Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.33 no.2 Bogotá Apr./June 2018
https://doi.org/10.22516/25007440.147
Case report
Transgastric endoscopic drainage of pancreatic pseudocyst in a pediatric patient
1Cirujano pediátrico Clínica Fundación Oftalmológica de Santander/Clínica Ardila Lulle (FOSCAL), Hospital universitario de Santander (HUS), jefe de cirugía pediátrica. Floridablanca, Colombia.
2Médico gastroenterólogo Clínica FOSCAL, FOSCAL Internacional. Floridablanca, Colombia.
3Médico internista, gastroenterólogo, Clínica FOSCAL, FOSCAL Internacional. Floridablanca, Colombia.
4Médico general, Clínica FOSCAL. Floridablanca, Colombia.
A pancreatic pseudocyst is an accumulation of fluid that is almost always sterile and is rich in digestive enzymes and pancreatic juice that is encapsulated in a wall of fibrous tissue and granulation tissue without an epithelial lining. They are generally oval or rounded. Pseudocysts can develop from complications in the pancreas that lead to obstruction or rupture of a pancreatic duct. We present the case of a 9-year-old male patient diagnosed with a pancreatic pseudocyst with progressive growth due to closed abdominal trauma. Multidisciplinary management determined treatment. Due to the characteristics of the pseudocyst, transgastric endoscopic drainage was used, and the procedure was carried out as described herein. The patient evolved satisfactorily.
Keywords: Pancreatic pseudocyst; treatment of pancreatic pseudocyst; endoscopic drainage of pancreatic pseudocyst; pancreatic trauma; cystogastrostomy
Un pseudoquiste pancreático es una acumulación de líquido casi siempre estéril, rico en enzimas digestivas y jugo pancreático encapsulado en una pared de tejido fibroso y de granulación sin revestimiento epitelial, generalmente de forma ovalada o redondeada. Los pseudoquistes se pueden desarrollar por complicaciones en el páncreas que generan obstrucción o ruptura de un conducto pancreático. Se presenta el caso de un paciente masculino de 9 años con diagnóstico de pseudoquiste pancreático con crecimiento progresivo, debido a trauma abdominal cerrado. Se realizó un manejo multidisciplinario para determinar el tratamiento. Por las características del pseudoquiste, se definió realizar un drenaje endoscópico transgástrico. El procedimiento llevado a cabo es descrito en el presente texto. El paciente evolucionó satisfactoriamente.
Palabras clave: Pseudoquiste pancreático; tratamiento de pseudoquiste pancreático; drenaje endoscópico de pseudoquiste pancreático; trauma pancreático; cistogastrostomía
Introduction
According to the Atlanta classification, a pancreatic pseudocyst is defined as a collection of fluid encapsulated within a well-defined inflammatory wall that does not contain solid elements. It is observed after a period of more than four weeks.1,2
Unlike a true cyst, a pseudocyst has no epithelial lining, 3 and the wall that encapsulates it is formed by fibrous and granulation tissue.4,5,6,7,8 A pancreatic pseudocyst usually has an oval or rounded shape formed by an accumulation of fluid which is almost always sterile, hypocellular, and rich in digestive enzymes and pancreatic juice.3,7,9
A pancreatic pseudocyst may develop due to complications in the pancreas and may be secondary to acute or chronic pancreatitis, chronic alcohol intake, or obstruction of the pancreatic duct due to a tumor or pancreatic trauma.3,5,9,10. Seventy to seventy-eight percent of cases are related to chronic pancreatitis of alcoholic origin, between 6% and 18% are related to idiopathic biliary pancreatitis, and between 5% and 10% are related to pancreatic trauma. 7 However, pancreatic pseudocysts in children are mainly due to accidental or non-accidental trauma (54% of cases). 11
A pseudocyst is formed as an inflammatory response when there is an obstruction or rupture of a pancreatic duct.5,7 They can also appear due to liquefaction of an area of necrosis and encapsulated secretions. 10 Symptoms associated with pancreatic pseudocysts include abdominal pain (75% -90%), nausea, early satiety and vomiting (50% -70%), weight loss (20% -50%), persistent fever (10%), jaundice (10%), and palpable masses (25% -45%). They may cause compression of adjacent organs. 5,6,7,9
