Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.33 no.2 Bogotá abr./jun. 2018
https://doi.org/10.22516/25007440.256
Case report
Megacolon toxic of idiophatic origin: case report
1Cirujano general, Clínica Belo Horizonte, Clínica Medilaser. Neiva, Colombia.
2Residente de cirugía general III año Universidad Surcolombiana, Hospital Universitario Hernando Moncaleano Perdomo. Epidemiólogo. Neiva, Colombia.
3Médico general, Universidad Surcolombiana. Neiva, Colombia.
Toxic megacolon is a pathology whose mortality rate is over 80%. A progressive inflammatory process compromises the colon wall, and secondary dilation of the intestinal lumen occurs due to inflammatory or infectious processes. Its clinical presentation is bizarre. but the basic pillars for management are opportune diagnosis and adequate medical management with antibiotics, water resuscitation, and metabolic correction. If necessary, effective surgical management can prevent the development of complications that worsen the disease and the prognosis of a patient. In this article we present the case of a patient who died after developing septic shock secondary to toxic megacolon. Cholangitis grade III was suspected, but discarded after ultrasonography, and this resulted in generated distortions in approach and initial management. Due to clinical deterioration and abdominal distension, the patient underwent diagnostic laparoscopy which revealed severe ischemic compromise of the entire colon but without involvement of the small intestine. For this reason, a total colectomy was performed. The pathology report and clinical history ruled out ulcerative colitis or Crohn’s disease which confirmed the diagnosis of toxic megacolon. The patient had no risk factors for the development of pseudomembranous colitis. We conclude that this was a case of idiopathic toxic megacolon.
Keywords: Toxic megacolon; infectious colitis; acute abdomen; colitis; cholangitis; sepsis
El megacolon tóxico (MT) es una patología con una tasa de mortalidad superior al 80% desencadenada por un proceso inflamatorio progresivo que compromete la pared del colon con dilatación secundaria de la luz intestinal debido a procesos inflamatorios o infecciosos. Su presentación clínica es infrecuente y los pilares básicos en su manejo son un diagnóstico oportuno, un manejo médico adecuado (antibiótico, reanimación hídrica y corrección metabólica) y, de ser necesario, un manejo quirúrgico eficaz que evite al máximo las complicaciones que empeoran el pronóstico de los pacientes. En este artículo se presenta un caso de una paciente con choque séptico secundario a MT, con desenlace fatal y con sospecha de un cuadro de colangitis grado III descartado por ecografía, lo cual generó distorsiones en su enfoque y manejo inicial. Por deterioro clínico y distensión abdominal, la paciente se llevó a laparoscopia diagnóstica en la que se evidenció un compromiso isquémico severo de todo el colon sin compromiso de intestino delgado, razón por la que se le realizó una colectomía total. El reporte de patología y la historia clínica descartan colitis ulcerativa o enfermedad de Crohn, lo que confirmó el MT. La paciente no presentaba factores de riesgo para el desarrollo de colitis pseudomembranosa. Se concluyó que fue la presentación de un caso de MT idiopático.
Palabras clave: Megacolon tóxico; colitis infecciosa; abdomen agudo; colitis; colangitis; sepsis
Introduction
Toxic megacolon (TM) was first described in 1950 as a complication due to Clostridium difficile infection characterized by progressive inflammation that compromises all four layers of the colon and is associated with dilation of more than six cm of a segment or the entire circumference of the opening. 1,2 Mortality rates range from 19% to 80%, and incidence varies depending on cause. For ulcerative colitis, the incidence ranges from 2.5% to 17% while for pseudomembranous colitis it ranges from 0.4% to 3%. Incidence has increased value due to indiscriminate use of antibiotics.3,4,5
The classic etiology of TM is ulcerative colitis, but Crohn’s disease has gradually taken its place since 1950 when it was discovered to predispose patients to TM.6 Other etiologies have also been identified. They include Shigella, salmonella, entamoeba, campylobacter, ischemic colitis, cytomegalovirus (CMV) and Kaposi’s sarcoma in immunosuppressed patients 2,6.
