Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.33 no.4 Bogotá Oct./Dec. 2018
https://doi.org/10.22516/25007440.172
Review articles
Crohn’s disease vs. intestinal tuberculosis: a challenging differential diagnosis
1 Internista, gastroenterólogo, Unidad de Gastroenterología y Endoscopia Digestiva, Hospital Pablo Tobón Uribe. Medellín, Colombia
2 Estudiante de pregrado de Medicina, Facultad de Medicina, Universidad de Antioquia. Medellín, Colombia
Crohn’s disease (CD) is a chronic, granulomatous and idiopathic disease that can affect the entire digestive tract. In recent years its incidence has increased, but the therapeutic arsenal has also grown bigger and now includes immunosuppressants. On the other hand, the World Health Organization (WHO) considers tuberculosis (TB) to be endemic in Colombia. CD and intestinal tuberculosis (ITB) are diseases that have clinical characteristics that are so similar that the endoscopic, imaging, and even histological findings may be indistinguishable in some patients. Immunosuppressed patients and patients with CD treated with immunomodulation have a higher risk of developing concomitant TB infections.
For this reason, we decided to review the literature to characterize the state of the art for both pathologies and to provide data that will allow clinicians to differentiate between them. We used the PUBMED, Scielo, Google Scholar databases to obtain information published in English and Spanish.
Keywords: Tuberculosis; gastrointestinal diseases; Crohn’s disease; differential diagnosis
La enfermedad de Crohn (EC) es una enfermedad crónica granulomatosa, idiopática y que puede afectar todo el tracto digestivo. Por una parte, en los últimos años la incidencia se ha incrementado y el arsenal terapéutico es mayor, incluidos los inmunosupresores. Por otra parte, Colombia es considerada por la Organización Mundial de la Salud (OMS) como un país endémico en infección por tuberculosis (TB). La EC y la tuberculosis intestinal (TBI) son enfermedades que tienen características clínicas similares y en algunos pacientes los hallazgos endoscópicos, imagenológicos e incluso histológicos podrían ser indistinguibles. Los pacientes inmunosuprimidos o con EC con terapias inmunomoduladoras tienen mayor riesgo de tener infección concomitante con TB.
Por esta razón, decidimos hacer una revisión de la literatura con términos libres, con el objetivo de poder hacer un estado del arte de ambas patologías y aportar datos que permitan al clínico diferenciarlas. Se utilizaron las bases de datos Pubmed, Scielo y Google Scholar para obtener información publicada en los idiomas inglés y español.
Palabras clave: Tuberculosis; enfermedades gastrointestinales; enfermedad de Crohn; diagnóstico diferencial
Introduction
TB, an infectious disease, is considered to be one of the main public health threats facing the world. 1 This epidemic has expanded to the point that up to a third of the world’s population, mostly in developing countries, is now infected. 2 TB is responsible for up to 9 million deaths per year. In 2011, the World Health Organization (WHO) registered 6.2 million cases of which 5.8 million had been recently diagnosed. Fifteen percent of these cases were extrapulmonary. 3
Its incidence is increasing in developed countries due to large-scale migration, the pandemic of human immunodeficiency virus (HIV) infection, and the increasing use of immunosuppressive therapies to treat various diseases. This problem is compounded by the emergence of multidrug-resistant TB (MDR TB) and extensively drug-resistant TB (XDR TB). 1,2
TB can occur throughout the body, but the foci of entry and the most frequently affected organs are the lungs. This is the primary form of the disease. Extrapulmonary involvement occurs in up to 20% of immunocompetent patients and in 50% of immunosuppressed patients. Intestinal TB is the sixth most common extrapulmonary TB. The ileocecal region is compromised in 90% of all cases of digestive tract TB. 4,5 Gastrointestinal symptoms vary and are nonspecific. Intestinal TB can mimic other disorders such as inflammatory bowel disease (IBD), malignant colon cancer and gastrointestinal infections. 3,4,5) Intestinal TB is mainly caused by Mycobacterium tuberculosis and, to a lesser extent, by Mycobacterium bovis. 4
