/NEJMoa0910812. [ Links ]
20. Lawlor G, Moss AC. Cytomegalovirus in inflammatory bowel disease: pathogen or innocent bystander? Inflamm Bowel Dis. 2010;16(9):1620-7. doi: 10.1002/ibd.21275. [ Links ]
21. Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34(8):1094-7. doi: 10.1086/339329. [ Links ]
22. Huston CD. Intestinal protozoa. En: Feldman M, Friedman LS, Brandt LJ (editores). Sleisenger and Fordtrans’s gastrointestinal and liver disease. Filadelfia: Elsevier Saunders ; 2010. pp. 1914-8. [ Links ]
23. Ma P. Cryptosporidiosis and immune enteropathy: a review. Curr Clin Top Infect Dis. 1987;8:99-153. [ Links ]
24. Rossignol JF. Nitazoxanide in the treatment of acquired immune deficiency syndrome-related cryptosporidiosis: results of the United States compassionate use program in 365 patients. Aliment Pharmacol Ther 2006;24(5):887-94. doi: 10.1111/j.1365-2036.2006.03033.x. [ Links ]
25. Silva CV, Ferreira MS, Borges AS, et al. Intestinal parasitic infections in HIV/AIDS patients: experience at a teaching hospital in central Brazil. Scand J Infect Dis. 2005;37(3):211-5. doi: 10.1080/00365540410020875. [ Links ]
26. Concha R, Harrington W Jr, Rogers AI. Intestinal strongyloidiasis: recognition, management, and determinants of outcome. J Clin Gastroenterol. 2005;39(3):203-11. doi: 10.1097/01.mcg.0000152779.68900.33. [ Links ]
27. Segarra-Newnham M. Manifestations, diagnosis, and treatment of Strongyloides stercoralis infection. Ann Pharmacother. 2007;41(12):1992-2001. doi: 10.1345/aph.1K302. [ Links ]
28. Casotti JA, Motta TQ, Ferreira CU Jr, et al. Disseminated histoplasmosis in HIV positive patients in Espirito Santo state, Brazil: a clinical-laboratory study of 12 cases (1999-2001). Braz J Infect Dis. 2006;10(5):327-30. doi: 10.1590/S1413-86702006000500005. [ Links ]
29. Assi M, McKinsey DS, Driks MR, et al. Gastrointestinal histoplasmosis in the acquired immunodeficiency syndrome: report of 18 cases and literature review. Diagn Microbiol Infect Dis. 2006;55(3):195-201. doi: 10.1016/j.diagmicrobio.2006.01.015. [ Links ]
30. Kartalija M, Sande MA. Diarrhea and AIDS in the era of highly active antiretroviral therapy. Clin Infect Dis 1999;28(4):701-5. doi: 10.1086/515191. [ Links ]
31. Cello JP, Day LW. Idiopathic AIDS enteropathy and treatment of gastrointestinal opportunistic pathogens. Gastroenterology. 2009;136(6):1952-65. doi: 10.1053/j.gastro.2008.12.073. [ Links ]
32. O’Brien ME, Clark RA, Besch CL, et al. Patterns and correlates of discontinuation of the initial HAART regimen in an urban outpatient cohort. J Acquir Immune Defic Syndr. 2003;34(4):407-14. doi: 10.1097/00126334-200312010-00008. [ Links ]
33. Wu X, Sun L, Zha W, et al. HIV protease inhibitors induce endoplasmic reticulum stress and disrupt barrier integrity in intestinal epithelial cells. Gastroenterology. 2010;138(1):197-209. doi: 10.1053/j.gastro.2009.08.054. [ Links ]
34. Macarthur RD. Management of noninfectious diarrhea associated with HIV and highly active antiretroviral therapy. Am J Manag Care. 2013;19(12 Suppl):s238-45. [ Links ]
35. National Institute of Allergy and Infectious Diseases. Division of AIDS table for grading the severity of adult and pediatric adverse events. US Department of Health and Human Services [internet] 2014 [acceso el 11 de noviembre de 2017]. Disponible en: Disponible en: http://rsc.tech-res.com/docs/default-source/safety/daids_ae_grading_table_v2_nov2014.pdf . [ Links ]
