Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.33 no.4 Bogotá Oct./Dec. 2018
https://doi.org/10.22516/25007440.191
Case report
Case report of a pulmonary air embolism secondary to endoscopic retrograde cholangiopancreatography in a liver transplant patient
1Médico internista, gastroenterólogo, Fundación Universitaria Santa Fe de Bogotá. Bogotá D. C., Colombia
2Médico internista, gastroenterólogo, jefe del servicio de gastroenterología y hepatología de la Fundación Universitaria Santa Fe de Bogotá. Bogotá D. C., Colombia.
3Residente de cirugía general, Universidad del Bosque. Bogotá D. C., Colombia
Endoscopic retrograde cholangiopancreatography (ERCP) is one of the most important therapeutic resources for management of biliary complications of liver transplantation. However, several complications including acute pancreatitis, hemorrhage, perforations, infections and cardiopulmonary adverse events can occur. Air embolisms occur very infrequently but are severe and potentially fatal complications. We report a case of post-ERCP embolism in a 55-year-old woman with a history of liver transplantation and stenosis of the biliary anastomosis. The clinical presentation, the diagnosis, the treatment and the possible mechanisms involved in this complication are discussed.
Keywords: Endoscopic retrograde cholangiopancreatography (ERCP); complications; air embolism; liver transplant
La colangiopancreatografía retrógrada endoscópica (CPRE) es uno de los recursos terapéuticos más importantes para el manejo de las complicaciones biliares del trasplante hepático. Sin embargo, se pueden presentar varias complicaciones: pancreatitis aguda, hemorragia, perforaciones, infecciones y eventos adversos cardiopulmonares. La embolia aérea es una complicación muy infrecuente, severa y potencialmente fatal. Se reporta un caso de embolia aérea post-CPRE en una mujer de 55 años con antecedente de trasplante hepático y estenosis de la anastomosis biliar. Se discute la presentación clínica, el diagnóstico, el tratamiento y los posibles mecanismos involucrados en esta complicación.
Palabras clave: Colangiopancreatografía retrógrada endoscópica (CPRE); complicaciones; embolia aérea; trasplante hepático
Introduction
Air embolisms occur as complications of endoscopic procedures an extremely infrequently but are potentially fatal. 1,2 A systematic review of the 41 cases of air embolism secondary to endoscopic procedures reported up to the 2013 date of publication found that 26 cases were secondary to endoscopic retrograde cholangiopancreatography (ERCP). 1 These embolisms were caused by direct communication between the air insufflated into the intestinal lumen and the blood vessels of the intestinal and/or biliary wall and were associated with pressure gradients favoring passage of air into the circulating blood and into altered intestinal or bile mucosa. The physiological effect of air embolism depends on the speed of infusion and the volume of air infused into circulation. Embolisms can be either venous or arterial and can produce ischemia in any organ. 1 Mechanisms for post-ERCP air embolisms include transection of duodenal root veins during sphincterotomy, dissection of intramural air into the portal vein, biliary-venous shunts or leaks, portacaval collateral veins, airflow directly into the hepatic vein or into the inferior vena cava, retrograde flow of air into the cerebral veins via the superior vena cava and inability of pulmonary circulation to filter out the air embolus. 1,2,3
Case report
The patient was a 55-year-old woman who had undergone liver transplantation in 2007 due to Budd-Chiari syndrome. She developed biliary anastomotic stenosis which was managed by ERCP for 1 year (pneumatic dilations and insertion of plastic biliary stents). Following recurrence of the biliary stenosis, a fully coated 80 mm by 10 mm self-expanding metal stent (SEMS) was inserted. Four months later she came to our clinic because of abdominal pain. Abdominal ultrasound showed a 20 mm dilation of the common bile duct with stones inside the duct. Paraclinical tests showed alkaline phosphatase levels three times higher than normal, normal bilirubin and transaminases (alkaline phosphatase: 290 IU/L, normal value: 32-91, aspartate aminotransferase: 25 U/L, normal value: 15-51) alanine aminotransferase: 31 U/L, normal value: 17-63, total bilirubin: 0.9 mg/dL, normal value: 0.3-1.2, direct bilirubin: 0.2 mg/dL, normal value: 0.1-0.5). An ERCP was performed under general anesthesia. The preliminary x-ray showed the metallic biliary stent and proximal pneumobilia (Figure 1A). After duodenoscopy found that the SEMS had migrated proximally. An extractor balloon was introduced, insufflated and retracted leaving its distal end partially exposed in the duodenal lumen. The SEMS was then extracted with a foreign body forceps. Upon examination, it could be seen that the SEMS had its lumen totally obstructed by stones and biliary sludge.