Classification of pancreatic pseudocysts facilitates treatment. From the beginning, they can be classified by their location as either intrapancreatic or extrapancreatic. The latter are usually found near the pancreatic gland. 10 According to their origin, they can be classified as acute or chronic. A pseudocyst is acute when it is secondary to acute pancreatitis or pancreatic trauma. It is chronic when it results from chronic pancreatitis. 12 Between 40% and 75% of pseudocysts are acute. Of these, 40% resolve spontaneously. Spontaneous resolution occurs infrequently in cases of chronic pseudocysts (3%). 12
The literature proposes seven classifications of pseudocysts according to the clinical picture, anatomical findings, deviations in the main pancreatic duct, and whether or not there is communication with the pseudocyst. The classification was done by Nealon and Walser (Table 1). 4,12)
Pseudocyst complications include infections, ruptures, bleeding, and intestinal and biliary obstructions. 6 Consequently, it is important to know the characteristics and typology of pseudocyst in order to make a good diagnosis and select the appropriate treatment. 11. The type of treatment is defined by symptoms, location, type of pseudocyst, size and whether or not it communicates with the main pancreatic duct. 11 Pancreatic pseudocysts should be drained only if they persist for more than 4 to 6 weeks, are larger than 6 cm, and cause symptoms or complications such as obstruction of the gastric outlet or biliary obstruction. 13
Diagnosis of a pseudocyst is based on symptoms and imaging results.5,6,11 Imaging techniques that can be used included abdominal computed tomography scans (CT scans), endoscopic ultrasonography (EUS) and magnetic resonance imaging (MRI). 10,14 Thoracic-abdominal CT scans are the most sensitive (90% -95%) with highly reliable diagnoses, followed by abdominal ultrasound with a sensitivity of 75%. 8
Pancreatic pseudocysts can be managed surgically or non-surgically, and surgical treatment can be laparoscopic or conventional. Non-surgical treatment can be performed by percutaneous drainage guided by ultrasound or CT or can be drained endoscopically.8,13 If the pseudocyst communicates with the pancreatic duct, it can be treated by endoscopy.
In general, endoscopic drainage is a technique that is performed with a lateral view endoscope and can be made done through the papilla, duodenum or stomach. Transduodenal or transgastric drainage is performed when the pseudocyst protrudes from the wall which allows observation through endoscopy. Transpapillary drainage is performed in cases where the pseudocyst communicates with the pancreatic duct. Transgastric drainage is called a cystogastrostomy, transduodenal drainage is a cystoduodenostomy, and drainage of the jejunum is a Roux-en-Y cystojejunostomy. 8,12
Case report
The patient was a 9-year-old boy who had fallen from a bicycle and suffered a closed abdominal trauma caused by the impact of the handlebar a month and a half prior to diagnosis and treatment. The patient developed severe abdominal pain, emesis and local ecchymosis, so the hospital nearest to Guaca in Málaga was consulted from which he was referred to the University Hospital of Santander.
Abdominal ultrasound documented acute traumatic pancreatitis. Evaluation by pediatricians and pediatric surgeons led to a decision to use medical treatment. Clinical evolution was slow due to persistence of pain and vomiting. Patient was given nasojejunal enteral nutrition starting the fourth week of trauma. A pancreatic pseudocyst of more than 4 cm, progressively growing as the result of systemic inflammatory response, was diagnosed sonographically. An upper digestive tract endoscopy found obvious bulging in the posterior region of the gastric corpus and antrum. A medical meeting of pediatrics, pediatric surgery, interventional radiology and gastrointestinal surgery decided that the patient was a candidate for transgastric endoscopic drainage.
