Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.33 no.3 Bogotá July/Sept. 2018
https://doi.org/10.22516/25007440.204
Originals articles
Experience of a liver transplant center in Medellín, Colombia with liver transplantation for autoimmune hepatitis and characteristics associated with post-transplant recurrence
1Residente de hepatología clínica, Universidad de Antioquia, Medellín, Colombia
2Grupo Gastrohepatología, Universidad de Antioquia. Medellín, Colombia
3Unidad de hepatología y trasplante hepático, Hospital Pablo Tobón Uribe, Medellín, Colombia
4 Unidad de epidemiología, Hospital Pablo Tobón Uribe. Medellín, Colombia
Introduction:
The recurrence of post-transplant autoimmune hepatitis implies risk of cirrhosis and graft loss. Risk factors have been proposed for recurrence, of which few data are known in Latin American patients.
Objectives:
To describe the characteristics of patients with liver transplantation for autoimmune hepatitis and to evaluate those associated with their recurrence during post-transplant.
Methods:
Historical cohort included patients with autoimmune hepatitis diagnosed after the age of 16 years and who were taken to liver transplant in a university hospital in Medellin, Colombia between January 2010 and September 2017. Collection of information from the registers of clinical history.
Results:
25 patients were included. Recurrence was diagnosed in 24%. Median follow-up was 59.5 months and recurrence 32.5 months (range 11-123 months). 100% of the recurrence group were women and none of these were transplanted due to acute liver failure. There were no differences in the pre-transplant and treatment characteristics, although a higher biochemical and histological inflammatory activity was found pre-transplant in the recurrence group. Of the group with recurrence, 100% received long-term glucocorticoids and 33.3% had graft loss related to recurrence requiring retransplantation (p = 0.008).
Conclusion:
The recurrence of autoimmune hepatitis after liver transplantation in our patients is similar to that reported worldwide, is a cause of graft dysfunction to be taken into account especially after the first year post-transplant, it predominates in women. 33.3% of patients require hepatic retransplantation due to graft dysfunction.
Keywords: Autoimmune hepatitis; liver transplant; Latin America; Colombia
Introducción:
la recurrencia de hepatitis autoinmune (HAI) postrasplante implica riesgo de cirrosis y pérdida del injerto. Se han propuesto factores de riesgo para su recurrencia, de los cuales pocos datos se conocen en pacientes latinoamericanos.
Objetivos:
describir las características de los pacientes con trasplante hepático por HAI y evaluar aquellas asociadas con su recurrencia durante el postrasplante.
Métodos:
cohorte histórica, incluyó pacientes con HAI diagnosticada a partir de los 16 años y que fueron llevados a trasplante hepático en un hospital universitario de Medellín, Colombia, entre enero de 2010 y septiembre de 2017. La recolección de la información se realizó a partir de los registros de historia clínica.
Resultados:
se incluyeron 25 pacientes. Se diagnosticó recurrencia en el 24%. La mediana de seguimiento fue de 59,5 meses y de recurrencia 32,5 meses (rango 11-123 meses). El 100% del grupo de recurrencia era de sexo femenino y ninguna de estas se trasplantó por insuficiencia hepática aguda. No hubo diferencias en las características pretrasplante y de tratamiento, aunque se encontró una mayor actividad inflamatoria bioquímica e histológica pretrasplante en el grupo de recurrencia. Del grupo con recurrencia, el 100% recibió glucocorticoides a largo plazo y el 33,3% tuvo pérdida del injerto relacionada con la recurrencia, por lo que requirieron retrasplante (p = 0,008).
Conclusión:
La recurrencia de HAI postrasplante hepático en nuestros pacientes es similar a la reportada mundialmente, es una causa de disfunción del injerto para tener en cuenta especialmente después del primer año postrasplante, predomina en mujeres. El 33,3% de los pacientes requiere retrasplante hepático por disfunción del injerto.
