Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.33 no.3 Bogotá jul./set. 2018
https://doi.org/10.22516/25007440.167
Case report
A case report of a neuroendocrine tumor in the small intestine with manifest dark digestive bleeding
1Medicina Interna, Gastroenterología, Hospital “Pablo Tobón Uribe”, Medellín, Colombia. Correo electrónico: gami8203@yahoo.com
2Médico, Hospital “Pablo Tobón Uribe”. Universidad de Antioquia, Medellín, Colombia. Correo electrónico: juanes1027@gmail.com
We present the case of a young patient with no history of illness who developed obvious bleeding in the form of melena, hematochezia, and severe anemia. Initial endoscopic studies were normal, but video capsule endoscopy (VCE) revealed an ulcerated subepithelial lesion in the jejunum leading to suspicion of GIST or a neuroendocrine tumor. It was marked in enteroscopy, extension studies showed that it was normal. Therefore, local resection was scheduled by laparoscopy, making diagnosis and management of a neuroendocrine tumor.
Keywords: Hemorrhage; small intestine; neoplasms of the jejunum; neuroendocrine tumor
Presentamos el caso de un paciente joven, sin historia de enfermedad, quien debutó con sangrado digestivo manifiesto por melenas, hematoquecia y anemia severa. Los estudios endoscópicos iniciales fueron normales. En el estudio del intestino delgado con videocápsula endoscópica (VCE) se evidenció una lesión subepitelial ulcerada en el yeyuno, cuyos diagnósticos diferenciales fueron tumor estromal gastrointestinal (GIST) versus tumor neuroendocrino, por lo que se marcó por enteroscopia. Se hicieron estudios de extensión que son normales, por lo que se programó a resección local por laparoscopia haciendo diagnóstico y tratamiento definitivo de un tumor neuroendocrino (TNE).
Palabras clave: Hemorragia; intestino delgado; neoplasias del yeyuno; tumor neuroendocrino
Introduction
In 10% to 20% of patients with gastrointestinal bleeding, the cause cannot be documented in initial endoscopic studies. For the doctor and patient, this may be a manifestation of obscure digestive bleeding if there is evidence of hemorrhaging (melena, hematochezia), or it could be a case of occult digestive bleeding if blood is found in fecal matter or if there is unexplained iron deficiency anemia without clinical evidence of bleeding. 1 Recent development of new diagnostic methods for exploring the small intestine including video capsule endoscopy (VCE), enteroscopy and angiography have made it possible to diagnose most sources of digestive bleeding in this segment. A recently published clinical guideline recommends change the term obscure digestive bleeding to bleeding from the small intestine. 2 It reserves the term obscure digestive bleeding for bleeding that cannot be identified after the entire gastrointestinal tract has been evaluated. 1,2,3 Of all the causes of gastrointestinal bleeding, only a small percentage (5%) is attributed to sources in the small intestine. In young people, the causes vary from tumors such as gastrointestinal stromal tumors (GIST), neuroendocrine tumors, lymphomas and adenocarcinomas, to inflammatory lesions especially due to the use of non-steroidal anti-inflammatory drugs (NSAIDs) and Crohn’s disease. Rarer causes have also been described. They include ulcerated or eroded Meckel’s diverticulum and the Dieulafoy’s lesion. In our environment, we cannot stop thinking about ancylostomiasis as a cause of anemia and obscure bleeding 3.
The initial approach to digestive bleeding from the small intestine should be to use VCE to establish the etiology and clarify the location in order to define the subsequent best approach.
Clinical case
The patient was a 26 year old man with no history of illness. He said that he did not take NSAIDs or smoke, but that he occasionally drank alcoholic beverages. He was admitted with digestive bleeding manifested by melena and hematochezia and had severe anemia. His initial hemoglobin level was 5.8 g/dL which required a transfusion of two units of red blood cells. Initial suspicion of upper gastrointestinal bleeding led to performance of esophagogastroduodenoscopy which was normal and without evidence of bleeding. A total ileocolonoscopy was also normal but showed blood coming from the small intestine. A VCE revealed a subepithelial ulcerated lesion with a visible vessel located in the jejunum at 52% of the transit of the VCE through the small intestine (Figure 1). Because of these findings, antegrade double-balloon enteroscopy was performed, the most distal site was marked, and local resection was requested. Staging studies with computed tomography (CT) of the thorax and contracted abdomen found no additional lesions are identified.
