Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.33 no.4 Bogotá oct./dic. 2018
https://doi.org/10.22516/25007440.314
Review articles
How can Helicobacter pylori eradication therapies be improved?
1Médico Cirujano, Universidad Nacional de Colombia. Bogotá D. C., Colombia
2Profesor Titular de Medicina, Unidad de Gastroenterología, Universidad Nacional de Colombia, Hospital Universitario Nacional. Gastroenterólogo, Clínica Fundadores. Bogotá D. C., Colombia
3Profesor Asociado de Medicina, Unidad de Gastroenterología, Universidad Nacional de Colombia, Hospital Universitario Nacional. Gastroenterólogo, Hospital de Kennedy. Bogotá D. C., Colombia
Since the discovery of Helicobacter pylori (H. pylori), eradication has been a constant challenge, and an ideal treatment is not yet available. The increasing resistance of the microorganism to the most frequently used antibiotics has most impacted the effectiveness of the various schemes. The objectives of this work are to provide a general review of H. pylori infections and the nomenclature of the various treatments, discuss the basic issues and components of eradication therapies together with the general characteristics of antibiotics, and finally to recommend treatments based on in the most important recent publications.
Keywords: Helicobacter; resistance; PPI; antibiotics.
Desde el descubrimiento de Helicobacter pylori, la erradicación del mismo ha sido un reto constante y el tratamiento ideal todavía no está disponible. La creciente resistencia del microorganismo a los antibióticos más frecuentemente utilizados es la circunstancia que más ha impactado en la eficacia de los diferentes esquemas. El objetivo del presente trabajo es revisar los aspectos generales de la infección por H. pylori, los aspectos básicos de los componentes de las terapias de erradicación, la nomenclatura de los distintos tratamientos, las características generales de los antibióticos y, finalmente, los tratamientos recomendados con base en las publicaciones recientes más importantes.
Palabras clave: Helicobacter; resistencia; inhibidores de la bomba de protones; antibióticos
Introduction
Helicobacter pylori is a gram-negative, microaerophilic bacterium capable of colonizing the stomach. 1 It produces chronic gastritis, peptic ulcers, gastric cancer (CG) and hematological diseases and is the second most common chronic bacterial infection in humans (after Streptococcus mutans, the producer of dental caries). 1,2,3,4 In 2015 there were approximately 4.4 billion people who were infected with Helicobacter pylori. 5 Prevalence is highest in Africa at 70% and lowest in Oceania at 24%. 5 Nigeria has the highest prevalence (87.7%) of any country, and Switzerland has the lowest (19%). The prevalence of H. pylori in countries with high incidences of GC is double those of countries with low incidences of GC. 6,7 Improvement of sanitary conditions and eradication of identified infections decrease prevalence of the disease. 7 The post-radiation reinfection rate is related to the socioeconomic levels of particular countries: in Japan, it is 2%, but in Latin America, it is 8%. 8,9,10
Warren and Marshall received the 2005 Nobel Prize for their research which demonstrated the etiological role of H. pylori in chronic gastritis and peptic ulcers in 1984. 11,12 Twenty percent of those infected develop digestive diseases such as peptic ulcers, GC or MALT lymphoma (lymphoma of lymphoid tissue associated with gastric mucosa). 13-16 In 1994, the World Health Organization (WHO) classified H. pylori as a type I defined carcinogen. 17
GC is a public health problem in many Latin American countries including Colombia, and the recent Colombian clinical practice guideline on GC recommends eradication of H. pylori in all those infected to reduce the risks of GC. 18 Taiwan and China have shown that eradicating H. pylori decreases the incidence of GC by 25% and 39%, respectively 19. The recent Kyoto consensus ratified the recommendation for eradication of H. pylori in all those infected, regardless of severity of gastritis and other symptoms. 20 Nevertheless, there are still no schemes available that are more than 98% effective, but this is required for eradication of infectious diseases. In fact, the rate of effectiveness is often 80% or less, which is unacceptable. 3,21
The frequent failures of therapies in use are related to both microorganism and host factors. 21-30 H. pylori factors include its acid habitat, its microaerophilic nature, its ability to produce biofilm which protects it from antibiotics, high bacterial loads, multiple dormant individuals (non-replicative stage), immunological evasion, and resistance to antibiotics which is the most important factor. 21-25 Throughout the world, therapy schemes have become increasingly less effective due to bacterial resistance to clarithromycin, metronidazole and most recently to quinolones. 3,4,21-27
This article reviews basic concepts of eradication therapies and additional factors involved in their success or failure.
