Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.33 no.3 Bogotá jul./set. 2018
https://doi.org/10.22516/25007440.284
Case report
Clinical case of pancreatic mucinous cystic neoplasms and literature review
1Médico, cirujano general de la Universidad del Rosario, residente de gastroenterólogo clínico quirúrgico de la Universidad de Caldas. Manizales, Colombia
2Médico, cirujano general, gastroenterólogo clínico quirúrgico, coordinador del programa de Gastroenterología Clínico-Quirúrgica, Universidad de Caldas. Manizales, Colombia
3Médico, patólogo anatómico y clínico de la Universidad Nacional de Colombia, docente catedrático de la Universidad de Caldas, Manizales, Colombia
4Médica, cirujana general, gastroenteróloga clínico-quirúrgica de la Universidad de Caldas. Manizales, Colombia
5Médico, cirujano general de la Universidad de Caldas, residente de gastroenterología clínico-quirúrgica de la Universidad de Caldas. Manizales, Colombia
Mucinous cystic neoplasms of the pancreas occur relatively frequently and mainly affect women in the transition to menopause. Most of these neoplasms are unique but are located in the body and tail of the pancreas and have no communication with the pancreatic ductal system. Less than 20% are malignant. Evaluation should include clinical presentation, imaging, endoscopic ultrasonography, puncture biopsies, cytology and chemical analysis of the liquid to measure angiotensin converting enzyme (ACE) levels. Complete surgical resection is the only treatment that can improve long-term survival in patients with malignant mucinous cystic lesions. This article includes a review of the literature related to presentation of a case diagnosed by the surgical clinical gastroenterology group at Clínica la Presentación in Manizales, Colombia.
Keywords: Cystadenoma; cystadenocarcinoma; mucinous cystic tumor of the pancreas
Las neoplasias quísticas mucinosas del páncreas son lesiones relativamente frecuentes, afectan principalmente a mujeres perimenopáusicas; la mayoría son únicos, localizados en el cuerpo y la cola del páncreas, y no tienen comunicación con el sistema ductal pancreático. Menos del 20% de los casos se asocian con malignidad. La evaluación debe incluir la presentación clínica, las imágenes, la utilización de la ultrasonografía endoscópica y toma de biopsias por punción, la citología y el análisis químico del líquido para la medición de niveles de antígeno carcinoembrionario (ACE). La resección quirúrgica completa es el único tratamiento que mejora la supervivencia a largo plazo en pacientes con lesiones quísticas mucinosas malignas. Se realiza una revisión de la literatura a propósito de un caso diagnosticado por el grupo de gastroenterología clínico-quirúrgica de la Clínica la Presentación, Manizales, Colombia.
Palabras clave: Cistoadenoma; cistoadenocarcinoma; tumor quístico mucinoso del páncreas
Introduction
For the most part, pancreatic cysts are found incidentally in images from computerized axial tomography (CAT) or magnetic resonance imaging (MRI). The etiology of these cysts can vary from benign in cases such as pancreatic pseudocysts and serous cystadenomas to premalignant or frankly malignant in cases such as mucinous cystic neoplasia or intraductal papillary mucinous neoplasia. The clinical challenge of 2017 was to classify them and diagnose their malignant potential with precision prior to a decision to carry out surgery, monitor or do nothing. 23
Cystic neoplasms of the pancreas may derive from the ductal epithelium (serous cystic neoplasms [SCNs], mucinous cystic neoplasms [MCNs], intraductal papillary mucinous neoplasms [IPMNs] and intraductal tubular neoplasms [ITNs]), from endocrine cells, from pancreatic acini (cystadenoma and cystadenocarcinoma of acinar cells) and elements of the mesenchyme. Some solid pseudopapillary tumors can form cysts that simulate SCNs or MCNs. 1
The 2010 World Health Organization (WHO) classification of tumors defines the MCN as a cyst-forming epithelial neoplasm that does not communicate with the pancreatic duct and that is composed of columnar epithelium producing mucin and whose stroma is similar stroma to that of the ovary. 2 Although they are less common than SCNs and IPMNs, they are classified as IPMNs depending on the degree of dysplasia (low, intermediate, high and MCN which is associated with invasive carcinoma). 1 The term in-situ carcinoma has been replaced by high-grade dysplasia, and the term invasive carcinoma is now reserved for malignancy. 2
This article describes the clinical case of a patient with MCN and reviews the literature.