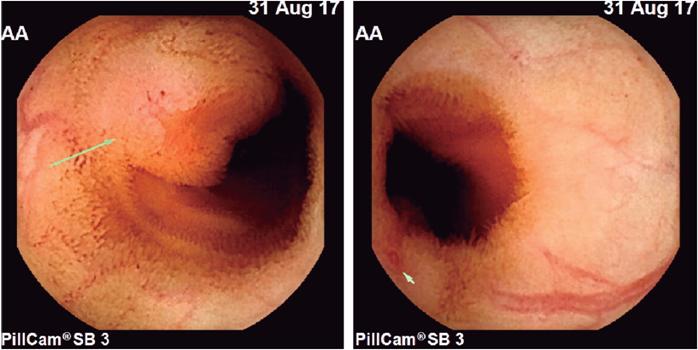
Figure 1 Endoscopic videocapsule shows ulcerated subepithelial lesion with a vessel visible in the jejunum.
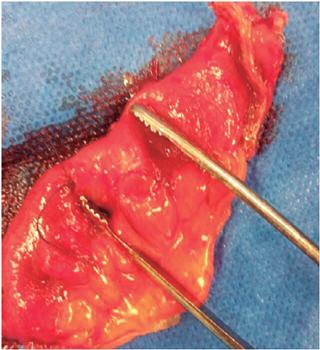
Laparoscopy identified the lesion and local oncological resection removed a 10 cm segment. The patient’s clinical evolution was satisfactory without new episodes of bleeding, and he was discharged two days later. Currently, he is asymptomatic.
Discussion
Obscure digestive bleeding is a great challenge for the clinician. In the case described, we adhere to the current recommendations of the guidelines of the American Society of Gastrointestinal Endoscopy (ASGE) for study of bleeding from the small intestine. 1 For patients with manifest and stable obscure bleeding, the initial approach should be made with VCE in order to determine the location and type of lesion and to determine the most appropriate method and approach. In this case, we documented an ulcerated subepithelial lesion in the jejunum. Bearing in mind that the most common causes of these lesions in this age group are GIST and neuroendocrine tumor, additional studies were carried out to define resectability. Given the absence of additional findings from these negative studies, we decided to perform local laparoscopic resection (Figure 2) after marking with ink using double balloon enteroscopy.
Currently, we have multiple techniques for evaluating the small intestine. These include: VCE, double balloon enteroscopy, enterography by multiphasic CT scan, magnetic resonance enterography (MRE) and, intraoperative enteroscopy which is rare. These modalities can recognize small bowel injuries and can impact therapeutic strategies. Unnecessary surgical intervention can often be avoided, but when necessary, surgical time can be decreased by better identification of the location of the lesion. Despite these advances, the most cost-effective approach for management of patients with suspected small bowel bleeding has not been fully determined. Still, the strongest recommendation at present is to address small bowel bleeding with VCE 1,3,4,5.
In cases of neuroendocrine tumors in the small intestine, a large number of authors propose a multi-image presurgical approach using transverse or functional images to define the extent of the disease. Once the diagnosis of tumor in the small intestine has been made, a CT scan or magnetic resonance imaging (MRI) are most commonly used during the preoperative approach.6,7,8 The value of each diagnostic tool varies. CT scans are usually better at identifying the primary lesion, and MRI seems to be better at evaluating liver metastases. However, up to 15% of lesions may not be evident in these studies, so the extent of abdominal metastases may be underestimated.7,8 CT enterography is a promising new modality for evaluating small bowel bleeding. It uses high-volume neutral oral contrast to distend the small intestine and thus improve evaluation of the wall. In addition, contrast material is administered intravenously, and images are typically acquired 30 seconds after the intravenous bolus in the arterial phase, 50 seconds after the bolus in the enteric phase, and 90 seconds after the bolus in the delayed phase. 9 This technique can detect inflammatory lesions, neoplasms and vascular lesions including angiectasis, varicose veins, Dieulafoy’s lesions, aortoenteric fistulas and pseudoaneurysms. 1,9 Like VCE, CT enterography scans can help clinicians determine whether an antegrade or retrograde approach is more appropriate for enteroscopy. A study published by Hakim et al. has even found higher rates of detection of small bowel tumors with CT enterography than with VCE. 10 CT enterography could an option in cases of digestive bleeding from the small intestine in young patients for whom a neoplastic etiology is considered in the differential diagnosis.
A study of 52 patients with digestive bleeding from the small bowel which could not be diagnosed by VCA, found that CT enterography could diagnose half of the cases. 11. Another study which included 30 patients with negative CT enterography results found that subsequent VCE had a diagnostic yield of 57%. 12 These data support the complementary role of the VCE and the CT enterography when one has negative results, thus adding a second examination to the diagnostic performance.
MRE could be an alternative when there is suspicion of obstruction in the small intestine and for young patients who require periodic monitoring as in the cases of polyposis and Crohn’s disease. Radiation exposure is much less than that in CT enterography although data related to MRE are still limited. 1,13,14 In all cases of obscure digestive bleeding, the individual characteristics of the patients, the resources available, and the experience in the institution with different diagnostic methods should all be taken into account in order to have a more rational and effective approach.