Discussion
The most frequent symptoms of pseudocysts, regardless of their origin, are pain, nausea, fever, weight loss, palpable masses and jaundice. The patient presented here had abdominal pain, a palpable mass and a slight fever. On admission to hospital, laboratory tests showed 702 U/L amylase, 35 U/L aspartate transaminase (AST), and 22 U/L alanine transaminase (ALT). The results for amylase indicated pancreatic compromise. Abdominal ultrasound showed the pancreatic body had grown to 6.5 cm in diameter and with a volume of 25 mL with heterogeneous echogenicity. Several days later, a new ultrasound showed that the volume of the pseudocyst on the pancreatic body had reached 230 mL and a diameter of 8.7 cm.
Monitoring of a pseudocyst allows determination of whether it will be reabsorbed or whether drainage is required. Drainage of a pseudocyst is recommended when symptoms persist, when it grows, and when it becomes complicated. After another gastroenterological assessment, it was decided to drain the pseudocyst because of its continued growth.
Pseudocysts can be drained surgically, percutaneously or endoscopically. In this case, drainage was endoscopic. It has a success rate of approximately 86% to 100%. A systematic review that compared the drainage of the pancreatic pseudocyst by endoscopic, percutaneous and surgical routes concluded that endoscopic and surgical drainage are equally effective, but that endoscopic drainage requires shorter hospital stays and lower costs and results in better patient quality of life. Surgical or percutaneous drainage should be considered in patients who have anatomical reasons for avoiding endoscopy. 15
The choice among transpapillary, transduodenal or transgastric drainage depends on the characteristics of the pseudocyst and its location.1,2,3 A joint medical decision was made to use transgastric drainage which can be done when the lesion can be seen endoscopically. 10
Drainage procedure:
Interventional radiology was requested for sonographic guidance of the procedure.
The patient was evaluated and found to be hemodynamically stable and in good general condition. The procedure began by means of upper digestive endoscopy with a lateral view endoscope. The bulge in the posterior face of the antrum and gastric corpus was identified, with fluoroscopic help.
The bulge in the gastric wall was punctured with a cystotome and the endoscope was advanced with a 0.33 hydrophilic guide to the pseudocyst (Figure 1). 10
The cystotome was removed and a hydrostatic balloon was used for a dilation to 9 mm and communication was opened between the pseudocyst and the gastric corpus.
The dilation balloon was removed and a completely covered self-expanding 10 mm x 80 mm self-expanding metal biliary stent was placed. Its distal crown remained in the pancreatic pseudocyst, but the body of the stent passed through the gastric wall and the proximal cup in the lumen of the stomach (Figure 2).
One hundred milliliters of purulent material was drained without bleeding from the wall, without pneumoperitoneum and without any other complications.
An endoscopic follow-up on the fourth day after the procedure found a well-positioned, functioning stent. Pain and fever disappeared, oral feeding was resumed and tolerated and inflammation decreased (Figure 3).
An ultrasonic follow-up two months after the procedure found that the pseudocyst had completely disappeared.
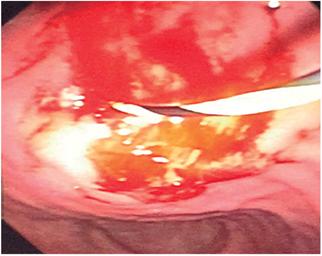
Figure 1 Passage of the hydrophilic guide into the pancreatic pseudocyst before cutting the gastric wall with the cystotome.
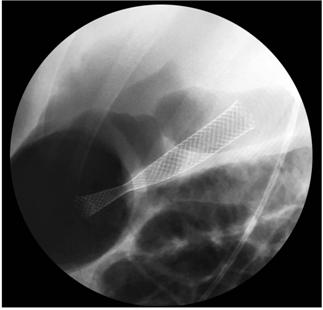
Figure 2 Fluoroscopic image. Fully covered metallic biliary stent draining the pancreatic pseudocyst into the stomach.
Conclusion
A fully covered biliary stent can be used to maintain the fistula created between the pseudocyst and the stomach open. The stent is easy to remove and unlikely to migrate.