Deep inhibition of acid secretion
Proton pump inhibitors (PPIs) include omeprazole, esomeprazole, lansoprazole, and rabeprazole, among others and are fundamental drugs in all treatment schemes. By inhibiting secretion of gastric acid and raising the pH above 6, the rate of H. pylori replication increases and the efficacy of the antibiotics increases. 3,24-30 However, the efficacy of PPIs is influenced by their metabolism in cytochrome P450 (CYP450) and the particular genotypes of CYP219C, CYP3A4, ABCB1 and others, as well as levels of IL-1β. 24-30 CYP2C19 is the most important, 24-29 and its impact is greatest on schemes that use omeprazole and lansoprazole, and less on those that include esomeprazole and rabeprazole. 25,26,27 This last drug has a metabolism that is independent of that enzyme system. 25,26,27 On the basis of individuals’ genetic polymorphisms of CYP2C19, people are classified into three categories of PPI metabolizers: rapid metabolizers (RM), intermediate metabolizers (IM) and slow metabolizers (SM). 2,28,29,30 The prevalence of these categories varies from population to population.28-31. Asians have a high SM prevalence whereas the majority are RM in Caucasian populations.29,30 Nevertheless, four doses a day of lansoprazole or omeprazole provides sufficient acid suppression for RMs. 24,26,27
Studies conducted in Japan more than 20 years ago showed that whether or not amoxicillin and PPI dual therapy eradicated H. pylori varied according to CYP2C19. 27,28 Eradication was 28%, 60% and 100%, respectively among RMs, IMs and SMs. The healing of peptic ulcers behaved in a similar way. 28 A recent meta-analysis has found that the therapeutic success rates of RMs were lower than those of IMs regardless of the type of PPI that was taken. 28,29 No significant differences were observed for esomeprazole and rabeprazole. 29 A South Korean study has found being RM is a risk factor for failure of eradication treatment (Odds ratio [OR]: 1.84, confidence interval [CI] 95%: 1.04-2.39). 30 However, the effect of CYP2C19 can be overcome with high doses of PPI such as 40 mg of omeprazole 2-3 times a day or equivalent doses of other PPIs. 21
Vonoprazan is a new PPI that inhibits the secretion of acid by competitively blocking the binding of potassium in the adenosine-triphosphatase (ATPase) of parietal cells. 31 It overcomes some drawbacks of conventional PPIs such as their short half-lives, their maximum inhibition of acid secretion after 4-5 doses and their need for protective wrapping of acid. In addition, its activity is independent of the CYP2C19 polymorphism.32,33. In Japan, vonoprazan is approved for treatment of peptic ulcers, gastroesophageal reflux disease, prophylaxis and treatment of gastroduodenal lesions due nonsteroidal anti-inflammatory drugs (NSAIDs) and for H. pylori eradication.34,35
In a Japanese study, 35 the success rate for eradication with a scheme that included vonoprazan was 92% versus 75.9% for a scheme with lansoprazole. When it was included in second-line therapies, the success rate was 98.0%. 35 A recent metaanalysis has found that vonoprazan therapies are more effective than those using conventional PPIs. 36
Antibiotics and their characteristics
The number of antibiotics used in H. pylori eradication schemes is small, but combinations and dosages vary. 19,21-24,37
Amoxicillin
Amoxicillin, a derivative of penicillin, inhibits the synthesis of the bacterial wall and has an average life of approximately one hour. 21 It’s bactericidal effect is time-dependent, so that it achieves adequate and sustained minimum inhibitory concentration (MIC) when it is administered 3 or 4 times a day.26,30 Its pharmacokinetic characteristics contrast with traditional twice-a-day dosing.26 It should be prescribed 3 or 4 times a day to continuously maintain therapeutic levels. 21,26,30,38 Primary resistance of H. pylori to this antibiotic is very rare worldwide. In Latin America it is 4%, 39 and in Colombia, it is less than 2%. 40 Consequently, it is an excellent medicine that can even be used in second line therapies after one scheme in which it has been used fails. 37,40
Clarithromycin
Clarithromycin, a macrolide that binds to the 50S unit of the bacterial ribosome, has been used around the world in standard triple therapy (STT) for several decades.21-25,41 Its half-life is approximately 5 hours, so it and it can be administered twice a day. 26 Because it has been broadly prescribed for numerous diseases, H. pylori has developed alarming resistance to it everywhere. 41 In Japan, H. pylori’s resistance increased from 1.8% in 1996 to 27.1% in 2008 while in China resistance was 14.8% in 2000 and 52.6% in 2014.41,42 In Latin America, average resistance was 12% until 2011 and is now 25% in Colombia.32,43. At the beginning of 2017, WHO included clarithromycin-resistant H. pylori on its list of 16 microorganisms that threaten humanity and for which eradication strategies are urgently needed. 44
Metronidazole
Metronidazole ruptures the double chain of bacterial DNA and has a plasma half-life of close to 8 hours so it can be administered 2 or 3 times a day. 9,26 Primary resistance to it is very high in Turkey, Iran, China and Latin America. 21 On this continent, the average resistance is 53%, and Colombia has the highest reported resistance of 83%. 39 It is the only drug whose in vitro resistance can be overcome by increasing the dosage and duration of treatment. 45 In quadruple therapy, 500 mg 4 times a day or 400 mg 4 times a day for 14 days achieves 92% success even with resistant strains. 46 These results are similar to those when there is no resistance the drug. 47 At this dosage, 10-14 day quadruple therapies schemes with bismuth have efficacy greater than 85% in regions with high resistance to metronidazole. 48-52
Quinolones
These drugs act on gyrase to cause breaks in bacterial DNA. 26 Their bactericidal effect depends more on maximum serum concentration than on duration of MIC, so they can be administered once a day. 26 Levofloxacin, the most commonly used quinolone, has been used in first and second line schemes when clarithromycin resistance rates are higher than 15% and when patients are allergic penicillin. 40,46 Like the case of clarithromycin, wide use to treat other diseases especially upper respiratory tract infections has led to development of resistance by H. pylori everywhere. 20 In Latin America the average resistance is 15%, 39 but in Bogotá, Colombia it is 27%. 48,49
Tetracycline antibiotics
The most important drugs in this group are tetracycline and doxycycline which act by binding to the 30S subunit of the bacterial ribosome to block protein synthesis. 26 Their half-lives are approximately six 6 hours, so they can be administered 2 or 3 times a day. Most commonly they are given 3 to 4 times a day. 26 Primary resistance is unusual and is not an obstacle to their use 46 In Latin America, the average resistance is 6%. 39
Bismuth
Bismuth salts have been used in gastroenterology since the 19th century as a primary or adjuvant treatment for dyspepsia and peptic ulcers. 47 Subsequently, they have been gradually replaced by antacids. 47 The most popular salts are bismuth subcitrate, bismuth subnitrate and bismuth subsalicylate which are hydrolyzed in the stomach to form insoluble polymers which have bactericidal effects. 47 Their most important mechanism of action in relation to H. pylori is preventing hydrogen ions from entering the cytoplasm thus prevent their replication. (50,51 To date, no H. pylori resistance to these drugs has been reported, and their safety and tolerance have been demonstrated. 38,50