Risk factors for development of TM in patients with infectious colitis include discontinuation of steroid treatment, use of barium enemas and drugs that reduce colon motility such as narcotics, antidiarrheal agents and anticholinergic agents. Since clinical presentation occurs very infrequently, the clinical criteria elaborated in 1969 continue to be accepted. The key indicators are a fever over 38.6 ° C (101.5 ° F), heart rate over 120 beats per minute, leukocytes over 10.5/μL, and anemia indicated by hemoglobin less than 7 g/dL. TM can also be associated with any of the following: dehydration, hypotension, electrolyte disturbances and changes in mental state. 7
The pillars of TM management are rehydration, correction of electrolytes, administration of blood products, management of immunosuppressive therapy and timely antibiotic treatment. 3,8,9. The absolute indications for surgery include signs of organ failure, shock, uncontrollable low gastrointestinal bleeding, evidence of perforation, acute abdomen and progressive colonic dilation after 24 to 72 hours of medical treatment. 10,11,12
We present the case of a patient with sepsis of abdominal origin. The initial focus was typical cholangitis, so diagnostic laparoscopy was performed due to clinical deterioration. Macroscopic evidence of TM led to total colectomy plus ileostomy. This diagnosis was subsequently confirmed by the pathology report. On this occasion, no clear etiology was found, so this episode of TM was classified as of possible idiopathic origin.
Clinical case
The patient was a 54-year-old woman with a history of hypertension, hypothyroidism and morbid obesity (body mass index [BMI]: 50.2). Her conditions were being managed with verapamil, levothyroxine, acetylsalicylic acid and atorvastatin. She was admitted to a level one emergency department after four days of fever, right hemisphere abdominal pain radiating to the ipsilateral lumbar region, asthenia, adynamia and tremors. The first level paraclinical tests found thrombocytopenia, so they remitted her to a more advanced level hospital due to their suspicion of arbovirus.
She was admitted to the emergency department in poor condition with rapid respiration, dehydration, fever and somnolence. She subsequently developed respiratory insufficiency and was rapidly intubed without complications. She was transferred to the intensive care unit (ICU), and additional paraclinical tests found thrombocytopenia (70,000), metabolic acidosis with hyperlactatemia and kidney injury Acute Kidney Injury Network (AKIN) level III kidney damage. It was considered that the patient was suffering from septic shock and possible pyelonephritis, so treatment with antibiotics using piperacillin tazobactam was combined with resuscitation by goals, inotropic support, management of comorbidities and microbiological tracking.
Her evolution during her hospital stay was torpid. She presented oligoanuria, distal hypoperfusion, borderline blood pressure and jaundice in the sclera. Folow up tests showed that her leukocytosis had increased and also indicated neutrophilia, thrombocytopenia, moderate metabolic acidosis with an elevated anion gap, normal transaminases and amylase, renal function deterioration, and direct hyperbilirubinemia with an obstructive pattern. Her chest X-rays were normal. Hepatobiliary ultrasound was requested due to a suspicion of type III cholangitis secondary to obstruction of the biliary tract, and antibiotic management was staggered with ertapenem. Due to deterioration of renal function, she was evaluated in the nephrology service which initiated renal replacement therapy with hemodialysis. The results of requested blood cultures reported findings of multi-resistant Escherichia coli. A urine culture was negative (Table 1).