Palabras clave: Hepatitis autoinmune; trasplante de hígado; Latinoamérica; Colombia
Introduction
Since autoimmune hepatitis (AIH) was first described in 1950, it has been understood as a chronic inflammatory liver disease of unknown etiology that causes acute liver failure and liver cirrhosis. It can be an indication for liver transplantation. 1,2
AIH has been described as an indication for liver transplantation in up to 5% of the series published internationally. 3 In Colombia, autoimmune liver diseases are the reasons for 12% of liver transplants in adults. 4 Post-transplant liver survival of patients treated for AIH is good, reaching 90% at one year and 80% at 5 years. 5 Nevertheless, recurrences of AIH occur in 12% to 46% of these patients and lead to cirrhosis and graft loss in up to 50% of the cases. 3,6,7,8,9 When this happens, retransplantation is required, and there is a risk of death.
Exactly which factors are associated with risks of AIH recurrence have not yet been clearly elucidated, but studies have described associations with early suspension of glucocorticoids in the post-transplant period, incompatibility of the donor’s human leukocyte antigen (HLA) system with that of the recipient, post-transplant immunosuppression schemes, and the amounts of biochemical and histological inflammatory activity at the time of transplant. 8,10
The objective of this study is to describe characteristics of patients who underwent liver transplantation to treat AIH at a referral hospital Colombia, evaluate factors associated with recurrences of AIH in the post-transplant period, and describe the post-transplant evolution of these patients.
Materials and methods
Population
This is an observational study of a historical cohort that included patients aged 16 and up who had undergone liver transplantation at the Hospital Pablo Tobón Uribe in Medellín, Colombia, between January 1, 2010 and September 30, 2017, to treat AIH. Diagnoses were made according to the simplified criteria for AIH diagnosis of the International Autoimmune Hepatitis Group (GIHA). 11
Patients without complete clinical, biochemical and histological data, and those who underwent liver transplantation because of overlap syndromes including autoimmune hepatitis and primary biliary cholangitis (AIH-PBC) or autoimmune hepatitis and primary sclerosing cholangitis (AIH-PSC), or other chronic liver diseases were excluded.
Variables
Data was collected through a review of the hospital’s electronic clinical records using a form designed for this purpose.
Demographic, clinical, surgical, serological, radiological, histological and treatment variables were collected for pre-transplant and post-transplant periods.
Recurrence of AIH
Recurrence of AIH was defined by hepatic histology (portal and periportal lymphoplasmacytic infiltrates), biochemistry (elevation of transaminases at least twice the upper limit of normal), and the absence of other causes of liver graft dysfunction.
Statistical analysis
The descriptive analysis of variables uses absolute and relative frequencies. Continuous variables are analyzed through means and standard deviations if they follow a normal or median distribution, and through interquartile ranges (ICR) if they do not follow normal distributions according to the Kolmogorov-Smirnov test. Qualitative variables were compared with Fisher’s exact test and continuous variables were compared with Student’s t test or the Mann-Whitney U test depending on their distributions. Patients were followed up until September 30, 2017 or until the last date that was documented in the clinical history in which case data were considered censored data if any of the measured outcomes were missing. The registered SPSS version 20 of the University of Antioquia was used.
The final manuscript adhered to the STROBE recommendations for reporting observational studies.
Results
A total of 25 patients with AIH had liver transplants between January 1, 2010 and September 30, 2017. Of these, post-transplant AIH recurrences were diagnosed in six (24%) during this period. The incidence of recurrence was 0.59 cases per 100 people/year. The median follow-up time was 59.5 months (IQR: 19.2-94). The most frequent comorbidity was hypothyroidism (7 patients, 22.5%). Patients’ characteristics at the time of diagnosis of AIH and prior to liver transplantation are described in Table 1.