Successful H. pylori eradication therapy with this drug was developed in Australia by Borody in 1989. 46 That scheme included bismuth subcitrate, metronidazole and tetracycline. 46 Subsequently, a PPI was added and duration was extended to 14 days in order to recover the efficacy lost due to resistance to metronidazole. 47 The dosage of this scheme is complex because of its duration and the large number of tablets per day: two tablets of bismuth four times a day, 500 mg of tetracycline four times a day, 500 mg of metronidazole three times a day and the PPI two times a day. Its complexity significantly affects adherence. In addition, it also produces adverse side effects which have been addressed by patient education, decreasing duration to 7-10 days, decreasing the dose of metronidazole when resistance to it is low, most recently development of a capsule called PYLERA which contains the three original medications. 46,47 The expected efficacy of quadruple therapy with bismuth for 14 days is greater than 95% even though there is high resistance to metronidazole. 52 This quadruple therapy is recommended as a second-line or first-line regimen when there is an allergy to penicillin, when resistance to clarithromycin is greater than 15%, and when there is dual resistance to clarithromycin and metronidazole. 46 It is also recommended as second-line therapy after a first-line therapy has failed. 46
Recently, bismuth has been added to triple therapies to counteract resistance to clarithromycin and levofloxacin. 53,54 This strategy is recommended in the Colombian guidelines, 55 but the initial studies were done in Italy and later in Spain. 56 Studies in China and Turkey have failed to demonstrate any advantages from adding bismuth to first-line triple therapies when there is resistance to clarithromycin or levofloxacin. 57,58
Rifabutin
Rifabutin, a metabolite of rifampicin, is similar to rifamycin. 59 It was discovered more than 20 years ago and has been used in fourth-line or salvage H. pylori eradication schemes. 40,59,60,61 Its bioavailability is low, and it has a large volume of distribution. 59 It is characterized by greater intracellular penetration capacity and tissue distribution than rifampicin, probably because it is more lipophilic. 59 It acts on the β subunit of the RNA polymerase dependent on bacterial DNA to inhibit synthesis and transcription of proteins. 59,61,62 Resistance of H. pylori to this medicine is only 1%, 50 but its drawbacks include high costs, lack of easy availability in many places, and adverse side-effects in more than 20% of patients. The most feared side-effect is myelotoxicity which occurs with doses of 600 mg per day or more and which disappears upon discontinuation. 63
Furazolidone
Furazolidone, a broad spectrum nitrofurans antimicrobial, 64 inhibits monoamine oxidase, is very effective against various microorganisms, and is very economical. 64-66 Its disadvantage is lack of availability. 64,65,66 Initially used in gastroenterology to treat diarrhea, 67 it has demonstrated efficacy against H. pylori, especially when it is used with bismuth. 67 Nevertheless, it frequently produces adverse effects when it interacts with soy derivatives and mature cheeses. Side-effects ranger from mild symptoms such as nausea and emesis to serious events such as hypertensive crisis and seizures. 64,67 This drug has been widely used in treatment schemes in China, Iran and Colombia. 64-68 Reported resistance in China is from 0% to 1% while in Brazil it is 3% on average. 39,67
Duration of eradication schemes
The initial duration of the STT was 14 days, but due to pressure from the pharmaceutical industry it was shortened to seven days because of commercial advantages. However, shorter duration is associated with lesser effectiveness. Reviews of 7-day therapies induced an increase to 10 days, but success of these schemes progressively decreased as resistance to clarithromycin, metronidazole and, ultimately, levofloxacin increased. 46 In the last decade, SST’s efficacy has become unacceptable (<80%). 45 Since 4th edition of the Maastricht consensus report, it has been recommended to extend duration to 14 days to increase efficiency by 5% on average. 53 Most studies have consistently demonstrated the advantages of longer duration. 24 A 2013 Cochrane metaanalysis has shown that an STT of 14 days is superior to those of 7-10 days by a 10% margin. 69 Extension of duration also increased adverse events from 15.5% to 19.4%, although they did not result in treatment discontinuations. 69 Other types of schemes have also been shown to be more successful when durations are increased to 14 days from shorter times. The only likely exception is quadruple therapy with bismuth, whose duration of 10 days remains effective. 70 The current recommendations of the most important international consensus on eradication of H. pylori and of the Colombian guidelines is that the duration for all therapy schemes, except those containing rifabutin, be 14 days. Those containing rifabutin can be 10 days. 38,52-55,70,71
Which eradication scheme should be tried first?
Ideally, H. pylori treatment should be chosen on the basis of susceptibility testing in a manner to the selection of treatment for any infectious disease. 16 However, culturing gastric biopsies and molecular methods such as polymerase chain reaction (PCR) and in-situ hybridization are not available everywhere, so treatment must be initiated empirically.46,47,55,72,73 Given this reality, the choice of therapy scheme should take into account the pattern of local resistance to antibiotics and should ideally consider whether effectiveness of the scheme to be used has been demonstrated independently of international consensus or guidelines. The treatment chosen must have a minimum efficacy of 90%. 74 To date, no scheme is 100% effective which is why second, third line and rescue treatments are needed. 40 The various schemes are described below.
Classic Quadruple Therapy
Classic quadruple therapy lasts 14 days and consists of a PPI twice a day, 550 mg of bismuth subsalicylate either four times/day or twice a day, 500 mg of metronidazole three times/day, and 500 mg of tetracycline HCl four times/day. 11,46,53,54,56,70,71) Bismuth can be given twice a day.
Hybrid Therapy
Hybrid therapy consists of two seven-day phases. 11,66,74. During the first seven-day phase, standard doses of amoxicillin are taken three or four times a day and a PPI is taken twice a day. In the second phase, two other antibiotics, usually 500 mg clarithromycin and 500 mg metronidazole or tinidazole, are also taken twice a day. Hybrid therapy’s effectiveness is 97%. 75 When there combined resistance to clarithromycin and metronidazole is greater than 9% (dual resistance), efficacy is less than 90%. 66 Recently, a 15-day hybrid therapy scheme studied in Colombia achieved an efficacy of 94%. 76 It included 500 mg of amoxicillin four times/day and 40 mg of esomeprazole twice a day for 15 days with the addition of two tablets of bismuth subsalicylate and 100 mg of Vibramycin® twice a day for the last ten days.
Concomitant Therapy
Concomitant therapy, the most commonly used scheme, combines PPIs with amoxicillin, clarithromycin and metronidazole. 46,53,54,70,71 It is recommended because dual resistance to clarithromycin and metronidazole is infrequent.26,46,53,54,70,71 When there is dual resistance, efficacy decreases markedly. 66,74,77 The scheme’s overall efficacy is 88% -90%. 38,46 As mentioned, a new strategy that began in Europe adds bismuth to different triple therapies to counteract resistance to clarithromycin and levofloxacin. It is currently valid and is recommended in the Colombian guidelines. 53-56
Standard Triple Therapy
This which includes a PPI, amoxicillin and clarithromycin scheme was very popular in recent decades, 53,54,55,70,71 but currently must be administered for 14 days when the local resistance level to clarithromycin is less than 15%. The scheme is amoxicillin 3-4 times/day, a PPI and 500 mg of clarithromycin twice a day. 53-55 If local resistance is more than 15%, two chewable tablets of bismuth subsalicylate (Bisbacter®) should be added before breakfast and dinner. Alternatively, clarithromycin can be replaced with 500 mg of levofloxacin/day. If the resistance to levofloxacin is greater than 20%, bismuth at the dosage described above can be added.