Clinical case
The patient was a 73-year-old woman who had been referred from a Level III hospital because of one year of severe diffuse abdominal pain with intermittent emesis that had been resistant to multiple schemes of analgesics. She had no constitutional symptoms or history of pancreatitis nor were there any masses or ascites found in the physical examination. Her blood count, bilirubin levels, transaminases, alkaline phosphatase, amylase, lipase and blood tumor markers (carcinoembryonic antigen [ACE] and carbohydrate antigen [Ca] 19-9) were all normal. An abdominal CT with contrast showed a well-demarcated, round, septated cystic lesion in the body and tail of the pancreas that measured 54 x 40 x 60 mm (Figure 1).
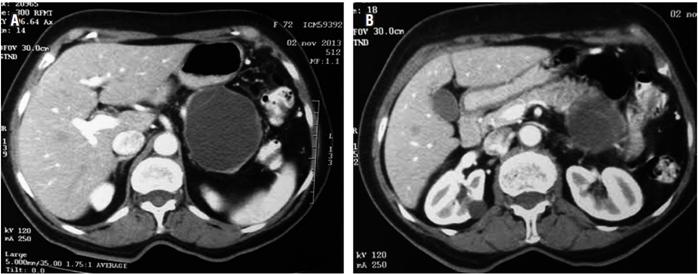
Figure 1A Pancreatic cystic mass in body and tail. 1B. Mass in the tail of the pancreas with septa inside.
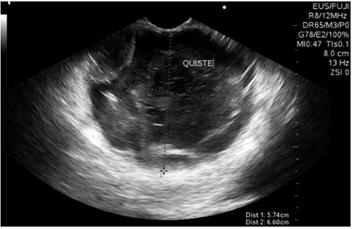
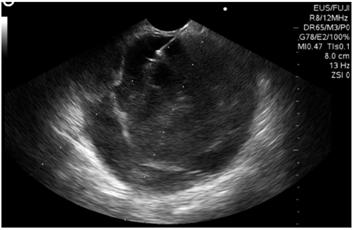
Biliopancreatic endoscopic ultrasonography showed a 60 x 60 mm solid cystic mass in the tail of the pancreas. Its walls were 4 mm thickness, echogenicity was mixed, there was no vascular component or calcifications, but there was a 30 mm polypoid lesion within the cyst (Figure 2). The cyst was punctured with a No. 19 G Wilson Cook needle (Figure 3), and 4 cc of mucoid fluid with multiple white-yellow fragments was obtained for cytology and cell block processing. Hematoxylin-eosin staining showed squamous and mononuclear epithelial cells, necrotic tissue, recent and old hemorrhaging, and a small number of glandular cells without atypia. There was no evidence of pancreatic tissue. No test was done for angiotensin converting enzyme (ACE) because it is not available at the institution.
Taking the patient’s chronic and intractable pain into account together with the findings from the CT scan, EUS and the inconclusive cytology for malignancy, a laparotomy was performed with the intention of removing the pancreas and regional lymph nodes. However, discovery of severe inflammation of the pancreas surrounding a tumor required a splenectomy en bloc. There were no complications or requirement for transfusion of blood products. The patient’s recovery was favorable, and she was discharged on the tenth day without ever developing a pancreatic fistula.