Although the prevalence of intestinal TB varies depending on the geographic location and population risk profiles, it is difficult to determine the number of individuals affected because patients with pulmonary TB can have asymptomatic intestinal TB with nonspecific and insidious digestive symptoms. Currently, there is no perfect test for establishing early diagnoses . 6
On the other hand, Crohn’s disease (CD) is an idiopathic, chronic and inflammatory pathology with a genetic background which is modified by multiple environmental factors. 4 It can affect any segment of the digestive tract from the mouth to the anus and is commonly accompanied by extraintestinal and even pulmonary manifestations. 7,8,9,10 In the natural course of the disease, outbreaks of inflammatory activity alternate with periods of remission and there are frequent recurrences even after surgical resection of affected sections. CD’s global incidence has also increased in recent decades, even in areas where it had been infrequently reported. 1 Today, TB and CD superimposed onto each other is a widely recognized phenomenon and rates of misdiagnosis of CD and intestinal TB range between 50% and 70% due to their non-specific and varied manifestations. 11
Both intestinal TB and CD are chronic granulomatous disorders that can compromise the intestinal walls which explains complications such as the formation of fistulas and stenoses. For this reason, the clinical, radiological, endoscopic and histological characteristics may be similar. Differentiation between these two entities is very difficult in some scenarios and requires a high index of suspicion since the repercussions of a misdiagnosis are serious. 1 One point to note is that CD is incurable and intestinal TB is a potentially curable infectious disease.
Pathogenesis
Mycobacterial infections in the gastrointestinal tract occur in several ways. A patient with active lung disease can swallow infected sputum, the disease can be spread through the blood or lymphatic system from a distant focus, the disease can direct extend from a contiguous site, or it can be spread by ingestion of infected dairy products in the case of M. bovis. The last mechanism is rare in the United States and other developed nations due to the pasteurization of milk. Dairy products continue to be a viable means of mycobacterial infection in some countries, particularly in those cultures where fresh or unpasteurized milk is consumed. 6
The entire gastrointestinal tract may be compromised by TB, but the ileocecal region is the most common location, accounting for 44% to 93% of all cases. 4,5,6 Each mycobacterium has a fat capsule that resists digestion and interferes with early release in the gastrointestinal tract. This explains the rarity of proximal gastrointestinal lesions. 6 In contrast, the greater vascularization, smaller diameter, and relative stasis of the ileocecal region allow the capsule to be digested so that the microorganism can be released resulting in a local infection. In addition, the germ has a special affinity for the lymphatic tissue which is abundant in the ileocecal region. Once in the submucosa, the bacillus colonizes Peyer’s patches and initiates an inflammatory response leading to the formation of granulomas. As the tuberculomas grow, the intestinal wall thickens markedly and small papillary elevations form in the mucosa. Endarteritis and lymphangitis may develop while the superficial mucosa becomes edematous and circumferentially ulcerated. As ulcers heal, deposition and contraction of collagen in the mucosa can lead to the formation of stenoses. Therefore, tuberculous enteritis can generally be classified as ulcerative, hypertrophic, mixed ulcer-hypertrophic or fibrotic. The ulcerative form is most commonly found in the small intestine while the hypertrophic form is more commonly found in the cecum. 6
In CD, the risk factors for development seem to be related to changes in the intestinal microbiome or alterations of the intestinal mucosa and genetics. Patients with IBD often seem to suffer dysbiosis in which there is a reduction in the diversity of the gut microbiome. Nevertheless, this has not yet been fully studied or understood. The best studied environmental risk factor is the habit of smoking which doubles the risk of occurrence of the disease. Gastrointestinal infections, nonsteroidal anti-inflammatory drugs (NSAIDs) and antibiotics have been implicated in development and outbreaks of IBD activity 7.