Cholangiography showed that the proximal extrahepatic bile duct was dilated to 18 millimeters in diameter and that there was a biliary anastomosis without stenosis (Figure 1B). The bile duct was instrumented with a balloon and the residual stones were extracted. The final radiographic plate showed serious pneumobilia that also compromised the small proximal intrahepatic bile ducts (Figure 1C). In the immediate postoperative period while the patient was still intubated, ventricular tachycardia followed by a trace of pulseless electrical activity was documented and desaturation, hypotension, cyanosis and marked jugular engorgement developed. Resuscitation measures were initiated, and spontaneous circulation returned after 2 minutes and administration of 1 mg of adrenaline, 300 mg of amiodarone, 60 meq of bicarbonate and infusion of norepinephrine (0.25 μg/k/m). The right subclavian central venous access was accessed.
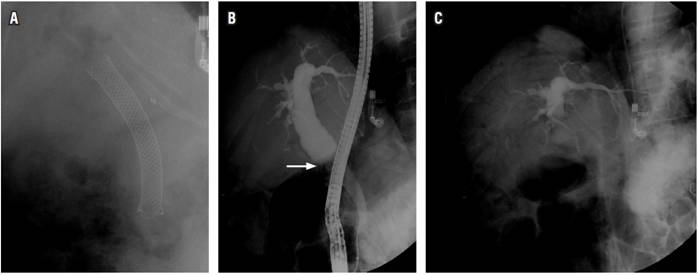
Figure 1A Preliminary radiograph, with SEMS and pneumobilia in the proximal bile duct, above the proximal edge of the stent. 1B. Cholangiogram after removal of the biliary prosthesis. Dilated proximal bile duct, biliary anastomosis (arrow) without stenosis. 1C. X-ray at the end of the procedure, with residual contrast medium in the proximal bile duct and extensive pneumobilia in the intrahepatic bile duct.
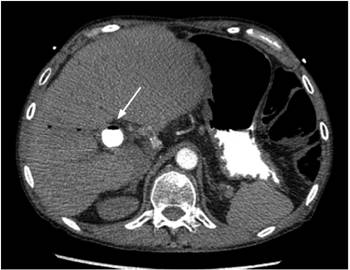
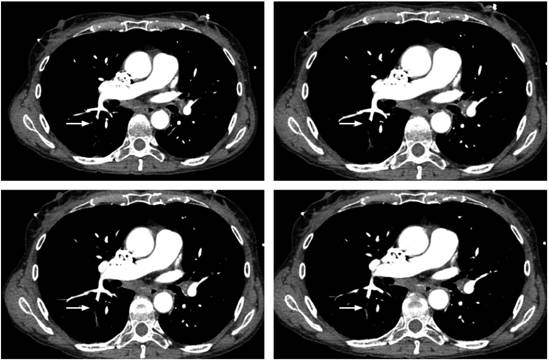
Focused assessment with sonography for trauma (FAST) ultrasound ruled out cardiac tamponade, but evidenced multiple air bubbles in the vena cava. The patient was immediately transferred to CT angiography due to suspicion of air embolism. CT angiography showed air in the intrahepatic vena cava (Figure 2) and air embolus in one of the subsegmental pulmonary arteries of the apical segment of the right lower lobe (Figure 3). The patient was transferred to the intensive care unit (ICU). A transthoracic echocardiogram showed collapse of the inferior cava, a finding expected in a post-resuscitation state, without left ventricular dysfunction. The patient progressively improved, was extubated on the second day, and was discharged after four days without additional complications or sequelae.
Discussion
Air embolisms are characterized by hemodynamic instability due to the arrival of air in the right ventricle with increased pressure in the pulmonary artery, obstruction of the outflow of the right ventricle and compromise of the pulmonary venous return to the left heart. This alters the preload causing decreased cardiac output, cardiovascular collapse and vascular insufficiency of organs such as the brain. 1,2 Possible consequences include arrhythmias, hypotension, myocardial ischemia, acute right heart failure, hypoxia, cyanosis, jugular engorgement, ocular deviation, mydriasis, altered consciousness, hypertonicity, hemiparesis, cerebral edema, coma and death. 3,4
Generally, the symptoms of air embolism during ERCP occur when the patient is repositioned in the supine position at the end of the procedure. 1,5 The majority of the symptoms are nonspecific and high clinical suspicion is required to make a correct diagnosis and initiate appropriate management. 6
Air embolisms occur more frequently in ERCPs than in any other endoscopic procedure, but they can occur during other procedures. 5 Associated risk factors include previous endoscopic or surgical intervention into the biliary tree, transjugular intrahepatic portosystemic shunts (TIPS), closed or penetrating liver trauma, inflammatory processes of the digestive tract (pylephlebitis, hepatic abscesses, inflammatory bowel disease, necrotizing enterocolitis, mesenteric ischemia), postoperative gastrointestinal fistula, gastrointestinal neoplasms, atresia of the biliary tract, interventional procedures (cholangioscopy, biliary sphincterotomy, percutaneous hepatic drainage, metallic biliary stent), liver biopsy, high-volume air insufflation and/or high pressure at site of a procedure above the level of the heart, and the use of nitrous oxide. 1,2 Insufflation with carbon dioxide (CO2) instead of air decreases the risks of air embolism because CO2 is absorbed more quickly and easily. Precordial Doppler cardiac monitoring during the procedure can detect air in the heart and in the pulmonary vasculature before symptoms appear. 5,6