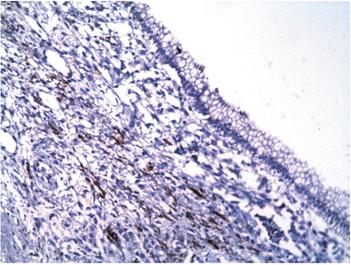
A 12 x 8 x 8 cm cystic lesion composed of a milky material and a 3-inch polypoid lesion were extracted. Microscopically, the cyst wall was lined by columnar epithelial cells with mucinous appearing cytoplasm and nuclei without significant atypia surrounding an ovarian stroma (Figure 4). Regional lymph nodes and pancreatic resection borders were not compromised by the tumor. Immunohistochemical studies showed reactivity in epithelial cells for CA 19-9 and cytokeratin 7 (Figures 5 and 6) as well as reactivity in the underlying stroma for progesterone receptors, smooth muscle actin and desmin (Figures 7, 8 and 9).
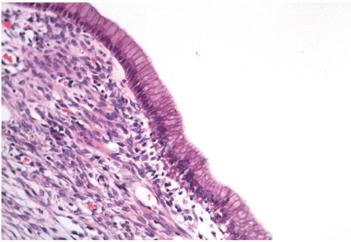
Figure 4 Microscopy (hematoxylin and eosin stain, 200x). Wall of the cyst lined by columnar epithelium surrounded by dense stromal tissue. The skin’s epithelial cells have mucinous appearing cytoplasm and nuclei without significant atypia. The underlying tissue looks similar to the ovarian stroma.
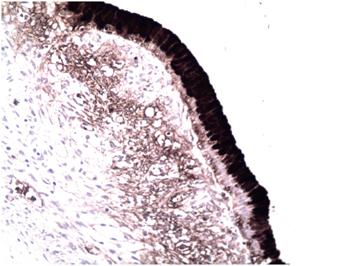
Figure 5 Immunohistochemical micrograph (200x). The epithelial cells that line the lesion show reactivity for CA 19-9.
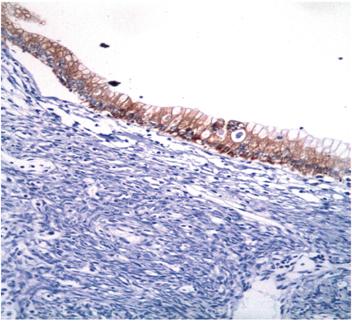
Figure 6 Immunohistochemical microphotography (200x). The epithelial cells that line the lesion show reactivity for cytokeratin 7.
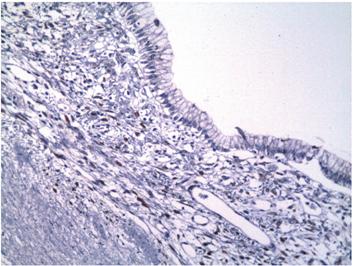
Figure 7 Immunohistochemical microphotography (200x). The stroma underlying the epithelial lining shows reactivity to progesterone receptors.
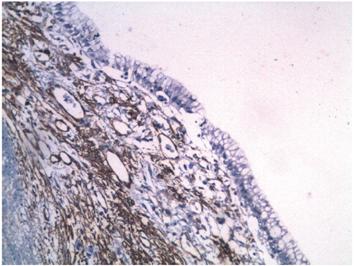
Figure 8 Immunohistochemical microphotography (200x). The stroma underlying the epithelial lining shows reactivity to smooth muscle actin
Discussion
MCNs mainly, but not exclusively, affect women in the fifth decade of life: twenty times as many women are affected as men.3,4 In general, they are single, solitary tumors located in the body and tail of the pancreas, a feature that confers nonspecific clinical presentation. In most cases, they are asymptomatic. They can be unilocular or multilocular, and they usually do not communicate with the pancreatic ductal system unless there are fistulas or erosions. However, a Japanese multi-institutional study has reported communication with the pancreatic duct in 18% of cases. Sizes vary and can reach diameters of up to 35 cm. Laboratory tests are nonspecific.