CD is characterized by discontinuous lesions and transmural inflammation which can affect any part of the gastrointestinal tract. The most common location is the ileocolonic region and the small intestine exclusively. These two locations account for almost 70% of all cases with up to 20% being isolated colonic CD. 5,7 In order of frequency, the CD’s forms of presentation (described in other countries) are inflammatory (almost 80% of cases) stenosing, and fistulizing. 12 However, in a study conducted at the Pablo Tobón Uribe Hospital, almost one third of the patients presented with the stenosing variety, but this data may be related to late diagnoses. 13
Epidemiology
Most of Colombia consists of highly endemic areas for TB. In the last annual report published by the WHO in 2015, 12,749 cases were noted, many of them with HIV co-infections. 14
Although the incidence rate decreased from 58.62 cases per 100,000 inhabitants in 1970 to 31 cases per 100,000 inhabitants in 2015, the number of cases detected annually has remained stable. 14,15,16 Intestinal TB represents 1% to 3% of all cases of TB and 11% of extrapulmonary TB cases. 11 As of yet, there are no epidemiological data on intestinal TB in Colombia.
At present, there are no data on the prevalence and incidence of IBD in Latin America, but incidence has been estimated at 0.5/100,000 inhabitants/year. 13,17 In addition, a growing number of case reports and descriptive studies show that a greater number of patients have ulcerative colitis than have CD in Colombia and other countries in the region. 13,18,19 The same is true for Japan and some areas in the Middle East. 3,20 Industrialization, improvements of a population’s quality of life and migration to countries with a high incidence of IBD may contribute increasing incidence of this entity similar to the phenomenon described in Hispanic children and children of immigrants who they move to countries with a high incidences of IBD. 21,22
Clinical manifestations
Clinical data for these two diseases can be so similar that they become indistinguishable, especially in patients in endemic areas of TB, patients with primary or acquired immunodeficiencies, and in patients who are taking immunosuppressive drugs.
The duration of symptoms in intestinal TB varies from one to twelve months, and relapses depend on the immune status of the host. Some non-specific manifestations of TB such as abdominal pain, fever and fatigue overlap with CD. Weight loss and nocturnal diaphoresis are more common symptoms in TB while malabsorption and protein loss are seen more frequently in CD. 11 A retrospective study conducted in Shanghai, China that included 141 patients with CD and 47 with intestinal tuberculosis showed that the strongest clinical data in favor of CD were stool with blood and perineal fistulas. Ascites, pulmonary TB and nocturnal diaphoresis were more indicative of intestinal TB. 23 A report by Makharia et al. found that longer-lasting symptoms such as chronic diarrhea, blood in the stool, perineal disease and extra-digestive manifestations were more common in CD. 24 Although it is worth noting that extra-digestive manifestations must be interpreted carefully, since TB can also compromise the joints, eyes, skin and other organs. 11
Primary sclerosing cholangitis (PSC) is a chronic inflammatory disease of the bile ducts that causes stenosis and recurrent cholangitis. It can lead to liver failure and/or cancer. It is closely correlated with IBD, and it is estimated that between 50% and 80% of PSC patients have concomitant IBD. 25 Therefore, the presence of PSC indicates that CD more often than it indicates intestinal tuberculosis. Biliary manifestations related to CD are underestimated since a large number of patients are asymptomatic from the biliary point of view. Individuals with IBD and PSC have worse prognoses since they have risks of developing colorectal neoplasia that are three times higher than those of patients with IBD without PSC. 26
Keys to diagnosis
Patients with chronic diarrhea, bloody stools, abdominal pain and those whose pathology reports show granulomatous ileocolitis without caseous necrosis are major diagnostic challenges. The clinical pictures in these cases may be similar, but the patient’s background, immunological status, any previous history of TB infection and extra-digestive manifestations must all be taken into account. 6