Table 1 Characteristics of AIH patients prior to transplantation
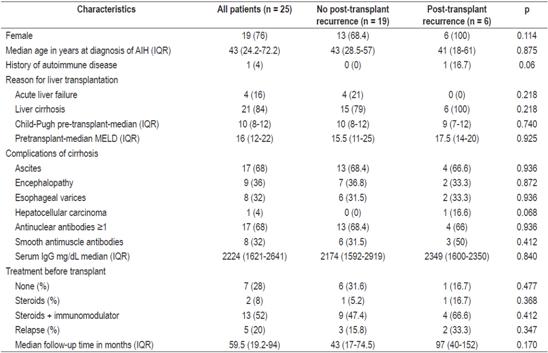
IgG: immunoglobulin G; MELD: model for end-stage liver disease.
Liver cirrhosis and its complications were the principal indication for transplantation in both groups. Sixteen percent of all transplant patients had experienced subacute liver failure. None of them developed post-transplant recurrences of AIH (p = 0.218).
Sixty-eight percent of all patients tested positive for antinuclear antibodies (ANA) while 32% tested positive for anti-smooth muscle antibodies (ASMA). There were no differences in the autoantibody profiles of two groups. Higher levels of pretransplant serum IgG in the AIH recurrence group were not statistically significant (p = 0.840).
The majority of patients in both groups received pharmacological treatment for AIH in the pre-transplant period, most frequently a combination of glucocorticoids and an immunomodulator (azathioprine [AZA] or mycophenolate mofetil [MMF]). In the group without recurrences, six patients (31.6%) did not receive pretransplant pharmacological treatment. Of these, the indication for transplant was acute liver failure in four patients (66.6%) while the other two patients had cirrhosis without inflammatory activity reflected in liver biochemistry.
Frequency of AIH relapses was higher in the post-transplant recurrence group (33.3% versus 15.8%, p = 0.347). The most important cause of relapses was irregular adherence to pharmacological treatment.
Table 2 describes patients’ characteristics at the time of liver transplantation and in the post-transplant period. The median age at the time of liver transplantation and the time between the diagnosis of AIH and transplantation were lower in the post-transplant recurrence group.
Table 2 Characteristics of AIH patients during and after liver transplantation
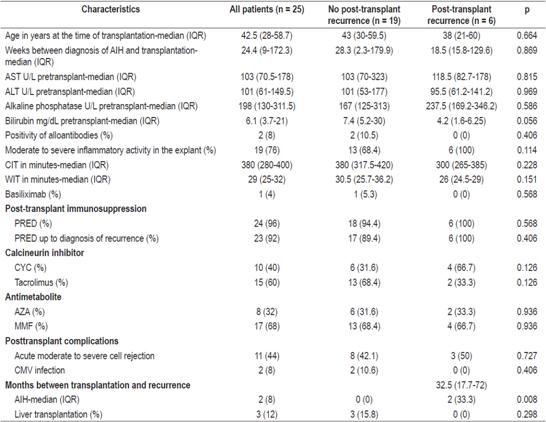
CYC: cyclosporine; CMV: cytomegalovirus; PRED: prednisolone; WIT: warm ischemia time; CIT: cold ischemia time.
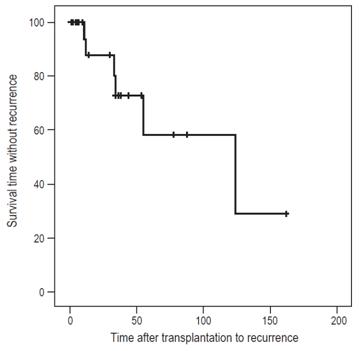
The median post-transplant AIH recurrence time was 32.5 months (IQR: 17.7-72) (Figure 1).
No significant difference between groups was found regarding alloantibodies (specific donor antibodies).
Comparison of the histological findings from the explanted livers from the two groups showed greater inflammatory activity in the patients who had post-transplant recurrences (p = 0.114).
More than 90% of patients received long-term prednisolone treatment during the post-transplant period. At the time of diagnosis of AIH recurrence, 100% of patients were receiving prednisolone.