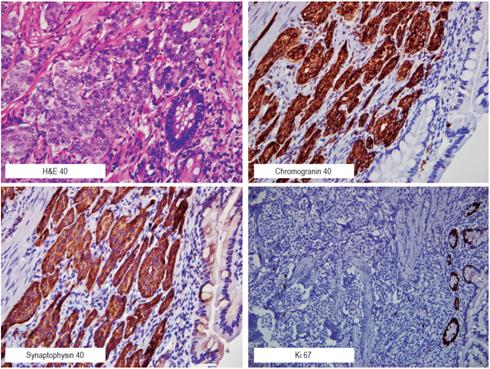
In our case, we defined the surgical treatment due to the suspicion of malignant lesion before we found the subepithelial lesion with a visible vessel in the jejunum. The pathology report showed a 15 x 10 x 5 mm unifocal lesion compatible with a well-differentiated neuroendocrine tumor. The tumor had deeply compromised the subserosa but had not reached the serosa. It had negative margins (markers of synaptophysin, chromogranin, CD56 with strong and diffuse positivity in the neoplastic cell, with Ki-67 proliferation index less than 1%) (Figure 3).
According to the literature, neuroendocrine tumors located in the duodenum or proximal jejunum are the rarest of those in the small intestine. It is estimated that their prevalence is between 5.7% and 7.9%.15,16 All except gangliocytic paragangliomas are considered to be malignant. Most patients with this type of tumor in the small intestine have metastatic disease or multiple lesions when the diagnosis is made. The case we have presented corresponds to grade 1 in the classification of neuroendocrine tumors which has excellent oncological prognosis and low risk of relapse.1,17 We recommend Table 1 where we summarize classifications of neuroendocrine tumors and the recent proposal to classify neuroendocrine carcinoma.9,18,19
Table 1 WHO Classification of Gastroenteropancreatic Neuroendocrine Tumors (9)
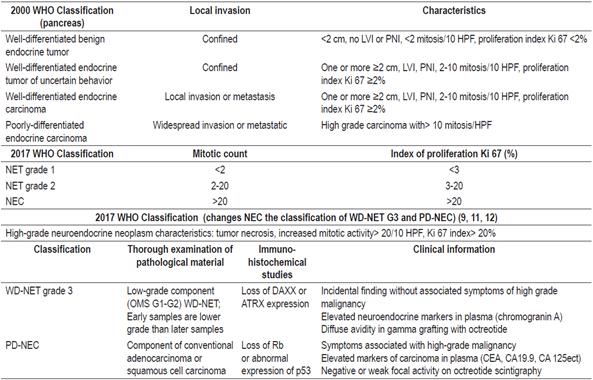
GEP-NET: gastroenteropancreatic neuroendocrine tumors; HPF: high power field(s); LVI: lymphovascular invasion; PNI: perineural invasion; NEC: neuroendocrine carcinoma; WHO: World Health Organization; PD-NEC: poorly differentiated neuroendocrine carcinoma; WD-NET: well differentiated neuroendocrine tumor.
REFERENCES
1. ASGE Standards of Practice Committee, Gurudu S, Bruining DH, Acosta RD, Eloubeidi MA, Faulx AL, et al. The role of endoscopy in the management of suspected small-bowel bleeding. Gastrointest Endosc. 2017;85(1):22-31. doi: 10.1016/j.gie.2016.06.013. [ Links ]
2. Gerson LB, Fidler JL, Cave DR, Leighton JA. ACG clinical guideline: diagnosis and management of small bowel bleeding. Am J Gastroenterol. 2015;110(9):1265-87. doi: 10.1038/ajg.2015.246. [ Links ]
3. Pennazio M, Spada C, Eliakim R, Keuchel M, May A, Mulder CJ, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2015;47(4):352-76. doi: 10.1055/s-0034-1391855. [ Links ]
4. Mosquera-Klinger G, Correa NF, Concha Mejía A. Sangrado digestivo oscuro y anemia crónica severa: discusión sobre dos causas gastrointestinales subvaloradas en Colombia. Univ Med. 2014;55(2):229-34. [ Links ]
5. Enns RA, Hookey L, Armstrong D, Bernstein CN, Heitman SJ, Teshima C, et al. Clinical practice guidelines for the use of video capsule endoscopy. Gastroenterology. 2017;152(3):497-514. doi: 10.1053/j.gastro.2016.12.032. [ Links ]
6. Chambers AJ, Pasieka JL, Dixon E, Rorstad O. Role of imaging in the preoperative staging of small bowel neuroendocrine tumors. J Am Coll Surg. 2010;211(5):620-7. doi: 10.1016/j.jamcollsurg.2010.07.016. [ Links ]
7. Ahdaleh FS, Lorenzen A, Rajput M, Carr JC, Liao J, Menda Y, et al. The value of preoperative imaging in small bowel neuroendocrine tumors. Ann Surg Oncol. 2013;20(6):1912-7. doi: 10.1245/s10434-012-2836-y. [ Links ]