36. Public Health England. UK Standards for Microbiology: investigation of specimens other than blood for parasites. NHS [internet] 2017 [acceso el 11 de noviembre de 2017]. Disponible en: Disponible en: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/622944/B_31i5.1.pdf . [ Links ]
37. American Society for Gastrointestinal Endoscopy. The role of endoscopy in the management of patients with diarrhea. Gastrointest Endosc. 2010;71(6):887-92. doi: 10.1016/j.gie.2009.11.025. [ Links ]
38. Cohen J, West AB, Bini EJ. Infectious diarrhea in human immunodeficiency virus. Gastroenterol Clin North Am. 2001;30(3):637-64. doi: 10.1016/S0889-8553(05)70203-X. [ Links ]
39. Olmos MA, Losso M, Ruvinsky S, et al. Decision analysis: diagnostic approach in HIV infection associated chronic diarrea. Acta Gastroenterol Latinoam. 2005;35(3):155-61. [ Links ]
40. Wilcox M, Schwartz D, Cotsonis G, et al. Chronic unexplained diarrhea in human immunodeficiency virus infection: determination of the best diagnostic approach. Gastroenterology. 1996;110(1):30-7. doi: 10.1053/gast.1996.v110.pm8536874. [ Links ]
41. Kearney DJ, Steuerwald M, Koch J, et al. A prospective study of endoscopy in HIV -associated diarrea. Am J Gastroenterol. 1999;94(3):596-602. doi: 10.1111/j.1572-0241.1999.00920.x. [ Links ]
42. Wei SC, Hung CC, Chen MY, et al. Endoscopy in acquired immunodeficiency syndrome patients with diarrhea and negative stool studies. Gastrointest Endosc. 2000;51(4 Pt 1):427. doi: 10.1016/S0016-5107(00)70443-3. [ Links ]
43. Orenstein JM, Dieterich DT. The histopathology of 103 consecutive colonoscopy biopsies from 82 symptomatic patients with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 2001;125(8):1042-6. doi: 10.1043/0003-9985(2001)125<1042:THOCCB>2.0.CO;2. [ Links ]
44. Bini EJ, Cohen J. Diagnostic yield and cost-effectiveness of endoscopy in chronic human immunodeficiency virus-related diarrea. Gastrointest Endosc. 1998;48(4):354-61. doi: 10.1016/S0016-5107(98)70003-3. [ Links ]
45. Oette M, Stelzer A, Göbels K, et al. Wireless capsule endoscopy for the detection of small bowel Diseases in HIV-1-infected patients. Eur J Med Res. 2009;14(5):191-4. [ Links ]
46. Burrill J, Williams CJ, Bain G, et al. Tuberculosis: a radiologic review. Radiographics. 2007;27(5):1255-73. doi: 10.1148/rg.275065176. [ Links ]
47. Restrepo CS, Martinez S, Lemos JA, et al. Imaging manifestations of Kaposi sarcoma. Radiographics. 2006;26(4):1169-85. doi: 10.1148/rg.264055129. [ Links ]
48. Ghai S, Pattison J, O’Malley ME, et al. Primary gastrointestinal lymphoma: spectrum of imaging findings with pathologic correlation. Radiographics. 2007;27(5):1371-88. doi: 10.1148/rg.275065151. [ Links ]
49. Hill A, Balkin A. Risk factors for gastrointestinal adverse events in HIV treated and untreated patients. AIDS Rev. 2009;11(1):30-8. [ Links ]
50. Clay PG, Crutchley RD. Noninfectious diarrhea in HIV seropositive individuals: a review of prevalence rates, etiology, and management in the era of combination antiretroviral therapy. Infect Dis Ther. 2014;3(2):103-22. doi: 10.1007/s40121-014-0047-5. [ Links ]
51. Mangili A, Murman DH, Zampini AM, et al. Nutrition and HIV infection: review of weight loss and wasting in the era of highly active antirretroviral therapy from the nutrition for healthy living cohort. Clin Infect Dis. 2006;42(6):836-42. doi: 10.1086/500398. [ Links ]