Macroscopically, MCNs are surrounded by a thick fibrous wall which is sometimes calcified. Their contents are mucinous or a mixture of mucin with blood or aqueous liquid. Microscopically, the cyst is covered by mucin-producing epithelial cells that may be flat, columnar or papillary with intestinal or gastric differentiation. 1 They are positive for epithelial membrane antigen, cytokeratins 7, 8, 18, 19 and CA 19-9. 5 The presence of a stroma similar to the ovary is characteristic and is necessary for establishing a diagnosis of MCN. Stromal cells may be reactive to estrogen receptors, progesterone receptors, vimentin, smooth muscle actin, desmin, calretinin, inhibin α, melan-A, tyrosine hydroxylase, CD 99, and B-cell CLL/lymphoma 2 (Bcl- 2). 1,6
Up to a third of cases harbor invasive carcinoma. Risk factors for malignancy are large lesions associated with nodules or masses, calcifications, walls thicker than 2 cm, septa and advanced age of patient. 1,7,8,9 Mucinous cystadenocarcinoma, the most common, resembles pancreatic ductal adenocarcinoma (ADP). Other types of neoplasms that occur include undifferentiated carcinomas with osteoclast-like giant cells, adenosquamous carcinoma, choriocarcinoma, and high-grade sarcoma. In all cases, several samples must be taken because the invasive component is small and can easily be overlooked.
Any pancreatic cyst that is larger than 1 cm should be characterized by an abdominal CT scan or abdominal MRI with gadolinium. The tomographic appearance of MCN varies, and they can look similar to pseudocysts or to serous cystadenomas. They can be unilocular or multilocular and may present as very large cysts (over 2 cm). Contrast enhances cystic walls which allows identification of solid septa and/or excrescences (Figure 1 B). 1 Liver involvement, peritoneal involvement and local invasion should be ruled out. 10
MRI has higher cyst detection rates (19.9%) than do CAT scans (1.2% -2.6%) plus MRI defines the relationship of the pancreatic duct with the lesion more precisely and can differentiate pseudocysts from IPMNs. MRI also characterizes the content of the cyst in T1 and T2 better which is its main advantage with respect to CT scans. The findings are similar to those of CT scans, but calcifications cannot be seen by this method. 11
Positron emission tomography (PET) with 18F-fluoro-deoxyglucose (18FDG) has a sensitivity of 94% and a specificity of 97% for diagnosis of cystic lesions. However, its role in MCN is not well established because some tumors do not capture (18FDG) leading to false negatives. 1
EUS is the ideal evaluation tool because of its low risks and the fact that biopsy samples can be taken. The criteria for differentiating mucinous cysts (macrocystic septa or adjacent masses) from non-mucinous cysts (unilocular, honeycomb or thickened wall) have a sensitivity of only 56% and a and specificity of only 45%, while diagnostic accuracy is 51%. 12 Superposition of morphological characteristics among the various types of cysts is the reason for this low precision despite adequate visualization of structures. 13
The presence of solid lesions within a cyst, irregularity, thickening of the wall, and/or an adjacent solid mass suggests malignancy. 2 Lack of communication with the pancreatic duct differentiates them from IPMN. 14
EUS-FNA using 19 or 22 gauge needles and a linear echoendoscope from the duodenum or stomach can obtain cytological material in real time in a safe way through opening of the cyst. It is ideal to puncture the cyst in a single step and aspirate the contents until the cyst collapses. This method prevents infectious complications. Biopsy samples of associated nodules, septa, and adjacent masses should be taken. A dose of prophylactic antibiotic should be administered during the procedure and for three to five days afterwards to prevent infection. 13
Cyst size and location are not predictors of successful aspiration, but 1.5 cm is the minimum size required to achieve a success rate of 85%. 15 EUS-FNA facilitates the evaluation of some characteristics in the fluid of the cyst, such as viscosity, cytology, chemistry, tumor markers and molecular analysis techniques.