8. Manguso N, Gangi A, Johnson J, Harit A, Nissen N, Jamil L, et al. The role of pre-operative imaging and double balloon enteroscopy in the surgical management of small bowel neuroendocrine tumors: is it necessary? J Surg Oncol. 2018;117(2):207-12. doi: 10.1002/jso.24825. [ Links ]
9. Huprich JE, Barkow JM, Hansel SL, Alexander JA, Fidler JL. Multiphase CT enterography evaluation of small-bowel vascular lesions. AJR Am J Roentgenol. 2013;201(1):65-72. doi: 10.2214/AJR.12.10414. [ Links ]
10. Hakim FA, Alexander JA, Huprich JE, Grover M, Enders FT. CT-enterography may identify small bowel tumors not detected by capsule endoscopy: eight years experience at Mayo Clinic Rochester. Dig Dis Sci. 2011;56(10):2914-9. doi: 10.1007/s10620-011-1773-0. [ Links ]
11. Agrawal JR, Travis AC, Mortele KJ, Silverman SG, Maurer R, Reddy SI, et al. Diagnostic yield of dual-phase computed tomography enterography in patients with obscure gastrointestinal bleeding and a non-diagnostic capsule endoscopy. J Gastroenterol Hepatol. 2012;27(4):751-9. doi: 10.1111/j.1440-1746.2011.06959.x. [ Links ]
12. Heo HM, Park CH, Lim JS, Lee JH, Kim BK, Cheon JH, et al. The role of capsule endoscopy after negative CT enterography in patients with obscure gastrointestinal bleeding. Eur Radiol 2012;22(6):1159-66. doi: 10.1007/s00330-011-2374-1. [ Links ]
13. Wiarda BM, Heine DG, Mensink P, Stolk M, Dees J, Hazenberg HJ, et al. Comparison of magnetic resonance enteroclysis and capsule endoscopy with balloon-assisted enteroscopy in patients with obscure gastrointestinal bleeding. Endoscopy. 2012;44(7):668-73. doi: 10.1055/s-0032-1309386. [ Links ]
14. Böcker U, Dinter D, Litterer C, Hummel F, Knebel P, Franke A, et al. Comparison of magnetic resonance imaging and video capsule enteroscopy in diagnosing small- bowel pathology: localization-dependent diagnostic yield. Scand J Gastroenterol. 2010;45(4):490-500. doi: 10.3109/00365520903567817. [ Links ]
15. Vinik AI, McLeod MK, Fig LM, Shapiro B, Lloyd RV, Cho K. Clinical features, diagnosis, and localization of carcinoid tumors and their management. Gastroenterol Clin N Am. 1989;18(4):865-96. [ Links ]
16. Kim JY, Hong SM, Ro JY. Recent updates on grading and classification of neuroendocrine tumors. Ann Diagn Pathol. 2017;29:11-36. doi: 10.1016/j.anndiagpath.2017.04.005. [ Links ]
17. Yin XN, Shen CY, Yin YQ, Chen HJ, Chen HN, Yin Y, et al. Prognoses in patients with primary gastrointestinal neuroendocrine neoplasms based on the proposed new classification scheme. Asia Pac J Clin Oncol. 2017;14(2):e37-344. doi: 10.1111/ajco.12760. [ Links ]
18. Tang L, Basturk O, Sue JJ, Klimstra DS. A Practical Approach to the Classification of WHO Grade 3 (G3) Well differentiated Neuroendocrine Tumor (WD-NET) and Poorly Differentiated Neuroendocrine Carcinoma (PD-NEC) of the Pancreas. Am J Surg Pathol. 2016;40(9):1192-202. doi: 10.1097/PAS.0000000000000662. [ Links ]
19. Basturk O, Yang Z, Tang LH, Hruban RH, Adsay V, McCall CM, et al. The High-grade (WHO G3) Pancreatic Neuroendocrine Tumor Category Is Morphologically and Biologically Heterogenous and Includes Both Well Differentiated and Poorly Differentiated Neoplasms. Am J Surg Pathol. 2015;39(5):683-90. doi: 10.1097/PAS.0000000000000408. [ Links ]
Received: October 04, 2017; Accepted: December 08, 2017











 texto em
texto em