First, second and third line choices
First Line Treatments
First line treatments, the initial treatment, should be based on susceptibility tests. 10,46 When they are, efficacy by intention to treat (ITT) is 94.7% (95% CI: 88.8% -100%) and efficacy per protocol (PP) is 96.4% (95% CI: 91.5% -100%) compared to 71.9% (95% CI: 60.2% -83.5%) and 73.2% (95% CI: 61.5% -84.8%) for empirical therapies. (78) When clarithromycin resistance is less than 15%, triple therapy with clarithromycin plus amoxicillin and PPI may be used for 14 days. 53 If it is greater than 15%, it is recommended that clarithromycin be replaced with levofloxacin or alternatively that bismuth be added. 53,54,70,71 Bismuth can be in the form of two chewable tablets of subsalicylate (Bisbacter®) before breakfast and two more before dinner.
In Colombia, the rate of resistance to clarithromycin is 20.5%, 79, the rate of resistance to levofloxacin is 27.3%, 80 and the rate of resistance to metronidazole is over 80%. 43 With this particular profile of resistance, the recommendation is to add two chewable tablets of subsalicylate (Bisbacter®) before breakfast and two more before dinner to the 14 day triple therapies. Quadruple therapies with bismuth do not need susceptibility testing. 52
Second and Third Line Treatments
When a first-line treatment fails, the second-line treatment is chosen from among the schemes that do not include the antibiotics used in the first line. Third-line treatments are chosen according to the same principle. Other possibilities include classical quadruple therapy, concomitant therapy or hybrid therapy. 40
Rescue Treatments
Rescue treatments are the fourth line used when the first three treatment lines have failed. (40 The antibiotic used in these schemes is furazolidone or rifabutin. 53,54,70,71,81
The recommended dose of furazolidone is 100 mg three times a day for 14 days. 43,64,65,66 It is used in quadruple therapies with a PPI, bismuth, two to four 4 times a day, and either amoxicillin or tetracycline. 43,64-66 This medication has been used in Colombia in 14 day quadruple therapy with eradication rates of 86% (95% CI: 65% -94%) for amoxicillin 82 and 91% for tetracycline. 83 The is 850 mg of amoxicillin three times a day or 500 mg of amoxicillin every 6 hours, 100 mg of furazolidone three times a day, two chewable tablets of subsalicylate (Bisbacter®) before breakfast and two more before dinner, and a PPI twice a day. For those who are allergic to penicillin, 500 mg of tetracycline every six hours replaces amoxicillin in the scheme.
Despite its efficacy, furazolidone is underused due to fear of adverse effects including speculation that it may have oncogenic effects (Although, there is no evidence for this.) 63,65,66,84,85 The International Agency for Research on Cancer of the WHO (IARC) has included furazolidone in category 3, “Not classifiable as a carcinogen for humans”. 84 More recent studies have confirmed furazolidone’s safety. 46,64,84
Rifabutin
Schemes with rifabutin call for 150 mg of rifabutin, amoxicillin, and a PPI twice a day for 10 days. 86 This triple therapy’s efficacy is 79% as a rescue treatment after three or four other schemes fail, 55 but the addition of bismuth increases efficacy to 96.6%. 87 Rifabutin is chosen for rescue therapy schemes for patients with important or serious pathologies such as complicated peptic ulcers, gastric MALT lymphomas and histories of CG. 55
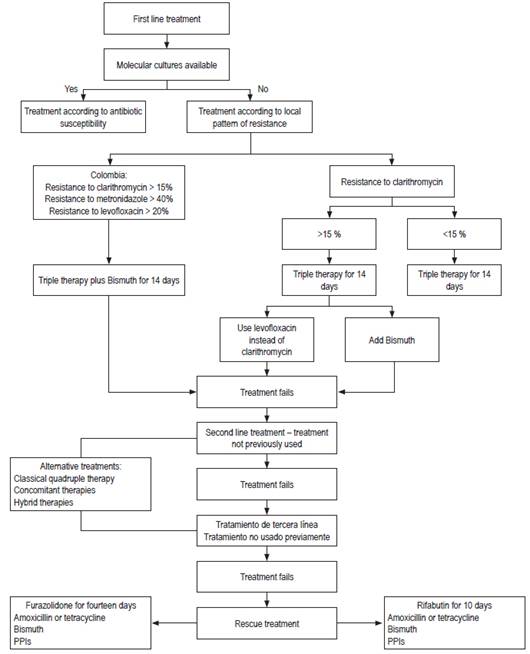
Figure 1 below shows a flow chart of suggested treatment schemes for H. pylori.