Viscosity is determined by the “sign of the string” and measurement of relative viscosity. The “sign of the string” is the measurement of the length of the string of fluid created by placing a drop between the index finger and the thumb and measuring it just before it breaks. Strings longer than 3.5 mm are associated with premalignant or malignant lesions. 16 Relative viscosity is measured with a viscometer using water whose relative viscosity is one as the standard measure. Values greater than 1.6 mm allow differentiating mucinous and non-mucinous cystic lesions with a sensitivity of 89% and a specificity of 100%. 17
Cytology is one of the most accurate diagnostic methods, but because it is difficult to obtain a sufficient sample due to the limited volume and low cellularity of the aspirate, sensitivity is less than 50%. Amylase levels may be elevated in lesions that communicate with the pancreatic ductal system, but they are not useful for differentiation of mucinous and non-mucinous cysts. Van der Waaij et al. have found that amylase levels less than 250 U/L are suggestive of SCN, MCN and mucinous cystadenocarcinomas with a sensitivity of 44% and a specificity of 98%. 13
Hammel et al. described the presence of high concentrations of tumor markers in the MCN fluid for the first time. Since then, multiple studies have been published that evaluate the use of ACE, CA 72-4, CA 125, CA 19-9 and CA 15-3 markers as predictors of mucinous and malignant lesions. ACE is the most accurate marker for differentiation of mucinous and non-mucinous cystic lesions. Optimal levels for characterizing these lesions vary greatly according to the series reviewed. In general, it is accepted that values greater than or equal to 192 ng/mL have a precision of 79%, sensitivity of 73% and specificity of 84%. 12 Other tumor markers have been evaluated in cystic lesions but have not shown satisfactory results. In molecular analysis, KRAS mutations, increased DNA levels, and the loss of two or more loci in the alleles are abnormalities found in mucinous neoplasms. KRAS mutation has the highest specificity (96%), but its sensitivity is only 45%. The greatest advantage of this diagnostic method is that only 0.2 mL of liquid is needed to perform the analysis.
The data obtained from a puncture sample should be analyzed with caution because lineability, precision, accuracy and stability have not been validated. ACE analysis has not been standardized, so some laboratories use undiluted samples while others use diluted or mixed samples. Approximately 0.5 to 1 mL of cystic fluid is needed for analysis of ACE. The liquid is diluted in saline solution, stirred vertically to achieve homogeneity, and ideally is laboratory processed undiluted to obtain more consistent and reliable measurements. (13 Despite the fact that ACE analysis is the most frequently used molecular test for diagnosing pancreatic cystic neoplasms, interpretation is difficult without the help of additional clinical information. Measurements depend on the series evaluated, as well as the superposition of the ACE results found in different types of cysts: more than 192 ng/mL in MCN and less than 5 ng/mL in SCN. 1 Despite this, a low level of ACE does not exclude the presence of a mucinous cyst. 18
The complication rate of EUS-FNA is approximately 2.2% and most of these are early complications. Hemorrhaging and infections occur in less than 1% of cases. 13
In 2010, the International Pancreatology Association recommended resection of all MCNs because of the low specificity of diagnostic tests and biopsy samples, the risk of malignant transformation (6% to 27%) based on the strict presence of the ovarian stroma, inability to differentiate non-invasive lesions from invasive lesions, and the good results of complete surgical resections. 1 The association also recommended that laparoscopic resection, parenchymal-sparing resection, and distal pancreatectomy with base preservation should be considered for lesions less than 4 cm without mural nodules. 18
Surgical options include conservative pancreatic resections (central pancreatectomy and enucleation), regional pancreatectomy (pancreatoduodenectomy and distal pancreatectomy), and total pancreatectomy. The choice of open or laparoscopic approach and the choice of procedure depend on the location of the lesion, whether the lesion is multifocal, the patient’s condition, quality of life (exocrine and endocrine function), the specific morbidity and mortality rates for each intervention, and the surgeon’s experience.
