Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.33 no.4 Bogotá oct./dic. 2018
https://doi.org/10.22516/25007440.206
Case report
Recurrent albendazole-induced acute hepatitis
1Núcleo de Estudios en Gastroenterología y Hepatología, Departamento de Medicina Interna, Universidad Federal de Santa Catarina. Florianópolis, Santa Catarina, Brasil.
2Departamento de Patología, Universidad Federal de Santa Catarina. Florianópolis, Santa Catarina, Brasil
3Programa de Posgrado en Ciencias Médicas, Centro de Ciencias de la Salud. Núcleo de estudios en gastroenterología y hepatología, Departamento de Medicina Interna, Universidad Federal de Santa Catarina. Florianópolis, Santa Catarina, Brasil.
Albendazole is used to treat helminth infections and usually has minimal or no side effects. A transient increase in liver enzymes is common following its use, but little evidence of albendazole-induced liver damage has been reported in the literature. This study presents a patient who developed acute hepatitis following self-medication with albendazole. The patient also had a history of similar episodes in the past after using the drug. After a thorough investigation and exclusion of all other causes of the patient’s clinical condition, the Roussel Uclaf Causality Assessment Method of the Council for International Organizations of Medical Sciences scale yielded a score of 10 points, indicating a high probability of albendazole-induced liver damage. In conclusion, expediting the process of combating helminths is ideal, but quality monitoring is required to avoid adverse reactions such as drug-induced hepatitis. Moreover, self-medication with any drug should always be discouraged.
Keywords: Drug-Induced Liver Injury; Albendazole; Alanine transaminase
El albendazol es un medicamento usado para tratar infecciones por helmintos y usualmente presenta pocos o ningún efecto secundario. A pesar de que hay un incremento transitorio de enzimas hepáticas luego de su uso, existe poca evidencia en la literatura en la que se reporte lesión hepática luego de automedicación con albendazol. En este informe, el paciente se presentó con hepatitis aguda luego de automedicarse con albendazol. El paciente cuenta además con una historia de episodios similares después de haber usado el fármaco. Se evaluada la causalidad con el método de evaluación de causalidad de Roussel Uclaf del Concejo para Organizaciones Internacionales de Ciencias Médicas, cuyo resultado fue un puntaje de 10, lo que indicó una alta probabilidad de lesión hepática inducida por albendazol al cabo de realizarse una investigación rigurosa y de excluir otras posibles causas de la condición física del paciente. En conclusión, aunque es ideal agilizar el proceso para combatir a los helmintos, es necesario intensificar la necesidad de monitorizaciones de calidad para evitar reacciones adversas como la hepatitis inducida por medicamentos. Asimismo, la automedicación de cualquier medicamento debe ser siempre evitada.
Palabras clave: Lesión hepática inducido por medicamentos; albendazol; alanina-aminotransferasa
Introduction
Albendazole is a widely used drug in the treatment of parasitosis. Its effectiveness is proven against several species of parasites and it’s the treatment of choice for cysticercosis and hydatid disease in the United States.1 It is also used to treat strongyloidiasis, taeniasis, ascariasis, hookworm, trichuriasis, oxyuriasis, among others.1 The absorption of this drug is erratic and is better absorbed when consumed with fatty foods.1,2,3 Albendazole is a prodrug that is converted into the active metabolite albendazole sulfoxide by first-pass metabolism in the liver, and its metabolites are excreted through the urine.1,2 Its effect is mediated by the inhibition of microtubule synthesis in the parasites’ cells.1-5
Albendazole’s side effects are rare, especially when administered for 1-3 days. When they do occur, they are usually mild. The most frequent side effects are epigastric discomfort, diarrhea, nausea, dizziness, headache, malaise, and insomnia. Prolonged therapy most commonly leads to adverse reactions, such as abdominal discomfort, headache, fever, fatigue, alopecia, increased liver enzymes, and pancytopenia.1,3,4
Reports of liver injury induced by albendazole are rare 6-14 and according to research, recurrent episodes are even rarer.10-14 We herein report a case of recurrent hepatitis related to seasonal empirical use of albendazole.