Laboratory tests do not provide enough information to distinguish between CD and intestinal tuberculosis. Blood tests such as anti-neutrophil cytoplasmic antibodies (ANCA) and anti-Saccharomyces cerevisiae antibodies (ASCA) have limited value since the former are found in less than 30% of patients with CD. ASCA can occur in up to 65% of cases but up to half of patients with intestinal TB also have ASCA. 1,27
Fecal calprotectin has been observed to increase from five to 40 times in infectious and inflammatory conditions. Its levels are markedly elevated in the feces of IBD patients, 28 and it has excellent negative predictive value for symptomatic patients while its positive predictive value is generally better than other markers of inflammation currently in use. Nevertheless, it can also be elevated in cases if intestinal TB. 29
The tuberculin skin test (PPD) has a low diagnostic yield for cases of active TB, and its use is gradually being restricted. 11 In countries like Colombia with high prevalences of TB, it has become essential to implement new tools such as interferon-gamma releasing assays (IGRA) Two well-known brands, QuantiFERON-TB Gold In-Tube (QFT-GIT; Cellestis, Carnegie, Australia) and T-SPOT.TB (Oxford Immunotec, Oxford, United Kingdom) are widely used in other countries. Interferon (IFN-γ) released by circulating T cells is measured after in vitro stimulation by Mtb antigens. According to a systematic review and metaanalysis how well IGRA and ASCA differentiate between CD and intestinal TB, the sensitivity of IGRA was 81% (95% CI: 75% to 86%) and its specificity was 85% (95% CI: 81% to 89%) for diagnosis of intestinal TB. ASCA’s sensitivity was only 33% (95% CI: 27% to 38%) although its specificity was 83% (95% CI: 77 to 88%) for CD. The advantage of QFT-G-IT is that it avoids cross-reactions with Bacillus Calmette-Guérin vaccinations and the majority of non-tuberculous mycobacteria. 11 Therefore, in countries with a high prevalence of TB in which there are also vaccines, this test could minimize false positives.
Similar results have been obtained from abdominal CT scans. Some studies suggest that CT data for intestinal tuberculosis such as asymmetry of the walls of the ileocecal region and a greater number and size of local adenopathies can be used for differential diagnosis between intestinal TB and CD. In contrast to intestinal TB, in cases of CD intestinal walls thicken symmetrically and concentrically with fibro-fat proliferation of the mesentery known as creeping fat. 31,32. A recent systematic literature review and metaanalysis regarding diagnostic accuracy of abdominal CT scans for differentiating between these 2 entities found that necrotic lymph nodes had a sensitivity of 23% and a specificity of 100% while the comb sign had a sensitivity of 81% and a specificity of 82%. These were best markers for this differential diagnosis of CD. 31 In addition, magnetic resonance enterography has excellent diagnostic accuracy for CD with a sensitivity of 78% and a specificity of 85%. Its sensitivity for detection of stenoses ranges between 75% and 100% while its specificity is between 91% and 100%. 33 There are no studies of magnetic resonance enterography’s ability to differentiate between these two diseases.
A number of endoscopic findings described in several studies can help differentiate between them. Intestinal TB causes circumferential ulcers, scar nodules, short stenoses and serious inflammation of the ileocecal valve (Figures 1A and 1B). In contrast, the most characteristic symptoms of CD are deep, discontinuous (segmental) longitudinal ulcers (Figure 2A), aphthous ulcers, and perianal lesions. Findings of perianal lesions almost rules out intestinal TB (Figure 2B). 3,11
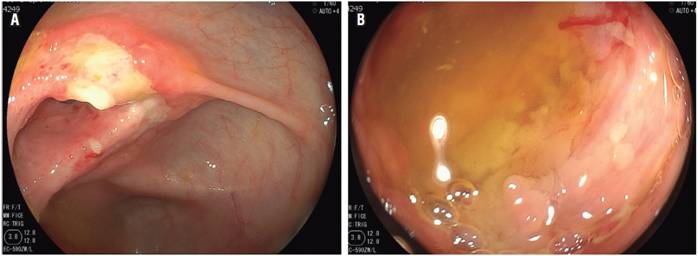
Figure 1A Inflammation and ulcer that compromises the ileocecal valve in patients with HIV and intestinal tuberculosis. Figure 1B. Inflammation, deep and circumferential ulcer in the distal ileum (patient with HIV and intestinal tuberculosis).