Segmental pancreatic resections include resection of the uncinate process, resection of the head with preservation of the duodenum, pancreatectomy directed by the ductal branch, and central or medial pancreatectomy. 1 Results depend on the experience and the learning curve, but rates of postoperative complications are high, and complications include fistulas in 15% to 50% of cases. 19 Regional resections include lymphadenectomies whose 30 day morbidity rate varies between 25% and 60%. Its mortality rate is between 2% and 5%. In the case of distal pancreatectomy, the fistula rate ranges between 5% and 20%. 20
EUS guided ablation of cystic pancreatic epithelium by injection of cytotoxic agents such as ethanol or paclitaxel shows promising early results although long-term follow-up data are required to evaluate efficacy and determine accurate indications for use of this therapy.2,21,22
Complete surgical resection is the only treatment that improves long-term survival in patients with malignant NQP. 21. Two year and five year survival rates for surgical resection of MCN with associated invasive carcinoma are 67% and 50%, respectively. 2
Curative resection of non-invasive MCN does not need any type of postoperative surveillance while follow-up for surgical resection of invasive MCNs must be imaging (CT/MRI) every 6 months and must not be based on recurrence of symptoms. 18
Conclusions
Our knowledge of the natural history of the MCN is incomplete, so management is constantly evolving. The presence of mucin is the most important predictor of mucinous neoplasms, followed by ACE levels over 192-200 ng/mL. The evaluation of mucinous lesions should include clinical factors, imaging, biopsy (EUS-FNA), measurement of ACE levels and cytological evaluation. EUS is the ideal evaluation tool and complete surgical resection is the only treatment that improves long-term survival.
REFERENCES
1. Roggin KK, Chennat J, Oto A, Noffsinger A, Briggs A, Matthews JB. Pancreatic cystic neoplasm. Curr Probl Surg. 2010;47(6):459-510. doi: 10.1067/j.cpsurg.2010.02.002. [ Links ]
2. Yoon WJ, Brugge WR. Pancreatic cystic neoplasms: diagnosis and management. Gastroenterol Clin North Am. 2012;41(1):103-18. doi: 10.1016/j.gtc.2011.12.016. [ Links ]
3. Suzuki M, Fujita N, Onodera H, Kayaba Y, Suzuki S, Kagaya H, et al. Mucinous cystic neoplasm in a young male patient. J Gastroenterol. 2005;40(11):1070-4. doi: 10.1007/s00535-005-1697-6. [ Links ]
4. Kobayashi T, Teruya M, Shimizu S, Miki K, Kobayashi K, Morita K. Mucinous cystic tumor of the pancreas in a man: a rare case. Pancreas. 2006;33(3):312-4. doi: 10.1097/01.mpa.0000229012.47291.8d. [ Links ]
5. Lüttges J, Feyerabend B, Buchelt T, Pacena M, Klöppel G. The mucin profile of noninvasive and invasive mucinous cystic neoplasms of the pancreas. Am J Surg Pathol. 2002;26(4):466-71. doi: 10.1097/00000478-200204000-00008. [ Links ]
6. Yeh MM, Tang LH, Wang S, Robert ME, Zheng W, Jain D. Inhibin expression in ovarian-type stroma in mucinous cystic neoplasms of the pancreas. Appl Immunohistochem Mol Morphol. 2004;12(2):148-52. doi: 10.1097/00129039-200406000-00009. [ Links ]
7. Crippa S, Salvia R, Warshaw AL, Domínguez I, Bassi C, Falconi M, et al. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg. 2008;247(4):571-9. doi: 10.1097/SLA.0b013e31811f4449. [ Links ]