The most frequently used post-transplant immunosuppression scheme was a combination of an antimetabolite (MMF or AZA) and a calcineurin inhibitor (tacrolimus or CIC). In both groups, the most frequently prescribed antimetabolite was MMF. The post-transplant recurrence group had a greater use of CIC calcineurin inhibitor while non-recurrence group was more likely to have received tacrolimus, but the difference was not statistically significant.
Two patients (33.3%) of the AIH recurrence group required retransplantation (p = 0.008) due to graft dysfunction. The retransplant incidence rate was 0.78 cases per 100 people/year. Both patients were female, had moderate inflammatory activity in the explant, and had histories of acute cellular rejection. The times between the recurrence of AIH and retransplant were 30 months and 113 months.
In total, 3 patients died (all from the group without AIH recurrence) due to infectious complications: two due to septic shock (at 7 and 17 months post-transplant), and one at 61 months due to pulmonary tuberculosis and a CMV infection.
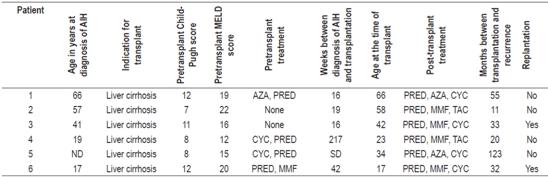
Individual characteristics of patients with post-transplant recurrences of AIH are described in Table 3.
Discussion
AIH is a major cause of liver transplantation in every part of the world, 2 but most data comes from studies of the Caucasian population, and little is known about the course of the disease in Latin American patients. 12 Studies that our group has recently published show that autoimmune liver diseases are the reason for 12% of liver transplants in Colombian adults, 4 and that 10.1% of patients with AIH (including AIH-PBC and AIH-PSC overlap syndromes) required liver transplantation with an incidence of 2.5 transplants per 100 patients/year (95% confidence interval: 1.7-2.7). 12 AIH recurrences were found in 24% of the transplant patients with a recurrence incidence rate of 0.59 cases per 100 people/year. These are similar to the figures reported elsewhere in the world literature. 6,7,8 Although our sample size in this study is too small and limited to find statistically significant differences between groups, there are several issues that deserve to be highlighted.
The median time until recurrence was 32.5 months (range 11-123 months) while the median reported in the literature is 26.4. 13 This suggests that recurrence of AIH should be considered when graft dysfunction occurs, especially after the first year after transplantation.
Pre-transplant characteristics associated with recurrence vary, as reported by Montano-Loza in Canadian patients. 8 In that study, none of the patients who underwent transplantation for acute liver failure had recurrences of AIH. Although no statistically significant association was found, this could be because of the limited number of patients included. In the same study, the pretransplant degree of liver inflammation (reflected by serological markers such as IgG or histology) was significantly associated with recurrence of AIH in the multivariate analysis. The histological hazard ratio (HR) was 6.9 and for moderate to severe inflammation, and the HR for serum IgG levels was 7.5. This suggests that reaching pre-transplant biochemical and histological remission could reduce the recurrence of AIH. In the AIH recurrence group, higher levels of serum IgG and higher inflammatory histological activity were found (100% vs. 68.4%). Both were without statistical significance, possibly due to the small number of patients.
Evaluation of post-transplant characteristics found no association between different regimens of post-transplant immunosuppressive treatment and recurrence of AIH, as described in systematic reviews throughout the world. 13,14 This is in contrast to other pathologies such as primary biliary cholangitis (PBC) for post-transplant recurrences have occurred more frequently with the use of tacrolimus than when cyclosporine was used. 14 Previous studies have shown that indefinite use of low doses glucocorticoids in the post-transplant period reduce the recurrence of AIH by 0% and at one year, but by 11% at 10 years. 15 In our study, despite the fact that 92% of all patients who underwent transplantation because of AIH and 100% of the recurrence group received low doses of prednisolone over the long term, there was a 24% recurrence rate. No analysis of the relation between recurrence of AIH and long-term use prednisolone could be made because indefinite administration of glucocorticoids is a long-established practice in our group for treating these patients.