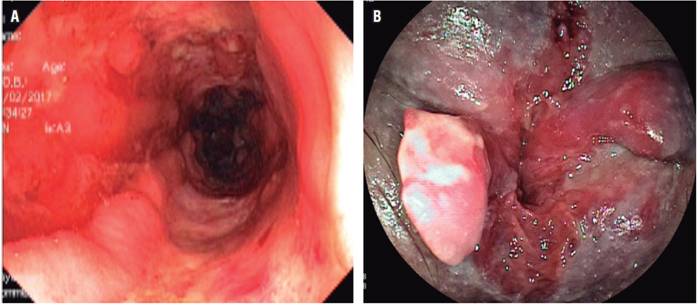
Figure 2A Severe inflammatory changes and deep, longitudinal ulcers in the colon (patient with a diagnosis of CD). Figure 2B. Perianal compromise due to CD.
Granulomatous inflammation can be observed histopathologically in both entities, but evidence of casein granuloma and acid-alcohol resistant bacilli by the Ziehl-Neelsen technique confirms a diagnosis of intestinal tuberculosis. Nevertheless, they are found in less than 30% of cases, so a positive culture for TB is the gold standard even though this usually takes three to eight weeks. 4,11,34
Typically, intestinal TB presents confluent caseous necrosis at four or more sites and has granulomas that are larger than 400 μm which are synchronously located in the mucosa, submucosa and/or granulation tissue. Bands of epithelioid histiocytes have also been described at the base of ulcers, and disproportionate amounts of submucosal inflammation have been found. In contrast, granulomas in cases of CD tend to be smaller than 200 μm and poorly organized, and they most frequently occur in the rectum and sigmoid colon (Table 1). 1,11,35
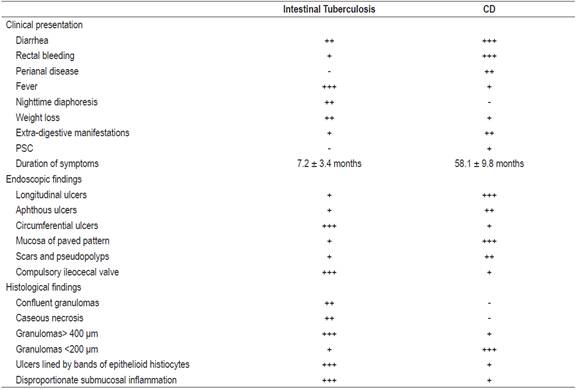
Table 1 Clinical, endoscopic and histological characteristics for differential diagnosis of CD and intestinal TB1,3,11
Polymerase chain reaction (PCR) tests of feces and tissue are being used more and more frequently because it noticeably increases diagnostic performance. Recently, the first metaanalysis of the diagnostic value of mycobacterial PCR for differentiating intestinal TB and CD was published. It demonstrates that it has high specificity but low sensitivity for intestinal tuberculosis. This suggests that PCR has potential diagnostic value for intestinal TB, but its low sensitivity means that a negative PCR does not rule out a diagnosis of TB. 36
A therapeutic test with antituberculosis drugs has been used in countries with high prevalences of TB under the assumption that improvement after treatment with antituberculosis drugs confirms a diagnosis of intestinal TB. 1,37 The Asia-Pacific consensus for CD diagnosis recommends an 8-12 week test with antituberculosis drugs for patients for whom it is not possible to safely differentiate between CD and intestinal TB (recommendation III, level of evidence C). 37 Recently, a paper published in India by Dr. Mouli et al. observed that there is a global symptomatic response of CD to antituberculosis therapy in 38% of patients at three months and in 37% of patients who complete six months of antituberculosis therapy. 38 In addition, 94% of patients with intestinal TB had global symptomatic responses at three months. Mucosal healing was observed endoscopically in only 5% of the patients with CD whereas it was observed in 100% of the patients with intestinal tuberculosis. These data seem to justify administering antituberculosis treatment to selected patients for whom it has not been possible to determine whether they have intestinal TB or CD with available diagnostic tools after a certain period of follow-up. However, this choise has detractors due to concern of the progressive appearance of MDR TB and XDR TB. 1,39
Conclusion
There are several controversial and very difficult scenarios facing clinicians. The first occurs when a patient who is from an area of endemic TB and/or is immunosuppressed presents nonspecific digestive manifestations and indeterminate findings for inflammatory diseases of the digestive tract. The second occurs when a patient who has been diagnosed with pulmonary TB develops digestive symptoms. The third occurs when a patient with CD is refractory to conventional medical treatment or has clinical, radiological or endoscopic worsening due to immunosuppressive therapies. The fourth occur when a patient with CD has latent TB. In these scenarios, TB should always be ruled out rationally through an approach in which clinical, endoscopic, and radiological and data are taken into account and confirmed by histology, PCR of fecal matter and/or tissue, as well as cultures of mycobacteria in the tissue of biopsies. In cases in which diagnostic doubt persists, the possibility empirical management with antituberculosis drugs should be evaluated.