52. Anastasi JK, Capili B, Kim AG, et al. Symptom management of HIV related diarrhea by using normal foods: a randomized controlled clinical trial. J Assoc Nurses AIDS Care. 2006;17(2):47-57. doi: 10.1016/j.jana.2006.01.005. [ Links ]
53. Huffman FG, Walgren ME. L-glutamine supplementation improves nelfinavir-associated diarrhea in HIV-infected individuals. HIV Clin Trials. 2003;4(5):324-9. doi: 10.1310/BFDT-J2GH-27L7-905G. [ Links ]
54. Anukam KC, Osazuwa EO, Osadolor HB, et al. Yogurt containing probiotic Lactobacillus rhamnosus GR-1 and L. reuteri RC-14 helps resolve moderate diarrhea and increases CD4 count in HIV/ AIDS patients. J Clin Gastroenterol. 2008;42(3):239-43. doi: 10.1097/MCG.0b013e31802c7465. [ Links ]
55. Salminen MK, Tynkkynen S, Rautelin H, et al. The efficacy and safety of probiotic Lactobacillus rhamnosus GG on prolonged, noninfectious diarrhea in HIV patients on antiretroviral therapy: a randomized, placebo-controlled, crossover study. HIV Clin Trials. 2004;5(4):183-91. doi: 10.1310/6F83-N39Q-9PPP-LMVV. [ Links ]
56. Hummelen R, Changalucha J, Butamanya NL, et al. Effect of 25 weeks probiotic supplementation on immune function of HIV patients. Gut Microbes. 2011;2(2):80-5. doi: 10.4161/gmic.2.2.15787. [ Links ]
57. Nwachukwu CE, Okebe JU. Antimotility agents for chronic diarrhoea in people with HIV/AIDS. Cochrane Database Syst Rev. 2008;(4):CD005644. doi: 10.1002/14651858.CD005644.pub2. [ Links ]
58. Sherman DS, Fish DN. Management of protease inhibitor-associated diarrhea. Clin Infect Dis. 2000;30(6):908-14. doi: 10.1086/313826. [ Links ]
59. Cello JP, Grendell JH, Basuk P, et al. Effect of octreotide on refractory AIDS-associated diarrhea. A prospective, multicenter clinical trial. Ann Intern Med. 1991;115(9):705-10. doi: 10.7326/0003-4819-115-9-705. [ Links ]
60. Beaugerie L, Baumer P, Chaussade S, et al. Treatment of refractory diarrhoea in AIDS with acetorphan and octreotide: a randomized crossover study. Eur J Gastroenterol Hepatol. 1996;8(5):485-9. [ Links ]
61. Castro JG, Chin-Beckford N. Crofelemer for the symptomatic relief of non-infectious diarrhea in adult patients with HIV/AIDS on anti-retroviral therapy. Expert Rev Clin Pharmacol. 2015;8(6):683-90. doi: 10.1586/17512433.2015.1082424. [ Links ]
62. Leonard C, Chordia P, MacArthur RD. Profile of crofelemer for the symptomatic treatment of diarrhea in HIV-infected persons. Botanics Target Ther. 2015;5:21-5. doi: 10.2147/BTAT.S42267. [ Links ]
63. Crutchley RD, Miller J, Garey KW. Crofelemer, a novel agent for treatment of secretory diarrhea. Ann Pharmacother. 2010;44(5):878-84. doi: 10.1345/aph.1M658. [ Links ]
64. Macarthur RD, Hawkins TN, Brown SJ, et al. Efficacy and safety of crofelemer for noninfectious diarrhea in HIV-seropositive individuals (ADVENT trial): a randomized, double-blind, placebo-controlled, two-stage study. HIV Clin Trials. 2013;14(6):261-73. doi: 10.1310/hct1406-261. [ Links ]
65. Hawkins T, MacArthur RD, Brown SJ, et al. Safety and tolerability of crofelemer 125 mg twice daily in the treatment of noninfectious diarrhea in HIV-seropositive patients on antiretroviral therapy: results from a Phase 3, 48-week open-label study. Meeting of the Infectious Diseases Society of America. 2013. [ Links ]
Received: December 14, 2017; Accepted: April 13, 2018











 texto en
texto en