Recurrence of AIH is an important cause of graft dysfunction, cirrhosis, need for liver retransplantation and death. 9 For these reasons, it is important to identify patients at risk of recurrence and establish actions to reduce these risks during both the pre-transplant and post-transplant periods. 14 Prior to transplantation an effort should be made to achieve biochemical and histological remission, while after transplantation long-term administration of low-dose glucocorticoids should be used. Follow-up liver biopsies are important since histological recurrence has been shown to precede clinical and biochemical recurrence. 16 The difference between requirements for liver retransplantation in the recurrence group, 33.3% of the group, and those of the non-recurrence group, 0%, p = 0.008, was statistically significant. Liver retransplantation, the therapeutic option described in up to 50% of patients who suffer recurrence, 9 is associated with higher costs and higher rates of morbidity. Moreover, it is not always available, especially in regions with low rates of organ donation.
This study suffers from the limitations of a retrospective study and from being done at a single center. The information bias resulting from collecting data from the hospital’s medical records registry and the small sample size are worth noting. Inferences requiring statistical significance could not be made for this reason. another limitation was that HLA was not evaluated in patients even though it is associated with recurrence and worse outcomes in the post-transplant period, especially for patients with HLA-DR3.17,18 In addition, there are no validated criteria for diagnosis of AIH recurrence, and the simplified criteria used for pretransplant diagnosis are not recommended following transplantation. 11 Nevertheless, findings of portal and periportal lymphoplasmacytic infiltrates, elevation of transaminases and serum IgG combined with the absence of other etiologies help confirm a diagnosis of recurrence. 14,15 Our patients met these criteria. Finally, follow-up biopsies were not performed per protocol in the absence of alteration of the hepatic biochemical profile, so the incidence of recurrence may have been underestimated since recurrence of AIH has been described on rare occasions in patients whose transaminase levels are not elevated. 19
We would also like to highlight this study’s strengths. It was conducted at a national referral center for liver diseases and transplantation that has the largest number of patients with AIH not only in Colombia, but in all of Latin America. 12 In addition, long follow-up times (median: 59.5 months) allowed us to document the behavior of the AIH in the adult population from pre-transplant through the post-transplant period.
Conclusions
AIH is an important indication for liver transplantation in Colombia worldwide and elsewhere. Post-transplant recurrence in our population is similar to that reported worldwide. It is a cause of graft dysfunction to be taken into account especially after the first year after transplantation, since 33.3% of patients with recurrence require retransplantation. A multicenter study of the transplant centers in Colombia with a larger number of patients would help determine factors associated with post-transplant AIH recurrence in the Colombian population.
REFERENCES
1. Heneghan MA, Yeoman AD, Verma S, Smith AD, Longhi MS. Autoimmune hepatitis. Lancet. 2013;382(9902):1433-44. doi: 10.1016/S0140-6736(12)62163-1. [ Links ]
2. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol. 2015;63(4):971-1004. doi: 10.1016/j.jhep.2015.06.030. [ Links ]