Treatment with immunosuppressants for CD can lead to fatal spread of TB. For this reason, and in view of evidence of inflammatory changes, erosions or ulcers in the distal ileum from a colonoscopy, more attention should be paid to diagnosis of CD in Colombia. Differentiation between CD and intestinal TB cannot be done with a single evaluation, since accurate diagnosis is most often established through the sum of clinical, endoscopic, radiological, laboratory and culture studies.
Referencias
1. Sood A, Midha V, Singh A. Differential diagnosis of Crohn’s disease versus ileal tuberculosis. Curr Gastroenterol Rep. 2014;16(11):418. doi: 10.1007/s11894-014-0418-9. [ Links ]
2. Epstein D, Watermeyer G, Kirsch R. Review article: the diagnosis and management of Crohn’s disease in populations with high-risk rates for tuberculosis. Aliment Pharmacol Ther. 2007;25(12):1373-88. doi: 10.1111/j.1365-2036.2007.03332.x. [ Links ]
3. Ahmed R, Shafique MS, Zafar S, Mehmood S, Mehmood S, Qureshi U, et al. Intestinal tuberculosis; pattern of presentation and surgical management. Professional Med J 2016;23(11):1334-1339. doi: 10.17957/TPMJ/16.3341. [ Links ]
4. Almadi MA, Ghosh S, Aljebreen AM. Differentiating intestinal tuberculosis from Crohn’s disease: a diagnostic challenge. Am J Gastroenterol. 2009;104(4):1003-12. doi: 10.1038/ajg.2008.162. [ Links ]
5. Hani A, Mosquera-Klinger G, Leguizamo AM. Presentación clínica de la enfermedad de Crohn. Aponte D, Gil F, Reyes G. Diagnóstico en enfermedad inflamatoria intestinal, edición 1. Bogotá: Pulso ediciones S.L; 2014. cap. 6. p. 81-90. [ Links ]
6. Choi EH, Coyle WJ. Gastrointestinal Tuberculosis. Microbiol Spectr. 2016;4(6). doi: 10.1128/microbiolspec.TNMI7-0014-2016. [ Links ]
7. Feuerstein JD, Cheifetz AS. Crohn Disease: Epidemiology, Diagnosis, and Management. Mayo Clin Proc. 2017;92(7):1088-1103. doi: 10.1016/j.mayocp.2017.04.010. [ Links ]
8. Veloso FT, Carvalho J, Magro F. Immune-related systemic manifestations of inflammatory bowel disease. A prospective study of 792 patients. J Clin Gastroenterol. 1996;23(1):29-34. [ Links ]
9. Vennera M, Picado C. Manifestaciones pulmonares de las enfermedades inflamatorias. Arch Bronconeumol. 2005;41 (2):93-8. [ Links ]
10. Storch I, Sachar D, Katz S. Pulmonary manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2003;9(2):104-15. [ Links ]
11. Ma JY, Tong JL, Ran ZH. Intestinal tuberculosis and Crohn’s disease: challenging differential diagnosis. J Dig Dis. 2016;17(3):155-61. doi: 10.1111/1751-2980.12324. [ Links ]
12. Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ. Crohn’s disease complicated by strictures: a systematic review. Gut. 2013;62(7):1072-84. doi: 10.1136/gutjnl-2012-304353. [ Links ]
13. Juliao F, Ruiz MH, Flórez JF, Donado JH, Marín JI, Monsalve C, et al. Fenotipo e historia natural de la enfermedad inflamatoria intestinal en un centro de referencia en Medellín-Colombia. Rev Col Gastroenterol. 2010;25(3):240-51. [ Links ]