Probiotics
Probiotics are live microorganisms or substances produced by them that have beneficial effects. 21,88 They have been used as adjuvants to antibiotic H. pylori eradication therapy whose attributed benefits include stimulation of the immune response, modulation of the microbiota and decreased adverse effects from antibiotics. 21 The species that has been studied most is Lactobacillus spp. Some studies have found improved efficacy rates, 88 and other studies and metaanalyses have confirmed the benefit of probiotics. These also include Biffidobacterium spp. and Saccharomyces boulardii. 88,89. The latter recently attracted attention when a 2015 metaanalysis covering more than 2200 participants found that the relative risk (RR) of success in the eradication of H. pylori in the group receiving this supplement was 1.11 (95% CI: 1.06-1.17) and that the RR of adverse effects related to therapy was 0.44 (95% CI: 0.31-0.64). The RR for diarrhea was 0.51 (95% CI: 0.42-0.62). 89 Probiotics’ mechanisms of action are not entirely clear and multiple strains, doses, and duration of probiotic therapy have been studied. 90
Currently there is no clear picture or uniform recommendation for use of these products. The Maastricht consensus only considered some specific strains to be useful for increasing efficacy and reducing the occurrence of adverse effects related to antibiotics, 52 but neither the Toronto consensus nor the Spanish guidelines recommend probiotics. 54,71
Biofilm’s Importance
Similar to microorganisms such as Pseudomonas aeruginosa and Staphylococcus aureus, H. pylori produces biofilm aggregates of organisms growing together to take advantage of surfaces or interfaces. 91 The biofilm is an ancient and integral component in the prokaryotic life cycle and is currently seen as an independent virulence factor which is recognized as a cause of exacerbation of chronic respiratory infections. 4 Biofilm notably favors H. pylori by making it difficult for antibiotics to reach the bacteria, decreasing replication due to the limitation of nutrients and protecting them from cellular and humoral immune responses. 91
The mucolytic N-acetylcysteine (NAC) has been used to weaken H. pylori’s biofilm, before initiation of antibiotics. 92 An unblinded clinical trial published in 2010 used this strategy after another treatment had failed and achieved an efficacy rate of 65% in the NAC group while the efficacy rate in the control group was only 20%. 93 Other biofilm degrading substances and enzymes have been tried in in-vitro studies but their results have yet to be validated in clinical trials. 4
Vaccines
Vaccination has emerged as a new preventive alternative focus in the fight against bacterial resistance. 94 H. pylori vaccines tested in animal models have achieved acceptable levels of protection, 23 but results in humans have varied depending on the route of immunization and the antigens used. 95 Recently, the first randomized double-blind clinical trial found efficacy obtained for immunogenicity to be 71% at one year and 55% at 3 years. 96 To date, no definitive vaccine has become available. 97
Referencias
1. Arévalo A, Trespalacios AA, Otero W. Importancia de la proteína CagA en la infección por Helicobacter pylori. Rev. Col Gastroenterol 2009; 24 (4): 388-95. [ Links ]
2. Trespalacios AA, Otero W, Caminos J, et al. Phenotypic and Genotypic Analysis of Clarthromycin-Resistant Helicobacter pylori from Bogotá D.C., Colombia. J Microbiol. 2013; 51 (4): 448-452. doi: 10.1007/s12275-013-2465-6. [ Links ]
3. Otero W, Trespalacios AA, Otero E. Helicobacter pylori: Tratamiento actual. Un importante reto en gastroenterología. Rev Colomb Gastroenterol. 2009; 24(3):279-92. [ Links ]
4. Cammarota G, Sanguinetti M, Gallo A, Posteraro B. Review article: biofilm formation by Helicobacter pylori as a target for eradication of resistant infection. Aliment Pharmacol Ther 2012; 36 (3): 222-230. doi: 10.1111/j.1365-2036.2012.05165.x. [ Links ]
5. Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153(2):420-429. doi: 10.1053/j.gastro.2017.04.022. [ Links ]
6. Cosme A, Montes M, Martos M, Gil I, Mendarte U, Salicio Y, et al. Usefulness of antimicrobial susceptibility in the eradication of Helicobacter pylori. Clinical Microbiology and Infection 2013;19(4):379-83. doi: 10.1111/j.1469-0691.2012.03844.x. [ Links ]
7. Peleteiro B, Bastos A, Ferro A, Nuno L. Prevalence of Helicobacter pylori Infection Worldwide: A Systematic Review of Studies with National Coverage. Dig Dis Sci 2014; 59 (8): 1698-1709. doi: 10.1007/s10620-014-3063-0. [ Links ]
8. Axon A. Helicobacter pylori and Public Health. Helicobacter 2014;19(Suppl 1): 68-73. doi: 10.1111/hel.12155. [ Links ]
9. Gisbert JP. Enfermedades relacionadas con la infección por Helicobacter pylori. Gastroenterol Hepatol. 2015;38(Supl 1):39-48. doi: 10.1016/S0210-5705(15)30018-2. [ Links ]
10. Gisbert JP. Enfermedades relacionadas con la infección por Helicobacter pylori. Gastroenterol Hepatol. 2014;37(Supl 3):40-52. doi: 10.1016/S0210-5705(14)70082-2. [ Links ]
11. Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-5. doi: 10.1016/S0140-6736(84)91816-6. [ Links ]
12. Kyle R, Steensma D, Shampo M. Barry James Marshall-Discovery of Helicobacter pylori as a Cause of Peptic Ulcer. Mayo Clin Proc. 2016;91(5):e67-e68. doi: 10.1016/j.mayocp.2016.01.025. [ Links ]
13. Otero W, Gómez M, Trespalacios AA. Helicobacter pylori: después de todo. Temas escogidos de gastroenterología. Asociación Colombiana de Gastroenterología. 2007;43-56. [ Links ]
14. Urrego J, Otero W, Gomez M. Helicobacter prlori y enfermedades hematológicas. Rev Col Gastroenterol. 2013;28(4):329-37. [ Links ]