Case report
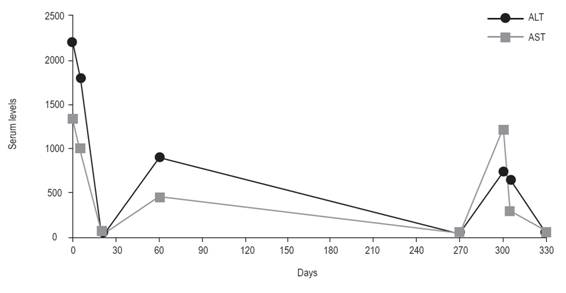
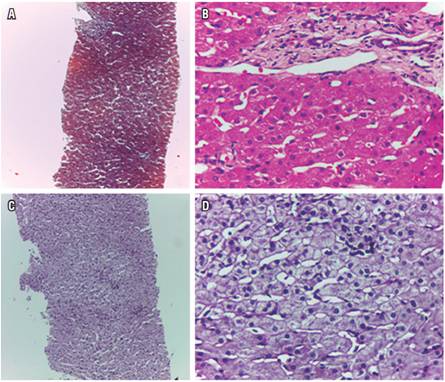
A 36-year-old woman presented to the specialist doctor’s office with a “liver problem.” Three weeks earlier, she developed a fever of 38°C, myalgia, and sore throat. She also reported dark urine, nausea, and asthenia. She recalled self-medicating with albendazole before the onset of her symptoms and reported using this medication every summer. She had a history of insomnia and recurrent episodes of diarrhea, fever, malaise, myalgia, and jaundice for 3 consecutive years during the months of November and January. She denied smoking, alcohol use, blood transfusions, or use of illicit drugs. There was no family history of liver disease. Physical examination of the patient showed anicteric sclerae without the stigmata of liver disease; she had no asterixis. Laboratory tests revealed hemoglobin level of 12.8 g/dL, hematocrit of 38.7%, leukocyte count of 4,830/mm3, and platelet count of 180,000/mm3. Hepatic biochemical tests revealed the following results: aspartate aminotransferase (AST) = 1,304 U\L, alanine aminotransferase (ALT) = 2,220 U\L, alkaline phosphatase (ALP) =158 U\L, gamma glutamyl transferase (GGT) =123 U\L, total bilirubin = 5.89 mg\L, direct bilirubin = 3.89 mg\L and prothrombin activity of 71%. Levels of ceruloplasmin (27.7 mg/dL), ferritin (145 μg/L), and transferrin saturation (49%) were normal. Anti-HAV IgG and IgM; HBsAg; IgM AntiHBc; and total anti-HBc, anti-HCV, and anti-HIV serology were negative. Tests for the following antibodies were negative: antinuclear antibodies, smooth muscle, anti-liver kidney microsomes 1, and anti-mitochondrial antibodies. Figure 1 shows the evolution of aminotransferase values to more than ten times the upper normal limit after the first exposure to albendazole. An exacerbation occurred 60 days after discontinuation of the drug, followed by progressive normalization. A new episode similar to the original appeared 10 months later after re-exposure (Figure 1). The patient underwent liver biopsy, which revealed minimal and unspecific reactive changes (Figure 2).
Figure 1. Evolution of aminotransferases after drug suspension and rechallenge 10 months later
Figure 2. Liver biopsy shows (A) preserved overall architecture, (B) port space maintained with few mononuclear cells and intact limiting plate, (C) light lobular changes, and (D) parenchyma with slight modifications and few mononuclear cells.
Discussion
The diagnosis of acute hepatitis is confirmed by the presence of jaundice or non-specific symptoms of acute illness accompanied by elevation of AST and/or ALT activities.15,16 The best discriminant values for recognizing acute hepatic injury appear to be 200 U/L for AST and 300 U/L for ALT.15 Different etiologies have similar clinical presentation, such as hepatitis by hepatotropic virus, drug induced liver injury (DILI), autoimmune hepatitis, ischemic hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, Wilson’s disease, alcoholic hepatitis, cardiac and thyroid diseases, etc. However, serum aminotransferase levels 10 times greater than the upper limit of normal and with near-normal GGT levels restrict the differential diagnosis to some specific etiologies, including hepatitis by hepatotropic virus; DILI; autoimmune hepatitis; ischemic hepatitis; alcoholic hepatitis; infectious causes, such as dengue, Epstein-Barr Virus, cytomegalovirus, toxoplasmosis.15,16)
DILI is a disease of variable clinical presentation, with a spectrum ranging from asymptomatic liver failure to chronic liver disease.6,15,16,17 It is a difficult condition to identify and its diagnosis is through exclusion, based on clinical history, history of drug use and evaluation of the patient’s hepatic biochemical profile.17,18
The drug classes responsible for cases of DILI in a population differ according to the geographical location. In Western countries, the majority of cases are associated with antibiotics, anticonvulsants, and psychotropic agents. In Asia, on the other hand, herbal dietary supplements rather than conventional medications are the most common causes of DILI.18
In younger patients, the hepatocellular pattern is more prevalent, and in older patients, cholestasis is more common.19 More than 17% of cases are attributed to idiosyncratic reactions, and liver failure is uncommon. There are no standardized and globally recognized ways to predict adverse reactions.6
In this case, the patient in question was asymptomatic and had a history of self-medication with over-the-counter albendazole. Hookworm infections, for instance, are considered to be a leading cause of pathological blood loss in tropical and subtropical countries.20 Sanitation alone can control hookworm disease, however this method is extremely low.20 The administration of anthelminthics as preventive treatment and mass anthelmintic therapy are recommended in specific settings.20,21 In the absence of adequate sanitation, the World Health Organization (WHO) advocates that anthelminthic treatment needs to be repeated every 6-12 months.20 However, these recommendations are not always followed, and the unrestricted and unsupervised use of an over-the-counter anthelminthic drug, at random dosages, can expose a subject to risky situations, such as the DILI. As reported in this study, albendazole can cause significant liver damage, and because the side effect is not trivial, albendazole-induced liver injury should always be considered by physicians when other causes have been excluded.