14. World Health Organization. Global Tuberculosis Report 2016. Génova: WHO; 2016. [ Links ]
15. Vera N. Tuberculosis en Colombia. Nova et Vetera. 2015;1(1). [ Links ]
16. Chaparro P, García I, Guerrero M, León C. Situación de la tuberculosis en Colombia, 2002. Biomédica. 2004;24 (Supl 1):102-14. [ Links ]
17. Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504-17. [ Links ]
18. Linares de la Cal JA, Cantón C, Hermida C, Pérez-Miranda M, Maté-Jiménez J. Estimated incidence of inflammatory bowel disease in Argentina and Panama (1987-1993). Rev Esp Enferm Dig. 1999;91(4):277-86. [ Links ]
19. Figueroa C, Quera R, Valenzuela J, Jensen Ch. Enfermedades in amatorias intestinales: Experiencia de dos centros chilenos. Rev Méd Chil. 2005;133(11):1295-304. doi: 10.4067/S0034-98872005001100004. [ Links ]
20. Navaneethan U, Cherian JV, Prabhu R, Venkataraman J. Distinguishing tuberculosis and Crohn’s disease in developing countries: how certain can you be of the diagnosis? Saudi J Gastroenterol. 2009;15(2):142-4. doi: 10.4103/1319-3767.49012. [ Links ]
21. Damas OM, Jahann DA, Reznik R, McCauley JL, Tamariz L, Deshpande AR, et al. Phenotypic manifestations of inflammatory bowel disease differ between Hispanics and non-Hispanic whites: results of a large cohort study. Am J Gastroenterol. 2013;108(2):231-9. doi: 10.1038/ajg.2012.393. [ Links ]
22. Lewis JD, Abreu MT. Diet as a Trigger or Therapy for Inflammatory Bowel Diseases. Gastroenterology. 2017;152(2):398-414.e6. doi: 10.1053/j.gastro.2016.10.019. [ Links ]
23. Zhao XS, Wang ZT, Wu ZY, Yin QH, Zhong J, Miao F, et al. Differentiation of Crohn’s disease from intestinal tuberculosis by clinical and CT enterographic models. Inflamm Bowel Dis. 2014;20(5):916-25. doi: 10.1097/MIB.0000000000000025. [ Links ]
24. Makharia GK, Srivastava S, Das P, Goswami P, Singh U, Tripathi M, et al. Clinical, endoscopic, and histological differentiations between Crohn’s disease and intestinal tuberculosis. Am J Gastroenterol. 2010;105(3):642-51. doi: 10.1038/ajg.2009.585. [ Links ]
25. Lunder AK, Hov JR, Borthne A, Gleditsch J, Johannesen G, Tveit K, et al. Prevalence of Sclerosing Cholangitis Detected by Magnetic Resonance Cholangiography in Patients With Long-term Inflammatory Bowel Disease. Gastroenterology. 2016;151(4):660-669.e4. doi: 10.1053/j.gastro.2016.06.021. [ Links ]
26. Zheng HH, Jiang XL. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: a meta-analysis of 16 observational studies. Eur J Gastroenterol Hepatol. 2016;28(4):383-90. doi: 10.1097/MEG.0000000000000576. [ Links ]
27. Ghoshal UC, Ghoshal U, Singh H, Tiwari S. Anti-Saccharomyces cerevisiae antibody is not useful to differentiate between Crohn’s disease and intestinal tuberculosis in India. J Postgrad Med. 2007;53(3):166-70. [ Links ]
28. Konikoff MR, Denson LA. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12(6):524-34. [ Links ]