15. Mégraud F, Bessède E, Varon C, Helicobacter pylori infection and gastric carcinoma. Clin Microbiol Infect 2015;21(11):984-90. doi: 10.1016/j.cmi.2015.06.004. [ Links ]
16. Graham DY. Helicobacter pylori Update: Gastric Cancer, Reliable Therapy, and Possible Benefits. Gastroenterol 2015;148(4):719-31. doi: 10.1053/j.gastro.2015.01.040. [ Links ]
17. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Schistosomes, liver flukes and Helicobacter pylori. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1-241. [ Links ]
18. Gomez M, Ruiz O, Hernandez D, Albis R, Otero W, Sabbagh L. Erradicación del Helicobacter pylori: encuesta realizada por la Asociación Colombiana de Gastroenterología. Rev. Col Gastroenterol. 2015;30(1):25-31. doi: 10.22516/25007440.19. [ Links ]
19. Malfertheiner P, Venerito M, Selgrad M. Helicobacter pylori infection: selected aspects in clinical management. Curr Opin Gastroenterol. 2013;29(6):669-75. doi: 10.1097/MOG.0b013e328365d443. [ Links ]
20. Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64(9):1353-67. doi: 10.1136/gutjnl-2015-309252. [ Links ]
21. Molina J, Shiotani A. Practical Aspects in Choosing a Helicobacter pylori Therapy. Gastroenterol Clin N Am 2015; 44:519-535. doi: 10.1016/j.gtc.2015.05.004. [ Links ]
22. Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010; 59: 1143-1153. doi: 10.1136/gut.2009.192757. [ Links ]
23. Malfertheiner P, Link A, Selgrad M. Helicobacter pylori: perspectives and time trends. Nat Rev Gastroenterol Hepatol. 2014;11(10):628-38. doi: 10.1038/nrgastro.2014.99. [ Links ]
24. Molina J, Gisbert JP. Optimizing clarithromycin-containing therapy for Helicobacter pylori in the era of antibiotic resistance. World J Gastroenterol. 2014;20(30):10338-47. doi: 10.3748/wjg.v20.i30.10338. [ Links ]
25. Sugimoto M, Furuta T. Efficacy of tailored Helicobacter pylori eradication therapy based on antibiotic susceptibility and CYP2C19 genotype. World J Gastroenterol. 2014;20(21):6400-11. doi: 10.3748/wjg.v20.i21.6400. [ Links ]
26. Furuta T, Graham DY. Pharmacologic Aspects of Eradication Therapy for Helicobacter pylori Infection. Gastroenterol Clin N Am. 2010;39(3):465-80. doi: 10.1016/j.gtc.2010.08.007. [ Links ]
27. Kim SY, Choi DJ, Chung JW. Antibiotic treatment for Helicobacter pylori: Is the end coming? World J Gastrointest Pharmacol Ther. 2015;6(4):183-98. doi: 10.4292/wjgpt.v6.i4.183. [ Links ]
28. Furuta T, Ohashi K, Kamata T, Takashima M, Kosuge K, Kawasaki T, et al. Effect of Genetic Differences in Omeprazole Metabolism on Cure Rates for Helicobacter pylori Infection and Peptic Ulcer. Ann Intern Med. 1998;129(12):1027-30. doi: 10.7326/0003-4819-129-12-199812150-00006. [ Links ]
29. Tang HL, Li Y, Hu YF, Xie HG, Zhai SD. Effects of CYP2C19 loss-of-function variants on the eradication of H. pylori infection in patients treated with proton pump inhibitor-based triple therapy regimens: a meta-analysis of randomized clinical trials. PLOS ONE. 2013;8(4):e62162. doi: 10.1371/journal.pone.0062162. [ Links ]
30. Lee JY, Kim N, Kim MS, Choi YJ, Lee JW, Yoon H, et al. Factors affecting first-line triple therapy of Helicobacter pylori including CYP2C19 genotype and antibiotic resistance. Dig Dis Sci. 2014;59:1235-43. doi: 10.1007/s10620-014-3093-7. [ Links ]
31. Echizen H. The first-in-class potassium-competitive acid blocker, vonoprazan fumarate: Pharmacokinetic and pharmacodynamic considerations. Clin Pharmacokinet. 2016;55:409-18. doi: 10.1007/s40262-015-0326-7. [ Links ]
32. Hori Y, Imanishi A, Matsukawa J, Tsukimi Y, Nishida H, Arikawa Y, et al. 1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamin e monofumarate (TAK-438), a novel and potent potassium-competitive acid blocker for the treatment of acid-related diseases. J Pharmacol Exp Ther. 2010;335(1):231-8. doi: 10.1124/jpet.110.170274. [ Links ]
33. Sakurai Y, Nishimura A, Kennedy G, Hibberd M, Jenkins R, Okamoto H, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single rising TAK-438 (Vonoprazan) doses in healthy male Japanese/ non-Japanese subjects. Clin Transl Gastroenterol 2015; 6:e94. doi: 10.1038/ctg.2015.18 [ Links ]
34. Garnock-Jones KP. Vonoprazan: first global approval. Drugs. 2015;75(4):439-43. doi: 10.1007/s40265-015-0368-z. [ Links ]
35. Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut. 2016;65(9):1439-46. doi: 10.1136/gutjnl-2015-311304. [ Links ]
36. Jung YS, Kim EH, Park CH. Systematic review with meta-analysis: the efficacy of vonoprazan-based triple therapy on Helicobacter pylori eradication. Aliment Pharmacol Ther.. 2017;46(2):106-14. doi: 10.1111/apt.14130. [ Links ]
37. Malfertheiner P, Selgrad M. Helicobacter pylori. Curr Opin Gastroenterol. 2014;30(6):589-95. doi: 10.1097/MOG.0000000000000128. [ Links ]
38. Gisbert JP, McNicholl AG. Optimization strategies aimed to increase the efficacy of H. pylori eradication therapies. Helicobacter.. 2017; 22(4). doi: 10.1111/hel.12392. [ Links ]
39. Camargo MC, García A, Riquelme A, et al. The Problem of Helicobacter pylori Resistance to Antibiotics: A Systematic Review in Latin America. Am J Gastroenterol. 2014;109(4):485-95. doi: 10.1038/ajg.2014.24. [ Links ]
40. Otero W, Otero L, Gómez M. Helicobacter pylori: tratamiento en 2018. En: Gómez M (editor). Temas Escogidos de Gastroenterología. 2018. p. 63-78. [ Links ]
41. Thung I, Aramin H, Vavinskaya V, Gupta S, Park JY, Crowe SE, et al. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. 2016;43(4):514-33. doi: 10.1111/apt.13497. [ Links ]
42. Horiki N, Omata F, Uemura M, Suzuki S, Ishii N, Iizuka Y, et al. Annual change of primary resistance to clarithromycin among Helicobacter pylori isolates from 1996 through 2008 in Japan. Helicobacter. 2009;14(5):86-90. doi: 10.1111/j.1523-5378.2009.00714.x. [ Links ]