Table 1 Principal paraclinical test results during hospital stay
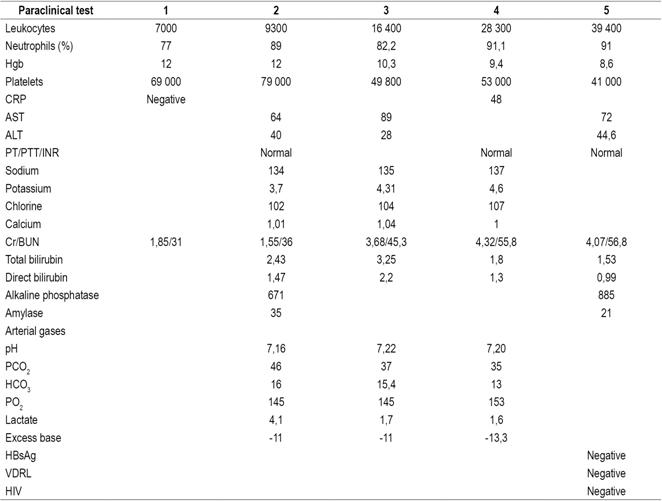
BUN: blood urea nitrogen; Cr: creatinine; HBsAg: hepatitis B surface antigen; HCO3: bicarbonate; INR: international normalized index; PCO2: partial carbon dioxide pressure; CRP: C-reactive protein; PO2: partial oxygen pressure; PT: prothrombin time; PTT: partial thromboplastin time; AST: Aspartate transaminase; ALT: alanine transaminase; VDRL: Venereal Disease Research Laboratory test; HIV: human immunodeficiency virus.
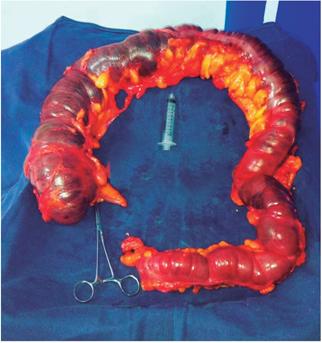
Since the ultrasound report did not show a vesicular lithiasis or dilation of the bile duct, a septic process of biliary origin was ruled out. Subsequently, abdominal distension became evident in the patient. The general surgery service performed diagnostic laparoscopy and found evident amounts of free fluid in the abdominal cavity, predominantly from a right parietal colic leak associated with marked distention of the entire colon but with no evidence of mechanical obstruction. The procedure was converted into exploratory laparotomy due to findings of TM (Figure 1) and a total colectomy plus ileostomy was performed. In addition, patient was treated with ceftriaxone, oral vancomycin and metronidazole for c. difficile, salmonella, Shigella and campylobacter.
Pathology report
The pathology report for the colon and a segment of the terminal ileum showed TM with severe luminal dilation of the cecum to the sigmoid measuring 22 cm in circumference and 107 cm in length. It also showed flattening of the mucosa and peripheral layers of the wall without inflammation of the mucosa or wall, suggestive of ulcerative colitis or Crohn’s disease.
During the postoperative period, the patient improved clinically in terms of leukocytosis, increased urinary output, decreased Cr and a better pattern of arterial blood gases. Vasopressor support was decreased. On the other hand, no signs of infection were observed in the surgical wound. On the eighth day following surgery the patient had no need for respiratory support and was hemodynamically stable. Sudden onset of dyspnea was observed with evidence of cardiorespiratory arrest. The blue code for advanced resuscitation maneuvers was activated. After 20 minutes without success, the patient succumbed with pulmonary thromboembolism as a possible cause of death.
Discussion
TM is a lethal disease whose general incidence is difficult to determine given current available literature. Nevertheless, it has been determined that its incidence is closely related to its cause. According to some studies, ulcerative colitis is six times as likely to lead to the development of TM than is Crohn’s disease. 3 One study that included 1,236 hospitalized patients has shown an incidence of 10% for these two pathologies. 4
At present, the incidence of TM secondary to pseudomembranous colitis is considered to be approximately 0.4% to 3% of cases. This rate has been increasing in the last decade due to the deliberate use of antibiotics which has caused adaptive genetic changes microorganisms resulting in higher levels of virulence and the consequent emergence of strains resistant to conventional treatments. These strains include BI/NAP1/027. 13,14,15
Although the pathophysiological mechanism of this disease has not yet been fully elucidated, some studies show severe and progressive infiltration of the neutrophil-mediated inflammatory response that manages to compromise the mucosa through the smooth muscle layer to the serosa. As inflammation progresses, neutrophils invade the muscle layer and cause additional damage by releasing proteolytic enzymes, cytokines, and leukotriene B4 (LTB4) resulting in dysmotility and consequent secondary dilation of the colon (Figure 2). 1 In addition, infectious agents such as pseudomembranous colitis and toxins A and B of C. difficile can interrupt the epithelial barrier and cause epithelial cell necrosis and electrophysiological changes in the colonic mucosa resulting in marked inflammation of the colon 8.