Teschke et al. performed a systematic review of 2906 cases of DILI 22 and observed that 14% of the presumptive diagnoses of this condition were made without satisfactorily eliminating other possible etiologies. In this case report, the episodes of altered liver enzymes coincided with the period in which the patient used the medication. The possibility of viral hepatitis was ruled out by negative serology. Moreover, because of disease recurrence, the main differential diagnosis would be autoimmune hepatitis, which was also eliminated by the negative autoantibody test results and the biopsy report that demonstrated no significant liver damage. Although the patient’s laboratory tests did not follow the classical presentation, less likely causes were also excluded; accumulation diseases were excluded because of the normal levels of ceruloplasmin and ferritin. Also, the near-normal GGT values were not suggestive of cholestatic diseases.
As DILI is a diagnosis of exclusion, there are no absolute criteria or specific diagnostic methods to accurately diagnose this condition. Therefore, diagnostic scales are used for this purpose. The most commonly used scale is the Roussel Uclaf Causality Assessment Method of the Council for International Organizations of Medical Sciences scale (RUCAM/CIOMS).23,24,25 This scale rates the likelihood of a drug being related to the occurrence of liver injury according to the score obtained. In this case, the patient had a score of 10 points, which indicates a high probability that albendazole was the cause of her liver injury. Although transient increases in aminotransferase levels is a common adverse effect (approximately 10% of cases), 26,27,28) the prevalence of acute hepatitis due to this drug is rare.
Conclusion
Wariness of pre-formed concepts about numerous medicines considered innocuous is necessary, as exemplified in this case. This report is not meant to discourage empirical or non-bureaucratic administration of anthelmintic drugs by teachers, family members, or other non-health professionals, including those recommended by WHO in endemic areas. However, extrapolating this recommendation for low-prevalence areas, such as Southern Brazil,29 may be, at least, incautious. Expediting the process of combating helminths is ideal; however, we must magnify the need for quality monitoring that avoids gaps that lead to adverse reactions, such as drug-induced hepatitis. Moreover, self-medication for any drug with harmful potential should always be discouraged.
Referencias
1. Katzung B, Trevor AJ. Basic & clinical pharmacology. Nueva York: McGraw-Hill; 2015. [ Links ]
2. Dayan AD. Albendazole, mebendazole and praziquantel. Review of non-clinical toxicity and pharmacokinetics. Acta Tropica. 2003;86:141-59. doi: 10.1016/S0001-706X(03)00031-7. [ Links ]
3. Venkatesan P. Albendazole. J Antimicrob Chemother. 1998;41:145-7. doi: 10.1093/jac/41.2.145. [ Links ]
4. Horton J. Albendazole: a review of anthelmintic efficacy and safety in humans. Parasitology. 2001;121:S113. doi: 10.1017/S0031182000007290. [ Links ]
5. Goodman LS, Brunton LL, Chabner B, Knollmann BC. Goodman & Gilman’s the pharmacological basis of therapeutics. 12.a edición. Nueva York: McGraw-Hill ; 2011. [ Links ]
6. Marin Zuluaga JI, Marin Castro AE, Perez Cadavid JC, Restrepo Gutierrez JC. Albendazole-induced granulomatous hepatitis: a case report. J Med Case Rep. 2013;7:201. doi: 10.1186/1752-1947-7-201. [ Links ]
7. Choi GY, Yang HW, Cho SH, Kang DW, Go H, Lee WC, et al. Acute drug-induced hepatitis caused by albendazole. J Korean Med Sci. 2008;23:903-5. doi: 10.3346/jkms.2008.23.5.903. [ Links ]
8. Koca T, Akcam M. Albendazole-induced autoimmune hepatitis. Indian Pediatr. 2015;52:78-9. [ Links ]