Appropriate diagnosis and multidisciplinary management allowed successful minimally invasive treatment of this pseudocyst due to trauma in a 9-year-old patient.
Referencias
1. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis-2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102-11. doi: 10.1136/gutjnl-2012-302779. [ Links ]
2. Rückert F, Lietzmann A, Wilhelm TJ, et al. Long-term results after endoscopic drainage of pancreatic pseudocysts: A single-center experience. Pancreatology. 2017;17(4):555-60. doi: 10.1016/j.pan.2017.06.002. [ Links ]
3. Pereda Rodríguez J, González Llorente J. Pseudoquiste pancreático gigante. Rev Argent Radiol. 2011;75(3):197-202. [ Links ]
4. Guardado Bermúdez F, Azuara Turribiates A, Ardisson Zamora FJ, et al. Pseudoquiste pancreático. Revisión y reporte de caso. Cir Cir. 2014;82(4):425-31. [ Links ]
5. Gabrielli M, Paz C, Troncoso P, et al. Manejo endoscópico del pseudoquiste pancreático. Cuad Cir (Valdivia). 2007;21(1):38-42. doi: 10.4206/cuad.cir.2007.v21n1-06. [ Links ]
6. Pérez Torres E, Bernal Sahagún F, García Guerrero VA, et al. Diagnóstico y tratamiento de los pseudoquistes del páncreas en el Servicio de Gastroenterología del Hospital General de México. Rev Med Hosp Gen Mex. 2005;68(2):76-81. [ Links ]
7. Cruz Salinas MA, Manjarrez Cuenca JA, González Acosta MA, et al. Drenaje abierto de pseudoquiste pancreático. Revista de Especialidades Médico-Quirúrgicas. 2011;16(4):256-9. [ Links ]
8. Alaéz AB, Ramiro C, Calero A, et al. Pseudoquiste de páncreas con extensión a mediastino. Rev Chil Cir. 2011;63(3):297-300. 10.4067/S0718-40262011000300010. [ Links ]
9. Crisanto Camposa BA, Rojano-Rodríguez ME, Cárdenas Lailsonc LE, et al. Drenaje laparoscópico de un seudoquiste pancreático: reporte de caso. Rev Gastroenterol Mex. 2012;77(3):148-52 doi: 10.1016/j.rgmx.2012.04.008. [ Links ]
10. Súbtil Iñigo JC. Manejo por ecoendoscopia de las colecciones del área pancreática en la patología inflamatoria del páncreas. Revista Española de Ecografía Digestiva. 2006;8(Supl 1):1-9. [ Links ]
11. Vaca C, Harris P, Barriga F, et al. Pancreatitis aguda grave y pseudoquiste pancreático por uso de drogas en niños: Presentación de tres casos clínicos y revisión de la literatura. Rev Chil Pediatr. 2001;72(3):235-43. doi: 10.4067/S0370-41062001000300009. [ Links ]
12. Correa Burciaga G, Garza G, Yañez Leija A. Cistogastroanastomosis por laparoscopía: manejo del pseudoquiste pancreático. Cir Gen. 2012;34(4):280-5. [ Links ]
13. Muniraj T, Jamidar PA, Nealon WH, et al. Endoscopic management of pancreatic fluid collections. J Clin Gastroenterol. 2017;51(1):19-33. doi: 10.1097/MCG.0000000000000644. [ Links ]
14. Brizuela Quintanilla R, Ruiz Torres J, Martínez López R, et al. Resultados del tratamiento endoscópico para los pseudoquistes del páncreas. Análisis de 73 pacientes. Endoscopia. 2012;24(1):7-12. [ Links ]
15. Nabi Z, Basha J, Reddy DN. Endoscopic management of pancreatic fluid collections-revisited. World J Gastroenterol. 2017;23(15):2660-72. doi: 10.3748/wjg.v23.i15.2660. [ Links ]
Received: May 24, 2017; Accepted: April 13, 2018











 text in
text in