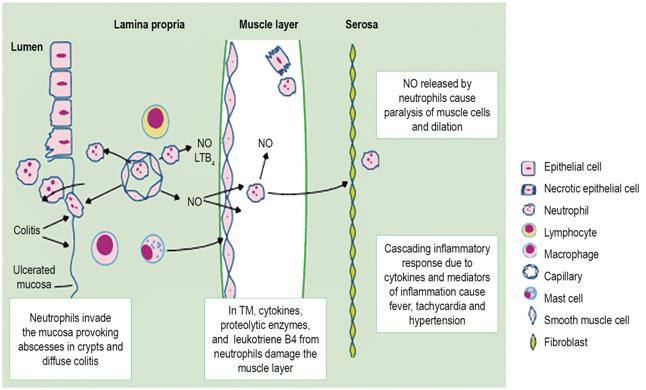
Figure 2 Neutrophil invasion through colon walls. LTB4: leukotriene B4; NO: nitric oxide. Taken from: Sheth SG et al. Lancet. 1998; 351 (9101): 509-13.
Commonly, these patients come to emergency services after suffering bloody diarrhea for more than a week. This is often associated with chills, fevers, abdominal pain, and intermittent colic. The onset of TM is inconsistent and can manifest as abdominal distension, diarrhea, constipation, decreased bowel sounds and systemic symptoms such as fever, tachycardia and hypotension. Symptoms may be masked by high doses of corticosteroids or an altered level of consciousness. (1,3,7 Diagnosis of TM is based on identification of the clinical picture and clinical criteria described by Jalan in 1969. 16 These criteria are associated with systemic toxicity and radiological and/or ultrasound evidence of dilation of the colon of more than six cm. 7,17
A simple abdominal x-ray can identify dilation of the colon up to 15 cm while ultrasound can identify dilation greater than six cm. 17 On the other hand, computerized axial tomography (CT) is useful for determining the causes of abdominal complications. 7 Laboratory results indicating possible TM include leukocytosis or leukopenia associated with neutrophilia and anemia, electrolyte alterations, and alterations of renal, hepatic and/or pulmonary functioning. Patients with leukocytosis of over 40,000 have been described as having poor prognoses. 9 It is necessary to take blood cultures to rule out bacteremia since septicemia occurs in up to 25% of patients with TM. 3 Stool samples should be sent for culturing, and assayed for C. difficile toxins A and B for patients with histories of antibiotic use or chemotherapy. Parasite infections should be considered in patients with HIV. 1,8
The central components of management include rehydration, correction of hydroelectrolytic disorder, colonic decompression (if possible), administration of antibiotics and pertinent consultation with the general surgery service. Anemia, dehydration and electrolyte deficits, particularly hypokalemia, aggravate dysmotility of the colon and must be treated aggressively. 18 The literature recommends administration of broad spectrum antibiotics such as ampicillin, sulbactam or third-generation cephalosporin together with an aminoglycoside or metronidazole. 9,19
The mainstay of medical management of patients with TM caused by ulcerative colitis is high doses of intravenous (IV) steroids. Most authors recommend a daily dose of 400 mg of hydrocortisone (100 mg every 6 hours) or 60 mg of IV methylprednisolone (1 mg/kg) for 5 days. If this management is not effective, rescue therapy with cyclosporine may be considered. 8,19
For cases of TM caused by pseudomembranous colitis, predisposing antibiotics should be identified and removed. The most common antibiotics associated with C. difficile are clindamycin, cephalosporins, and fluoroquinolones. 20 Vancomycin associated with metronidazole should be administered as first-line therapy in accordance with current guidelines issued by the Society for Healthcare Epidemiology of America and the Infectious Disease Society of America. In cases in which resistant C. difficile strains NAP1/ BI/027 are suspected or confirmed in cases of TM, Fidaxomicin or a macrolide are the antibiotics of choice. 18,21