29. Gomollón F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J Crohns Colitis. 2017;11(1):3-25. doi: 10.1093/ecco-jcc/jjw168. [ Links ]
30. Ng SC, Hirai HW, Tsoi KK, Wong SH, Chan FK, Sung JJ, et al. Systematic review with meta-analysis: accuracy of interferon-gamma releasing assay and anti-Saccharomyces cerevisiae antibody in differentiating intestinal tuberculosis from Crohn’s disease in Asians. J Gastroenterol Hepatol. 2014;29(9):1664-70. doi: 10.1111/jgh.12645. [ Links ]
31. Kedia S, Sharma R, Sreenivas V, Madhusudhan KS, Sharma V, Bopanna S, et al. Accuracy of computed tomographic features in differentiating intestinal tuberculosis from Crohn’s disease: a systematic review with meta-analysis. Intest Res. 2017;15(2):149-159. doi: 10.5217/ir.2017.15.2.149. [ Links ]
32. Boudiaf M, Zidi SH, Soyer P, Lavergne-Slove A, Kardache M, Logeay O, et al. Tuberculous colitis mimicking Crohn’s disease: utility of computed tomography in the differentiation. Eur Radiol. 1998;8(7):1221-3. doi: 10.1007/s003300050539. [ Links ]
33. Fiorino G, Bonifacio C, Peyrin-Biroulet L, Minuti F, Repici A, Spinelli A, et al. Prospective comparison of computed tomography enterography and magnetic resonance enterography for assessment of disease activity and complications in ileocolonic Crohn’s disease. Inflamm Bowel Dis. 2011;17(5):1073-80. doi: 10.1002/ibd.21533. [ Links ]
34. Dilauro S, Crum-Cianflone NF. Ileitis: when it is not Crohn’s disease. Curr Gastroenterol Rep. 2010;12(4):249-58. doi: 10.1007/s11894-010-0112-5. [ Links ]
35. Pulimood AB, Peter S, Ramakrishna B, Chacko A, Jeyamani R, Jeyaseelan L, et al. Segmental colonoscopic biopsies in the differentiation of ileocolic tuberculosis from Crohn’s disease. J Gastroenterol Hepatol. 2005;20(5):688-96. doi: 10.1111/j.1440-1746.2005.03814.x. [ Links ]
36. Jin T, Fei B, Zhang Y, He X. The diagnostic value of polymerase chain reaction for Mycobacterium tuberculosis to distinguish intestinal tuberculosis from crohn’s disease: A meta-analysis. Saudi J Gastroenterol. 2017;23(1):3-10. doi: 10.4103/1319-3767.199135. [ Links ]
37. Ooi CJ, Makharia GK, Hilmi I, Gibson PR, Fock KM, Ahuja V, et al. Asia Pacific Consensus Statements on Crohn’s disease. Part 1: Definition, diagnosis, and epidemiology: (Asia Pacific Crohn’s Disease Consensus--Part 1). J Gastroenterol Hepatol. 2016;31(1):45-55. doi: 10.1111/jgh.12956. [ Links ]
38. Pratap Mouli V, Munot K, Ananthakrishnan A, Kedia S, Addagalla S, Garg SK, et al. Endoscopic and clinical responses to anti-tubercular therapy can differentiate intestinal tuberculosis from Crohn’s disease. Aliment Pharmacol Ther. 2017;45(1):27-36. doi: 10.1111/apt.13840. [ Links ]
39. Pulimood AB, Amarapurkar DN, Ghoshal U, Phillip M, Pai CG, Reddy DN, et al. Differentiation of Crohn’s disease from intestinal tuberculosis in India in 2010. World J Gastroenterol. 2011;17(4):433-43. doi: 10.3748/wjg.v17.i4.433. [ Links ]
Received: November 03, 2017; Accepted: February 06, 2018











 text in
text in 