One of the most common characteristics of patients who develop post-ERCP air embolisms is a history of previous endoscopic or surgical interventions into the biliary tree. 5 In the case reported here, there were several associated factors that could act together in the development of the air embolism: multiple previous interventions in the biliary tract (liver transplantation with end-to-end biliary anastomosis, several ERCPs for the management of biliary stenosis, insertion of a SEMS), gallstones, a SEMS occluded in the lumen, and the consequent inflammatory process in the walls of the bile duct. 4 Due to the proximal migration of the biliary stent and the maneuvers necessary for its removal, trauma of the biliary mucosa and the duodenal papilla occurred during stent extraction in a previously inflamed mucosa. This favored exposure of mucosal and submucosal blood vessels to contact with air and subsequent intravascular diffusion. 1,2
Extraction of a blocked stent and instrumentation of the bile duct with a balloon, together with the broad opening between the bile duct and the duodenal lumen resulting from the previous papillotomy, produced negative pressure in the biliary tree that facilitated the rapid passage of air from the duodenum into the intrahepatic biliary tract. This is demonstrated in the radiographic plate from the ERCP (important pneumobilia that involves the small intrahepatic bile ducts in a generalized manner). This abundant air rapidly entered the bile duct and was able to diffuse easily into the systemic circulation (right cardiac cavities) through the hepatic veins which could constitute another mechanism for air embolism. 3
The diagnosis of this entity is difficult because air can be rapidly absorbed from the circulation while diagnostic tests are being performed. Transthoracic or transesophageal echocardiography has been used to identify air in dilated right ventricles and hypertension of the pulmonary artery. This can be used to rule out abnormalities of cardiac contractility,1,7 but CT angiography can detect an air embolism with greater sensitivity. 2,6
In the case presented, a FAST ultrasound showed air in the vena cava, which, together with the clinical picture, suggested the diagnosis of an air embolism. This was confirmed with CT angiography performed immediately after the initial phase of cardiorespiratory resuscitation.
Management of a patient when an air embolism is suspected requires maneuvers to reduce the physiological impact. The endoscopic procedure should be stopped immediately, 100% oxygen should be administered, administration of large volumes of saline solution should be initiated, the patient should be placed in the Trendelenburg position and left lateral decubitus to minimize migration of air to the brain, and nitrous oxide should be suspended if it is being used.1,4 Air aspiration can be performed through a central catheter and hyperbaric oxygen begun. This reduces the size of bubbles, accelerates reabsorption of nitrogen and decreases the risk of ischemia.1,2 If cardiorespiratory arrest occurs, advanced cardiopulmonary resuscitation should be initiated. This also decreases the size of large bubbles and forces air out of the right ventricle into the pulmonary veins. 1
REFERENCES
1. Donepudi S, Chavalitdhamrong D, Pu L, Draganov PV. Air embolism complicating gastrointestinal endoscopy: A systematic review. World J Gastrointest Endosc. 2013;5(8):359-65. doi: 10.4253/wjge.v5.i8.359. [ Links ]
2. Kwon CI, Song SH, Hahm KB, Ko KH. Unusual complications related to endoscopic retrograde cholangiopancreatography and its endoscopic treatment. Clin Endosc. 2013;46(3):251-9. doi: 10.5946/ce.2013.46.3.251. [ Links ]
3. Cha ST, Kwon CI, Seon HG, Ko KH, Hong SP, Hwang SG, et al. Fatal biliary-systemic air embolism during endoscopic retrograde cholangiopancreatography: a case with multifocal liver abscesses and choledochoduodenostomy. Yonsei Med J. 2010;51(2):287-90. doi: 10.3349/ymj.2010.51.2.287. [ Links ]
4. Bisceglia M, Simeone A, Forlano R, Andriulli A, Pilotto A. Fatal systemic venous air embolism during endoscopic retrograde cholangiopancreatography. Adv Anat Pathol. 2009;16(4):255-62. doi: 10.1097/PAP.0b013e3181aab793. [ Links ]
5. Green BT, Tendler DA. Cerebral air embolism during upper endoscopy: case report and review. Gastrointest Endosc. 2005;61(4):620-3. [ Links ]
6. Bechi A, Nucera MP, Olivotto I, Manetti R, Fabbri LP. Complete neurological recovery after systemic air embolism during endoscopic retrograde cholangiopancreatography. Minerva Anestesiol. 2012;78(5):622-5. [ Links ]
7. Rangappa P, Uhde B, Byard RW, Wurm A, Thomas PD. Fatal cerebral arterial gas embolism after endoscopic retrograde cholangiopancreatography. Indian J Crit Care Med. 2009;13(2):108-12. doi: 10.4103/0972-5229.56061. [ Links ]
PosterPresented as number 0223 at the Pan American Congress of Gastroenterology in Cartagena, Colombia September 10-13, 2016
Received: December 07, 2017; Accepted: April 17, 2018











 text in
text in