Timely medical treatment reduces the need for surgery by 50%, 1 but surgical intervention may be necessary in up to 80% of patients, mainly in patients with TM secondary to C. difficile. The surgical treatment of choice for a large number of surgeons is subtotal colectomy plus a mucosal fistula and ileostomy because the rate of morbidity and mortality is less than those of total proctocolectomies. It is very important to have a priority assessment done by the general surgery service since complications and intestinal perforations cause very high percentages of mortality (from 8% to approximately 40%). 1
Morbidity and mortality rates for TM patients are high, and patients who survive an episode of TM after responding to medical treatment also have poor prognoses of six to twelve months survival. Recurrence rates are over 18%, and recurrence may require colectomy. Among patients with ulcerative colitis who initially respond to medical therapy, 60% will require a colectomy in the following 12 months and 80% will require a colectomy within 5 years of the first event. 8
In relation to the case presented here, there is very little literature regarding TM cases masked by suspected acute cholangitis. 22. It is important to emphasize that the patient had poor prognostic factors including age over forty, hypoalbuminemia, renal failure and hyperlactatemia. These factors increased her risk of mortality exponentially. In addition, no risk factors for pseudomembranous colitis were identified. After the surgical intervention, the patient presented clear clinical improvement, but a secondary entity, possibly pulmonary thromboembolism, caused death.
Conclusions
TM is a well-recognized and highly lethal complication of acute colitis. Physicians should expect an increase in the incidence of TM due to the increasing number of acute colitis cases associated with the use of broad spectrum antibiotics and co-infections of strains of hypervirulent C. difficile resistant to conventional therapies.
It is essential that patients with TM be diagnosed quickly and correctly, and managed comprehensively to reduce morbidity and mortality. Emergency physicians can minimize excessive delays in diagnosis by suspecting and ruling out this entity in all patients with abdominal distension, acute or chronic diarrhea, and signs of systemic inflammatory response and, thus, improve the prognoses of these patients.
Referencias
1. Sheth SG, LaMont JT. Toxic megacolon. Lancet. 1998;351(9101):509-13. doi: 10.1016/S0140-6736(97)10475-5. [ Links ]
2. Kwok M, Maurice A, Lisec C, et al. Campylobacter colitis: Rare cause of toxic megacolon. Intern J Surg Case Rep. 2016;27:141-3. doi: 10.1016/j.ijscr.2016.08.030. [ Links ]
3. Gan SI, Beck PL. A new look at toxic megacolon: an update and review of incidence, etiology, pathogenesis, and management. Am J Gastroenterol. 2003;98(11):2363-71. doi: 10.1111/j.1572-0241.2003.07696.x. [ Links ]
4. Greenstein AJ, Sachar DB, Gibas A, et al. Outcome of toxic dilatation in ulcerative and Crohn’s colitis. J Clin Gastroenterol. 1985;7(2):137-43. doi: 10.1097/00004836-198504000-00007. [ Links ]