9. Rios D, Restrepo JC. Albendazole-induced liver injury: a case report. Colomb Med (Cali). 2013;44:118-20. [ Links ]
10. Nandi M, Sarkar S. Albendazole-induced recurrent hepatitis. Indian Pediatr 2013;50:1064. doi: 10.1007/s13312-013-0285-8. [ Links ]
11. Amoruso C, Fuoti M, Miceli V, Zito E, Celano MR, De Giorgi A, et al. Acute hepatitis as a side effect of albendazole: a pediatric case. Pediatr Med Chir. 2009;31(6):262-4. [ Links ]
12. Ben Fredj N, Chaabane A, Chadly Z, Ben Fadhel N, Boughattas NA, Aouam K. Albendazole-induced associated acute hepatitis and bicytopenia. Scand J Infect Dis. 2014;46(2):149-51. doi: 10.3109/00365548.2013.835068. [ Links ]
13. Gozukucuk R, Abci I, Guclu M. Albendazole-induced toxic hepatitis: A case report. The Turkish Journal of Gastroenterology. 2009;24:82-84. doi: 10.4318/tjg.2013.0426. [ Links ]
14. Shah C, Mahapatra A, Shukla A, Bhatia S. Recurrent acute hepatitis caused by albendazole. Trop Gastroenterol. 2013;34(1):38-9. doi: 10.7869/tg.2012.90. [ Links ]
15. Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. II. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clin Chem. 2000;46(12):2050-68. [ Links ]
16. Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clin Chem. 2000;46(12):2027-49. [ Links ]
17. Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135(6):1924-34, 1934.e1-4. doi: 10.1053/j.gastro.2008.09.011. [ Links ]
18. Fontana RJ, Seeff LB, Andrade RJ, Björnsson E, Day CP, Serrano J, et al. Standardization of nomenclature and causality assessment in drug-induced liver injury: summary of a clinical research workshop. Hepatology. 2010;52(2):730-42. doi: 10.1002/hep.23696. [ Links ]
19. Ghabril M, Chalasani N, Bjornsson E. Drug-induced liver injury: a clinical update. Curr Opin Gastroenterol. 2010;26:222-6. doi: 10.1097/MOG.0b013e3283383c7c. [ Links ]
20. Pawlowski ZS, Schad GA, Stott GJ. Hookworm infection and anaemia: approaches to prevention and control. Génova: World Health Organization; 1991. [ Links ]
21. Checkley AM, Chiodini PL, Dockrell DH, Bates I, Thwaites GE, Booth HL, et al. Eosinophilia in returning travellers and migrants from the tropics: UK recommendations for investigation and initial management. J Infect. 2010;60(1):1-20. doi: 10.1016/j.jinf.2009.11.003. [ Links ]
22. Teschke R, Frenzel C, Wolff A, Eickhoff A, Schulze J. Drug induced liver injury: accuracy of diagnosis in published reports. Ann Hepatol. 2014;13(2):248-55. [ Links ]
23. Danan G, Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323-30. doi: 10.1016/0895-4356(93)90101-6. [ Links ]
24. Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs--II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol. 1993;46:1331-6.doi: 10.1016/0895-4356(93)90102-7. [ Links ]
25. Benichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990;11:272-6. doi: 10.1016/0168-8278(90)90124-A. [ Links ]
26. Farrar J, Hotez PJ, Junghanss T, Kang G, Lalloo D, White N. Manson›s tropical diseases. 23.a edición. Elsevier; 2014. [ Links ]
27. Morris DL, Dykes PW, Marriner S, Bogan J, Burrows F, Skeene-Smith H, et al. Albendazole--objective evidence of response in human hydatid disease. JAMA. 1985;253(14):2053-7. [ Links ]
28. Morris DL, Smith PG. Albendazole in hydatid disease--hepatocellular toxicity. Trans R Soc Trop Med Hyg. 1987;81:343-4. doi: 10.1016/0035-9203(87)90259-8. [ Links ]
29. Bueno GCL, Reis M, Dantas-Corrêa EB, Schiavon LL, Narciso-Schiavon JL. The prevalence of intestinal parasitosis according to gender in a University Hospital in Southern Brazil. Rev Patol Trop. 2015;44(4):441-52. doi: 10.5216/rpt.v44i4.39240. [ Links ]
Received: February 05, 2018; Accepted: March 23, 2018











 texto en
texto en