43. Trespalacios AA, Otero W, Mercado M, et al. Impacto de la resistencia de Helicobacter pylori a los antimicrobianos en la eficacia de la terapia triple estándar y en dos triples terapias con levofloxacina en pacientes colombianos. Gastroenterol Latinoam. 2012;23:S35. [ Links ]
44. World Health Organization. WHO publishes list of bacteria for which new antibiotics are urgently needed. WHO [internet] 2017 [acceso el 20 de diciembre de 2017]. Disponible en: Disponible en: www.who.int/entity/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/ . [ Links ]
45. Wu JY, Liou JM, Graham DY. Evidence-based recommendations for successful Helicobacter pylori treatment. Expert Rev Gastroenterol Hepatol. 2014;8(1):21-8. doi: 10.1586/17474124.2014.859522. [ Links ]
46. Graham DY, Dore MP. Helicobacter pylori therapy: a paradigm shift. Expert Review of Anti-infective Therapy 2016, 14(6):577-85. doi: 10.1080/14787210.2016.1178065. [ Links ]
47. Graham DY, Lee SY. How to Effectively Use Bismuth Quadruple Therapy: The Good, the Bad, and the Ugly. Gastroenterol Clin North Am. 2015; 44: 537-563. doi: 10.1016/j.gtc.2015.05.003. [ Links ]
48. Trespalacios-Rangél AA, Otero W, Arévalo-Galvis A, Poutou-Piñales RA, Rimbara E, Graham DY. Surveillance of Levofloxacin Resistance in Helicobacter pylori Isolates in Bogotá-Colombia (2009-2014). PLoS One. 2016;11(7):e0160007. doi: 10.1371/journal.pone.0160007. [ Links ]
49. Sugimoto M, Sahara S, Ichikawa H, Kagami T, Uotani T, Furuta T. High Helicobacter pylori cure rate with sitafloxacin-based triple therapy. Aliment Pharmacol Ther. 2015;42(4):477-83. doi: 10.1111/apt.13280. [ Links ]
50. Marcus EA, Sachs G, Scott DR. Eradication of Helicobacter pylori infection. Current gastroenterology reports. 2016;18(7):33. doi: 10.1007/s11894-016-0509-x. [ Links ]
51. Keogan DM, Griffith DM. Current and Potential Applications of Bismuth-Based Drugs. Molecules 2014; 19: 15258-97. doi: 10.3390/molecules190915258. [ Links ]
52. Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66(1):6-30. doi: 10.1136/gutjnl-2016-312288. [ Links ]
53. Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, et al. Management of Helicobacter pylori infection-the Maastricht IV/ Florence Consensus Report. Gut. 2012;61(5):646-64. doi: 10.1136/gutjnl-2012-302084. [ Links ]
54. Gisbert JP, Molina-Infante J, Amador J, Bermejo F, Bujanda L, Calvet X, et al. IV Conferencia Española de Consenso sobre el tratamiento de la infección por Helicobacter pylori. Gastroenterol Hepatol. 2016;39(10):697-721. doi: 10.1016/j.gastrohep.2016.05.003. [ Links ]
55. Otero W, Trespalacios AA, Otero L, Vallejo M, Torres M, Pardo R, et al. Guía de práctica clínica para el diagnóstico y tratamiento de la infección por Helicobacter pylori en adultos. Rev Col Gastroenterol. 2015;30(Suppl 1):17-33. [ Links ]
56. Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut. 2016;65(5):870-8. doi: 10.1136/gutjnl-2015-311019. [ Links ]
57. Zhang W, Chen Q, Lian X, et al. Bismuth, lansoprazole, amoxicillin and metronidazole or clarithromycin as first-line Helicobacter pylori therapy. Gut. 2015;64:1715-20. doi: 10.1136/gutjnl-2015-309900. [ Links ]
58. Gokcan H, Oztas E, Onal IK. Different bismuth-based therapies for eradicating Helicobacter pylori: Randomized clinical trial of efficacy and safety. Clin Res Hepatol Gastroenterol. 2016;40:124-31. doi: 10.1016/j.clinre.2015.06.014. [ Links ]
59. O’Morain, C. and Montague, S. Challenges to Therapy in the Future. Helicobacter. 2000;5:23-6. doi: 10.1046/j.1523-5378.2000.0050S1023.x. [ Links ]
60. Perri F, Festa V, Andriulli A. Treatment of antibiotic-resistant Helicobacter pylori. N Engl J Med 1998; 339:53. doi: 10.1056/NEJM199807023390116. [ Links ]
61. Crabol Y, Catherinot E, Veziris N, et al. Rifabutin: where do we stand in 2016? J Antimicrob Chemother. 2016;71(7):1759-71. doi: 10.1093/jac/dkw024. [ Links ]
62. Della Bruna C, Schioppacassi G, Ungheri D, et al. LM 427, a new spiropiperidylrifamycin: in vitro and in vivo studies. J Antibiot (Tokyo). 1983;36:1502-6. doi: 10.7164/antibiotics.36.1502. [ Links ]
63. Gisbert JP, Calvet X. Review article: rifabutin in the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther. 2012;35:209-21. doi: 10.1111/j.1365-2036.2011.04937.x. [ Links ]
64. Graham DY, Lu H. Furazolidone in Helicobacter pylori therapy: misunderstood and often unfairly maligned drug told in a story of French bread. Saudi J Gastroenterol. 2012; 18:1-2. doi: 10.4103/1319-3767.91724. [ Links ]
65. Cheng H, HU FL.Furazolidone , amoxicillin, bismuth and rabeprazole quadruple rescue therapy for the eradication of Helicobacter pylori. World J Gastroenterol. 2009;15:860-64. doi: 10.3748/wjg.15.860. [ Links ]
66. Lu H, Zhang W, Graham DY. Bismuth containing quadruple therapy for Helicobacter pylori: lessons from China. Eur J Gastroenterol Hepato 2013;25:1134-40. doi: 10.1097/MEG.0b013e3283633b57. [ Links ]
67. Mohammadi M, Attaran B, Malekzadeh R, et al. Furazolidone, an Underutilized Drug for H. pylori Eradication: Lessons from Iran. Dig Dis Sci. 2017;62(8):1890-6. doi: 10.1007/s10620-017-4628-5. [ Links ]
68. Hu Y, Zhu Y, Lu N-H. Novel and Effective Therapeutic Regimens for Helicobacter pylori in an Era of Increasing Antibiotic Resistance. Front. Cell. Infect. Microbiol. 2017; 7:168. doi: 10.3389/fcimb.2017.00168. [ Links ]
69. Yuan Y, Ford AC, Khan KJ, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev. 2013;12:CD008337. doi: 10.1002/14651858.CD008337.pub2. [ Links ]
70. Chey WD, Leontiadis GI, Howden CW, et al. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol 2017;112:212-39. doi: 10.1038/ajg.2016.563. [ Links ]