3. Reich DJ, Fiel I, Guarrera JV, Emre S, Guy SR, Schwartz ME, et al. Liver transplantation for autoimmune hepatitis. Hepatology. 2000;32(4 Pt 1):693-700. doi: 10.1053/jhep.2000.16666. [ Links ]
4. Santos O, Londoño M, Marín J, Muñoz O, Mena Á, Guzmán C, et al. An experience of liver transplantation in Latin America: a medical center in Colombia. Colomb Med (Cali). 2015;46(1):8-13. [ Links ]
5. Mottershead M, Neuberger J. Transplantation in autoimmune liver diseases. World J Gastroenterol. 2008;14(21):3388-95. doi: 10.3748/wjg.14.3388. [ Links ]
6. Liberal R, Zen Y, Mieli-Vergani G, Vergani D. Liver transplantation and autoimmune liver diseases. Liver Transpl. 2013;19(10):1065-77. doi: 10.1002/lt.23704. [ Links ]
7. Czaja AJ. Diagnosis and Management of Autoimmune Hepatitis: Current Status and Future Directions. Gut Liver. 2016;10(2):177-203. doi: 10.5009/gnl15352. [ Links ]
8. Montano-Loza AJ, Mason AL, Ma M, Bastiampillai RJ, Bain VG, Tandon P. Risk factors for recurrence of autoimmune hepatitis after liver transplantation. Liver Transpl. 2009;15(10):1254-61. doi: 10.1002/lt.21796. [ Links ]
9. Ratziu V, Samuel D, Sebagh M, Farges O, Saliba F, Ichai P, et al. Long-term follow-up after liver transplantation for autoimmune hepatitis: evidence of recurrence of primary disease. J Hepatol. 1999;30(1):131-41. doi: 10.1016/S0168-8278(99)80017-8. [ Links ]
10. Czaja AJ. Recurrent autoimmune hepatitis after liver transplantation: a disease continuum or a fresh start? Liver Transpl. 2009;15(10):1169-71. doi: 10.1002/lt.21809. [ Links ]
11. Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48(1):169-76. doi: 10.1002/hep.22322. [ Links ]
12. Díaz-Ramírez GS, Marín-Zuluaga JI, Donado-Gómez JH, Muñoz-Maya O, Santos-Sánchez Ó, Restrepo-Gutiérrez JC. Characterization of patients with autoimmune hepatitis at an university hospital in Medellín-Colombia: cohort study. Gastroenterol Hepatol. 2018;41(2):87-96. doi: 10.1016/j.gastrohep.2017.09.003. [ Links ]
13. Gautam M, Cheruvattath R, Balan V. Recurrence of autoimmune liver disease after liver transplantation: a systematic review. Liver Transpl. 2006;12(12):1813-24. [ Links ]
14. Montano-Loza AJ, Bhanji RA, Wasilenko S, Mason AL. Systematic review: recurrent autoimmune liver diseases after liver transplantation. Aliment Pharmacol Ther. 2017;45(4):485-500. doi: 10.1111/apt.13894. [ Links ]
15. Krishnamoorthy TL, Miezynska-Kurtycz J, Hodson J, Gunson BK, Neuberger J, Milkiewicz P, et al. Longterm corticosteroid use after liver transplantation for autoimmune hepatitis is safe and associated with a lower incidence of recurrent disease. Liver Transpl. 2016;22(1):34-41. doi: 10.1002/lt.24323. [ Links ]
16. Duclos-Vallée JC, Sebagh M, Rifai K, Johanet C, Ballot E, Guettier C, et al. A 10 year follow up study of patients transplanted for autoimmune hepatitis: histological recurrence precedes clinical and biochemical recurrence. Gut. 2003;52(6):893-7. https://doi.org/10.1136/gut.52.6.893. [ Links ]
17. González-Koch A, Czaja AJ, Carpenter HA, Roberts SK, Charlton MR, Porayko MK, et al. Recurrent autoimmune hepatitis after orthotopic liver transplantation. Liver Transpl. 2001;7(4):302-10. doi: 10.1053/jlts.2001.21449. [ Links ]
18. Narumi S, Hakamada K, Sasaki M, Freise CE, Stock PG, Roberts JP, et al. Liver transplantation for autoimmune hepatitis: rejection and recurrence. Transplant Proc. 1999;31(5):1955-6. doi: 10.1016/S0041-1345(99)00227-4. [ Links ]
19. Yao H, Michitaka K, Tokumoto Y, Murata Y, Mashiba T, Abe M, et al. Recurrence of autoimmune hepatitis after liver transplantation without elevation of alanine aminotransferase. World J Gastroenterol. 2007;13(10):1618-21. doi: 10.3748/wjg.v13.i10.1618. [ Links ]
Received: January 30, 2017; Accepted: March 28, 2018











 text in
text in