8. Goh BK, Tan YM, Chung YF, Chow PK, Cheow PC, Wong WK, et al. A review of mucinous cystic neoplasms of the pancreas defined by ovarian-type stroma: clinicopathological features of 344 patients. World J Surg. 2006;30(12):2236-45. doi: 10.1007/s00268-006-0126-1. [ Links ]
9. Sarr MG, Carpenter HA, Prabhakar LP, Orchard TF, Hughes S, van Heerden JA, et al. Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas: can one reliably differentiate benign from malignant (or premalignant) neoplasms? Ann Surg. 2000;231(2):205-12. doi: 10.1097/00000658-200002000-00009. [ Links ]
10. Procacci C, Carbognin G, Accordini S, Biasiutti C, Guarise A, Lombardo F, et al. CT features of malignant mucinous cystic tumors of the pancreas. Eur Radiol. 2001;11(9):1626-30. doi: 10.1007/s003300100855. [ Links ]
11. Minami M, Itai Y, Ohtomo K, Yoshida H, Yoshikawa K, Iio M. Cystic neoplasms of the pancreas: comparison of MR imaging with CT. Radiology. 1989;171(1):53-6. doi: 10.1148/radiology.171.1.2928546. [ Links ]
12. Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126(5):1330-6. doi: 10.1053/j.gastro.2004.02.013. [ Links ]
13. Samarasena JB, Nakai Y, Chang KJ. Endoscopic ultrasonography-guided fine-needle aspiration of pancreatic cystic lesions: a practical approach to diagnosis and management. Gastrointest Endosc Clin N Am. 2012;22(2):169-85, vii. doi: 10.1016/j.giec.2012.04.007. [ Links ]
14. Hutchins GF, Draganov PV. Cystic neoplasms of the pancreas: a diagnostic challenge. World J Gastroenterol. 2009;15(1):48-54. doi: 10.3748/wjg.15.48. [ Links ]
15. Walsh RM, Zuccaro G, Dumot JA, Vargo J, Biscotti CV, Hammel J, et al. Predicting success of endoscopic aspiration for suspected pancreatic cystic neoplasms. JOP. 2008;9(5):612-7. [ Links ]
16. Leung KK, Ross WA, Evans D, Fleming J, Lin E, Tamm EP, et al. Pancreatic cystic neoplasm: the role of cyst morphology, cyst fluid analysis, and expectant management. Ann Surg Oncol. 2009;16(10):2818-24. doi: 10.1245/s10434-009-0502-9. [ Links ]
17. Lewandrowski KB, Southern JF, Pins MR, Compton CC, Warshaw AL. Cyst fluid analysis in the differential diagnosis of pancreatic cysts. A comparison of pseudocysts, serous cystadenomas, mucinous cystic neoplasms, and mucinous cystadenocarcinoma. Ann Surg. 1993;217(1):41-7. doi: 10.1097/00000658-199301000-00008. [ Links ]
18. Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12(3):183-97. doi: 10.1016/j.pan.2012.04.004. [ Links ]
19. Assalia A, Gagner M. Laparoscopic pancreatic surgery for islet cell tumors of the pancreas. World J Surg. 2004;28(12):1239-47. doi: 10.1007/s00268-004-7617-8. [ Links ]
20. Kooby DA, Gillespie T, Bentrem D, Nakeeb A, Schmidt MC, Merchant NB, et al. Left-sided pancreatectomy: a multicenter comparison of laparoscopic and open approaches. Ann Surg. 2008;248(3):438-46. doi: 10.1097/SLA.0b013e318185a990. [ Links ]
21. Oh HC, Seo DW, Lee TY, Kim JY, Lee SS, Lee SK, et al. New treatment for cystic tumors of the pancreas: EUS-guided ethanol lavage with paclitaxel injection. Gastrointest Endosc. 2008;67(4):636-42. doi: 10.1016/j.gie.2007.09.038. [ Links ]
22. Sharma RR, London MJ, Magenta LL, Posner MC, Roggin KK. Preemptive surgery for premalignant foregut lesions. J Gastrointest Surg. 2009;13(10):1874-87. doi: 10.1007/s11605-009-0935-2. [ Links ]
23. Farrell JJ. Pancreatic cysts and guidelines. Dig Dis Sci. 2017;62(7):1827-39. doi: 10.1007/s10620-017-4571-5. [ Links ]
Received: August 22, 2017; Accepted: October 30, 2017











 texto em
texto em