5. Sayedy L, Kothari D, Richards RJ. Toxic megacolon associated Clostridium difficile colitis. World J Gastrointest Endosc. 2010;2(8):293-7. doi: 10.4253/wjge.v2.i8.293. [ Links ]
6. Meyers MA, Alonso DR, Morson BC, et al. Pathogenesis of diverticulitis complicating granulomatous colitis. Gastroenterology. 1978;74(1):24-31. [ Links ]
7. Earhart MM. The identification and treatment of toxic megacolon secondary to pseudomembranous colitis. Dimens Critical Care Nurs. 2008;27(6):249-54. doi: 10.1097/01.DCC.0000338869.70035.2b. [ Links ]
8. Halaweish I, Alam HB. surgical management of severe colitis in the intensive care unit. J Intensive Care Med. 2015;30(8):451-61. doi: 10.1177/0885066614534941. [ Links ]
9. Levine CD. Toxic megacolon: diagnosis and treatment challenges. AACN Clin Issues. 1999;10(4):492-9. doi: 10.1097/00044067-199911000-00008. [ Links ]
10. Fornaro R, Caratto M, Barbruni G, et al. Surgical and medical treatment in patients with acute severe ulcerative colitis. J Dig Dis. 2015;16(10):558-67. doi: 10.1111/1751-2980.12278. [ Links ]
11. Tapani MJ, Olavi KH. Surgical management of toxic megacolon. Hepatogastroenterology. 2014;61(131):638-41. [ Links ]
12. Teeuwen PH, Stommel MW, Bremers AJ, et al. Colectomy in patients with acute colitis: a systematic review. J Gastrointest Surg. 2009;13(4):676-86. doi: 10.1007/s11605-008-0792-4. [ Links ]
13. Dobson G, Hickey C, Trinder J. Clostridium difficile colitis causing toxic megacolon, severe sepsis and multiple organ dysfunction syndrome. Intensive Care Med. 2003;29(6):1030. doi: 10.1007/s00134-003-1754-7. [ Links ]
14. Synnott K, Mealy K, Merry C, et al. Timing of surgery for fulminating pseudomembranous colitis. Br J Surg. 1998;85(2):229-31. doi: 10.1046/j.1365-2168.1998.00519.x. [ Links ]
15. Zilberberg MD, Shorr AF, Kollef MH. Increase in adult clostridium difficile-related hospitalizations and case-fatality rate, United States, 2000-2005. Emerg Infect Dis. 2008;14(6):929-31. doi: 10.3201/eid1406.071447. [ Links ]
16. Jalan KN, Sircus W, Card WI, et al. An experience of ulcerative colitis. I. Toxic dilation in 55 cases. Gastroenterology. 1969;57(1):68-82. [ Links ]
17. Maconi G, Sampietro GM, Ardizzone S, et al. Ultrasonographic detection of toxic megacolon in inflammatory bowel diseases. Dig Dis Sci. 2004;49(1):138-42. doi: 10.1023/B:DDAS.0000011615.64250.6e. [ Links ]
18. Leifeld L, Kruis W. Current management of toxic megacolon. Z Gastroenterol. 2012;50(3):316-22. doi: 10.1055/s-0031-1299079. [ Links ]
19. Sobrado CW, Sobrado LF. Management of acute severe ulcerative colitis: a clinical update. Arq Bras Cir Dig. 2016;29(3):201-5. doi: 10.1590/0102-6720201600030017. [ Links ]
20. Yu S, Abdelkarim A, Nawras A, et al. Fecal transplant for treatment of toxic megacolon associated with Clostridium difficile colitis in a patient with duchenne muscular dystrophy. Am J Ther. 2016;23(2):e609-13. doi: 10.1097/MJT.0000000000000062. [ Links ]
21. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307(18):1959-69. doi: 10.1001/jama.2012.3507. [ Links ]
22. Juel J, Woyen AV, Vyberg M, et al. Primary manifestation of Chrohn’s disease with toxic megacolon in a patient with long-time primary sclerosing cholangitis. Ugeskr Laeger. 2013;175(35):1965-6. [ Links ]
Received: August 08, 2017; Accepted: April 13, 2018











 texto em
texto em