71. Fallone C, Chiba N, van Zanten S, Fischbach L, Gisbert JP, Hunt R, et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults, Gastroenterology 2016; 151, 51-69.e14. doi: 10.1053/j.gastro.2016.04.006. [ Links ]
72. Calvet X. Diagnosis of Helicobacter pylori Infection in the proton pump Inhibitor era. Gastroenterol Clin N Am. 2015;44(3):507-18. doi: 10.1016/j.gtc.2015.05.001. [ Links ]
73. Malfertheiner P, Mégraud F, O’Morain C, et al. Current European concepts in the management of Helicobacter pylori infection-the Maastricht Consensus Report. The European Helicobacter pylori Study Group (EHPSG). Eur J Gastroenterol Hepatol. 1997;9:1-2. doi: 10.1097/00042737-199701000-00002. [ Links ]
74. Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. 2014;12:177-186. doi: 10.1016/j.cgh.2013.05.028. [ Links ]
75. Hsu PI, Wu DC, Wu JH, et al. Modified sequential Helicobacter pylori therapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days. Helicobacter 2011;16:139-45. doi: 10.1111/j.1523-5378.2011.00828.x. [ Links ]
76. Otero W, Gutiérrez O, Saabagh LC: Efectiveness of a hibrid therapy in eradicating Helicobacter pylori in a Colombian population. Gastroenterology. 2016;150:S246. doi: 10.1016/S0016-5085(16)30889-7. [ Links ]
77. Ducoumau A, Bénéjat L, Sifré LE, Lehours P, Mégraud F. Helicobacter pylori resistance to antibiotics in 2014 in France detected by phenotypic and genotypic methods. Clin Microbiol Infect 2016;22:715-8. doi: 10.1016/j.cmi.2016.06.003. [ Links ]
78. Park CS, Lee SM, Park CH, et al. Pretreatment Antimicrobial Susceptibility-Guided Vs. Clarithromycin-Based Triple Therapy for Helicobacter pylori Eradication in a Region With High Rates of Multiple Drug Resistance. Am J Gastroenterol. 2014;109:1595-602. doi: 10.1038/ajg.2014.222. [ Links ]
79. Arévalo A, Trespalacios AA, Otero W. Personalized therapy for Helicobacter pylori: influence of cyp2c19 genotype in first triple therapy. Sometido y aceptado para publicación. Helicobacter. 2019. [ Links ]
80. Trespalacios-Rangél AA, Otero W, Arévalo-Galvis A, Poutou-Piñales RA, Rimbara E, Graham DY. Surveillance of Levofloxacin Resistance in Helicobacter pylori Isolates in Bogotá-Colombia (2009-2014). PLoS One. 2016;11(7):e0160007. doi: 10.1371/journal.pone.0160007. [ Links ]
81. Villoria A, García P, Calvet X, Gisbert J, Vergara M. Meta-analysis: high dose proton pump inhibtor vs standard dose in triple therapy for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2008;28:868-77. doi: 10.1016/S0016-5085(08)61572-3. [ Links ]
82. Segura AM, Gutiérrez O, Otero W, Angel LA, Genta R, Graham DY. Furazolidone, amoxycillin, bismuth triple therapy for Helicobacter pylori infection. Aliment Pharmacol Ther 1997;11:529-32. doi: 10.1046/j.1365-2036.1997.00172.x. [ Links ]
83. Otero W, Gutierrez O, Sierra F. Erradicación de H pylori con Terapia Triple: Bismuto, Furazolidona, Tetraciclina. Acta Med Col. 1996;21:218 (Res). [ Links ]
84. Some food additives, feed additives and naturally occurring substances. IARC Monogr Eval Carcinog Risk Chem Hum. 1983;31:1-291. [ Links ]
85. Auro A, Sumano H, Ocampo L, Barragán A. Evaluation the carcinogenic effects of furazolidone and its metabolites in two fish species. Pharmacogenomics J. 2004;4:24-8. doi: 10.1038/sj.tpj.6500216. [ Links ]
86. Sun Q, Liang X, Zheng Q. High efficacy of 14 -day triple therapy-based, bismuth-containing quadruple therapy for initial Helicobacter pylori eradication. Helicobacter 2010;15:233-8. doi: 10.1111/j.1523-5378.2010.00758.x. [ Links ]
87. Ciccaglione AF, Tavani R, Grossi L, et al. Rifabutin containing triple therapy and rifabutin with bismuth Containing quadruple therapy for third-line treatment of Helicobacter pylori infection: Two pilot studies. Helicobacter. 2016;21:375-81. doi: 10.1111/hel.12296. [ Links ]
88. O’Connor A., Vaira D., Gisbert JP, O’Morain C. Treatment of Helicobacter pylori infection 2014. Helicobacter. 2014;19(Suppl 1):38-45. doi: 10.1111/hel.12163. [ Links ]
89. Szajewska H, Horvath A, Kołodziej M. Systematic review with meta-analysis: Saccharomyces boulardii supplementation and eradication of Helicobacter pylori infection. Aliment Pharmacol Ther 2015;41:1237-45. doi: 10.1111/apt.13214. [ Links ]
90. Luther J, Chey WD, Saad RJ. A clinician’s guide to salvage therapy for persistent Helicobacter pylori infection. Hosp Pract (1995). 2011;39:133-140. doi: 10.3810/hp.2011.02.383. [ Links ]
91. Yonezawa H, Osaki T, Kamiya S. Biofilm Formation by Helicobacter pylori and its involvement for antibiotic resistance. Biomed Res Int 2015;2015:914791. doi: 10.1155/2015/914791. [ Links ]
92. Cammarota G, Ianiro G, Bibbò S, et al. Culture-guided treatment approach for Helicobacter pylori infection: Review of the literature. World J Gastroenterol. 2014;20(18):5205-11. doi: 10.3748/wjg.v20.i18.5205. [ Links ]
93. Cammarota G, Branca G, Ardito F, et al. Biofilm demolition and antibiotic treatment to eradicate resistant Helicobacter pylori: a clinical trial. Clin Gastroenterol Hepatol. 2010;8:817-20. doi: 10.1016/j.cgh.2010.05.006. [ Links ]
94. Graham DY, Calvet X. Guide Regarding Choice of Second-line Therapy to Obtain a High Cumulative Cure Rate. Helicobacter. 2012;17:243-5. doi: 10.1111/j.1523-5378.2012.00952.x. [ Links ]
95. Urrego JA, Otero W, Trespalacios A. Avances en la búsqueda de la vacuna contra Helicobacter pylori. MÉD.UIS. 2017;30(3):111-20. [ Links ]
96. Zeng M, Mao XH, Li JX, Tong WD, Wang B, Zhang YJ, et al. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386(10002):1457-64. doi: 10.1016/S0140-6736(15)60310-5. [ Links ]
97. Sutton P. At last, vaccine-induced protection against Helicobacter pylori. Lancet. 2015;386(1002):1424-5. doi: 10.1016/S0140-6736(15)60579-7. [ Links ]
Received: February 14, 2018; Accepted: May 02, 2018











 texto en
texto en